

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type-1 angiotensin II receptor A (RAT) | BDBM50082568 (2-({3-[4-(2-Azido-benzenesulfonylaminocarbonyl)-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A. Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. | J Med Chem 42: 4572-83 (1999) BindingDB Entry DOI: 10.7270/Q2XW4J1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50082567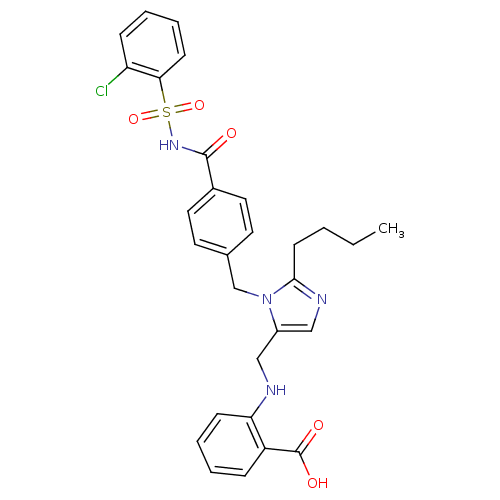 (2-({2-Butyl-3-[4-(2-chloro-benzenesulfonylaminocar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A. Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. | J Med Chem 42: 4572-83 (1999) BindingDB Entry DOI: 10.7270/Q2XW4J1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50082570 (2-[2-butyl-1-(4-carboxybenzyl)-1H-5-imidazolylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A. Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. | J Med Chem 42: 4572-83 (1999) BindingDB Entry DOI: 10.7270/Q2XW4J1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor A (RAT) | BDBM50082569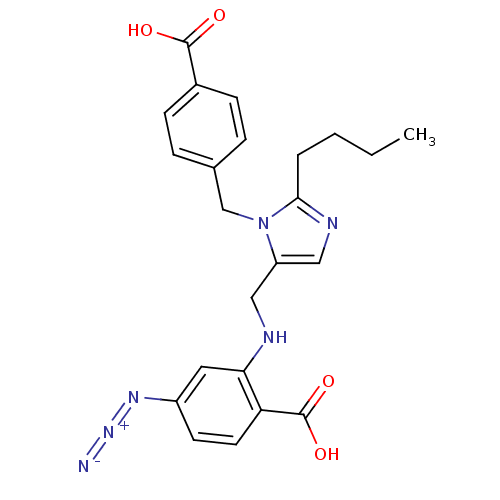 (4-Azido-2-{[2-butyl-3-(4-carboxy-benzyl)-3H-imidaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratoires Fournier S.A. Curated by ChEMBL | Assay Description The compound was evaluated for the inhibition of [125I]-Sar-AII binding to Angiotensin II receptor, type 1 from purified rat liver membranes. | J Med Chem 42: 4572-83 (1999) BindingDB Entry DOI: 10.7270/Q2XW4J1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50009777 ((ponalrestat)[3-(4-Bromo-2-fluoro-benzyl)-4-oxo-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068078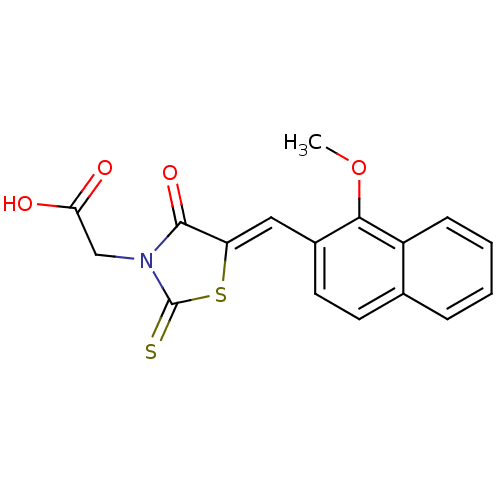 (CHEMBL142239 | {5-[1-(1-Methoxy-naphthalen-2-yl)-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-2 adrenergic receptor activity was assessed from the ability to inhibit [3H]yohimbine binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM31046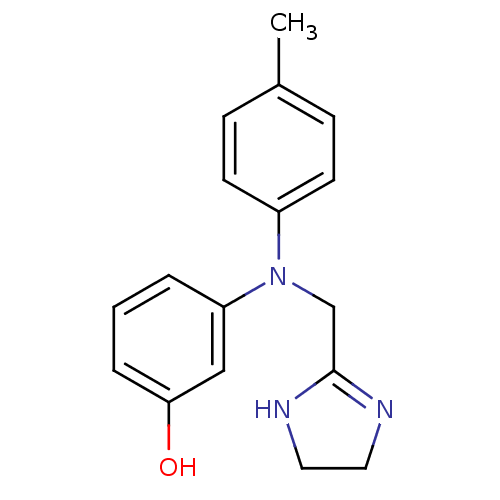 (3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-2 adrenergic receptor activity was assessed from the ability to inhibit [3H]yohimbine binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM31046 (3-[4,5-dihydro-1H-imidazol-2-ylmethyl-(4-methylphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068085 (CHEMBL358284 | {5-[1-(1-Bromo-naphthalen-2-yl)-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068083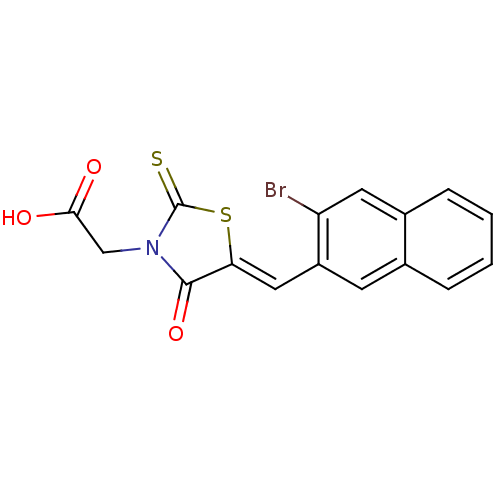 (CHEMBL358825 | {5-[1-(3-Bromo-naphthalen-2-yl)-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068086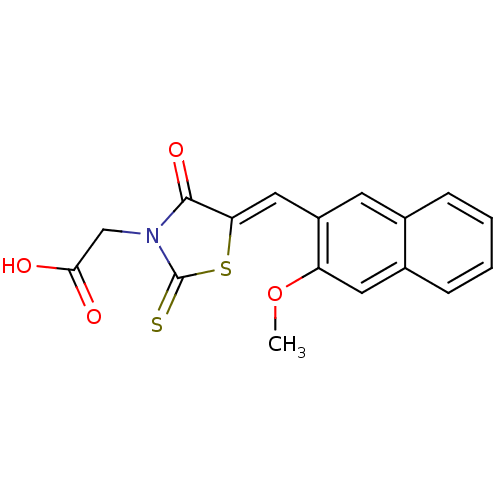 (CHEMBL142701 | {5-[1-(3-Methoxy-naphthalen-2-yl)-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228240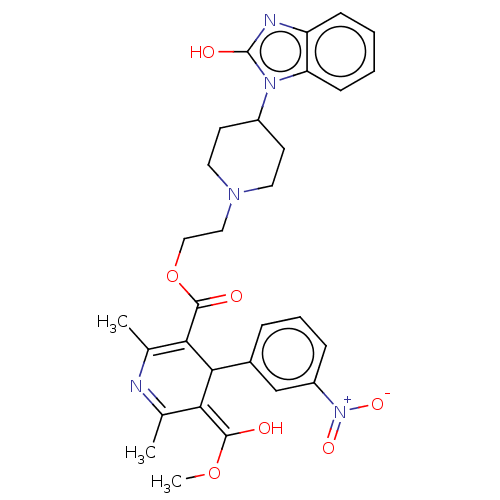 (CHEMBL284056) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068074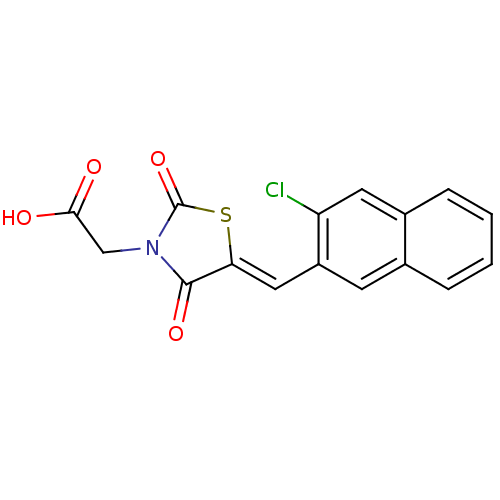 (CHEMBL142001 | {5-[1-(3-Chloro-naphthalen-2-yl)-me...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068075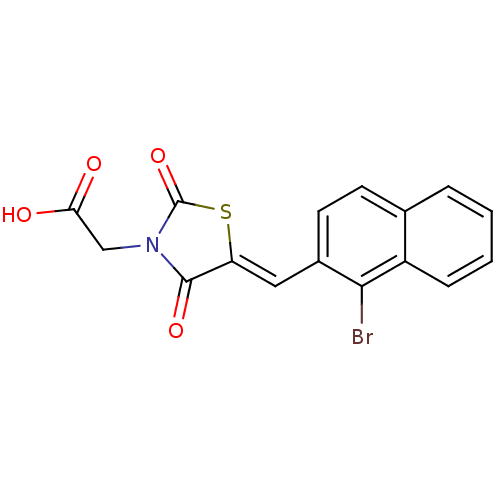 (CHEMBL336874 | {5-[1-(1-Bromo-naphthalen-2-yl)-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068073 (CHEMBL143658 | {5-[1-(6-Methoxy-naphthalen-2-yl)-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068076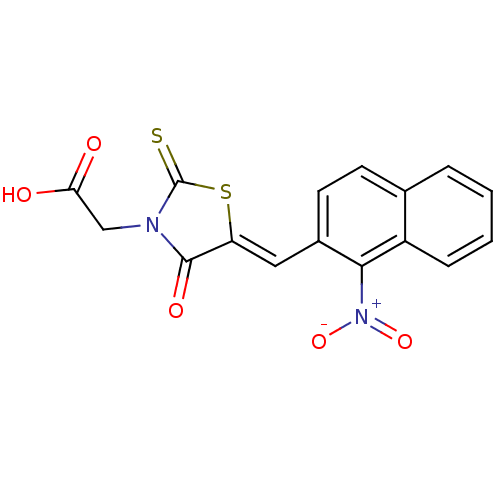 (CHEMBL143933 | {5-[1-(1-Nitro-naphthalen-2-yl)-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068080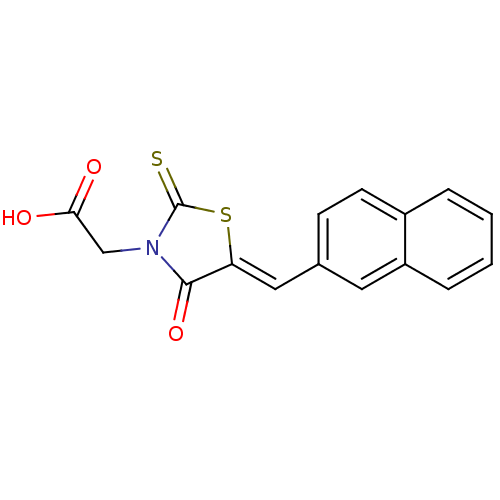 (CHEMBL142615 | {5-[1-Naphthalen-2-yl-meth-(Z)-ylid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068084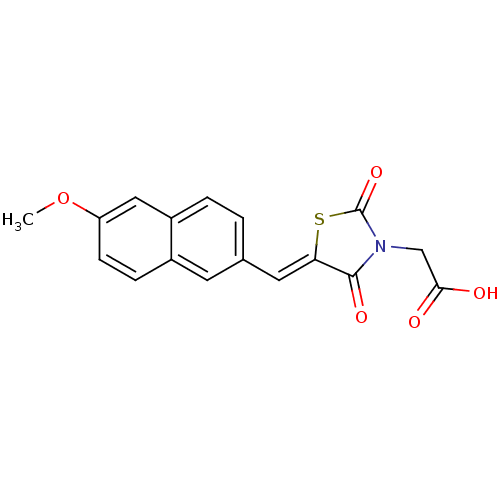 (CHEMBL142869 | {5-[1-(6-Methoxy-naphthalen-2-yl)-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068081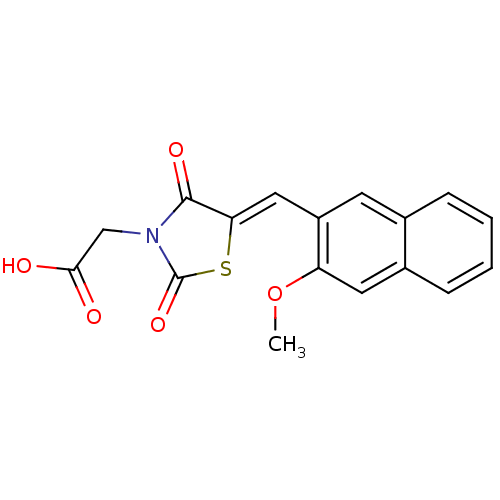 (CHEMBL142390 | {5-[1-(3-Methoxy-naphthalen-2-yl)-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 128 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-2 adrenergic receptor activity was assessed from the ability to inhibit [3H]yohimbine binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068077 (CHEMBL139720 | {5-[1-(1-Methoxy-naphthalen-2-yl)-m...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068082 (CHEMBL345945 | {5-[1-(3-Bromo-naphthalen-2-yl)-met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 153 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228236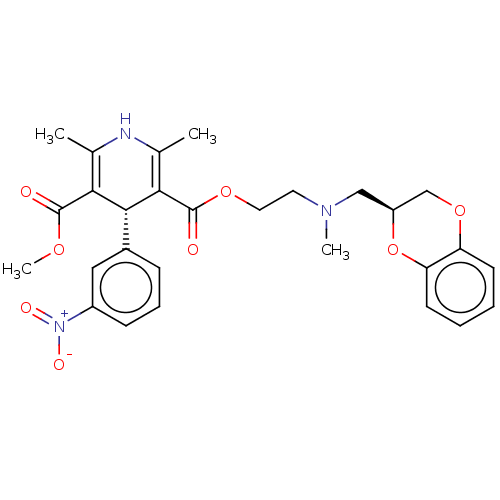 (CHEMBL3350238) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-2-adrenolytic activity was assessed from the ability to inhibit [3H]yohimbine binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member B1 [K65Q] (Bos taurus (Cattle)) | BDBM50068079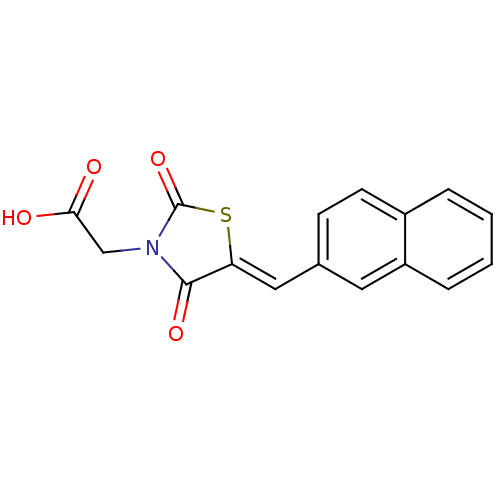 (CHEMBL142385 | {5-[1-Naphthalen-2-yl-meth-(Z)-ylid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 269 | n/a | n/a | n/a | n/a | n/a | n/a |
EP811-CNRS Curated by ChEMBL | Assay Description Evaluated in vitro for the inhibition of Aldose reductase. | J Med Chem 41: 4706-15 (1998) Article DOI: 10.1021/jm9801399 BindingDB Entry DOI: 10.7270/Q2JQ1040 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228238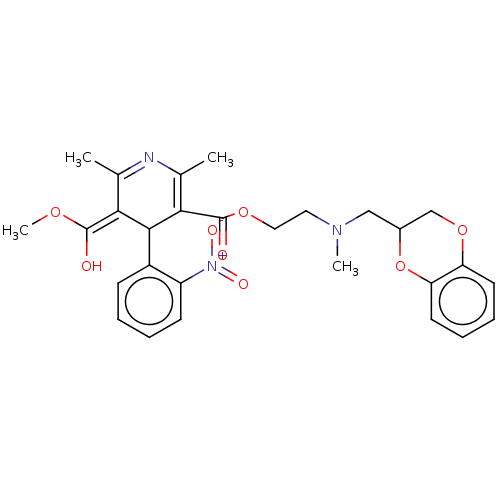 (CHEMBL30911) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Ability to inhibit [3H]yohimbine binding to alpha-2 adrenergic receptor of rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228238 (CHEMBL30911) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228242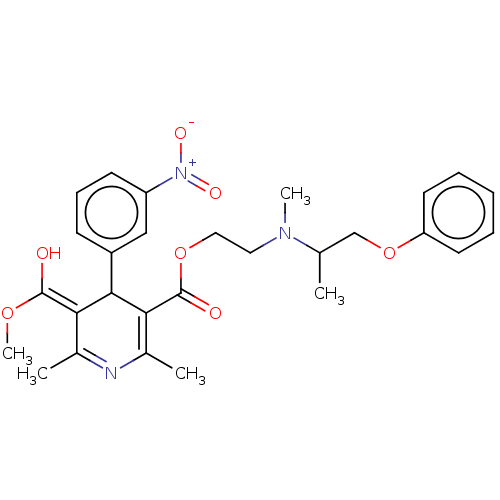 (CHEMBL542812) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228244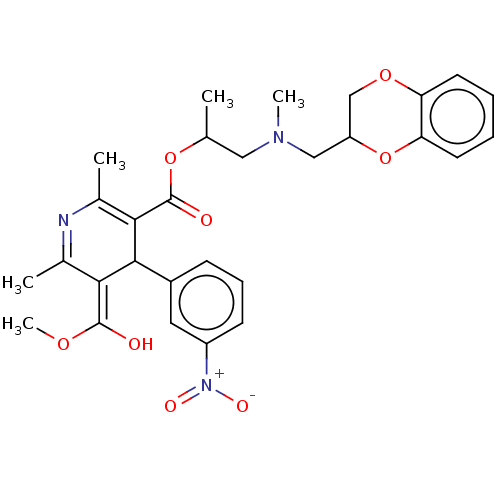 (CHEMBL553942) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Ability to inhibit [3H]yohimbine binding to alpha-2 adrenergic receptor of rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228237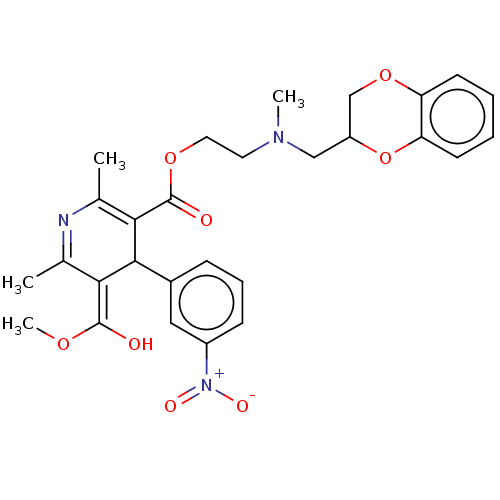 (CHEMBL554455) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Ability to inhibit [3H]yohimbine binding to alpha-2 adrenergic receptor of rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228242 (CHEMBL542812) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Ability to inhibit [3H]yohimbine binding to alpha-2 adrenergic receptor of rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228237 (CHEMBL554455) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228241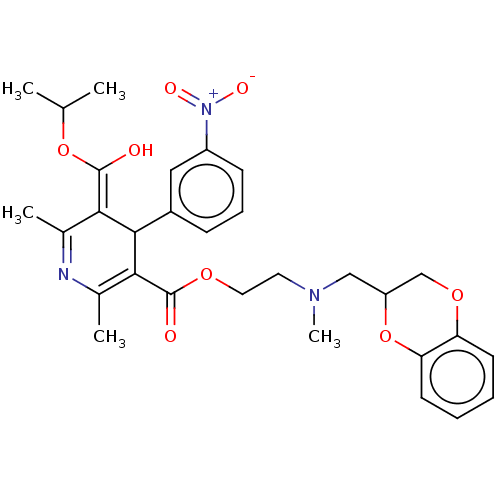 (CHEMBL540511) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-2-adrenolytic activity was assessed from the ability to inhibit [3H]yohimbine binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228244 (CHEMBL553942) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50228238 (CHEMBL30911) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description In vitro ability to inhibit norepinephrine binding to alpha-2 adrenergic receptor of guinea pig vas deferens | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50228237 (CHEMBL554455) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description In vitro ability to inhibit norepinephrine binding to alpha-2 adrenergic receptor of guinea pig vas deferens | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50228241 (CHEMBL540511) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-2-adrenolytic activity was assessed in vitro from the ability to inhibit norepinephrine binding to guinea pig vas deferens | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50101815 (CHEBI:7550 | Nicardipine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-2-adrenolytic activity was assessed from the ability to inhibit [3H]yohimbine binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50064234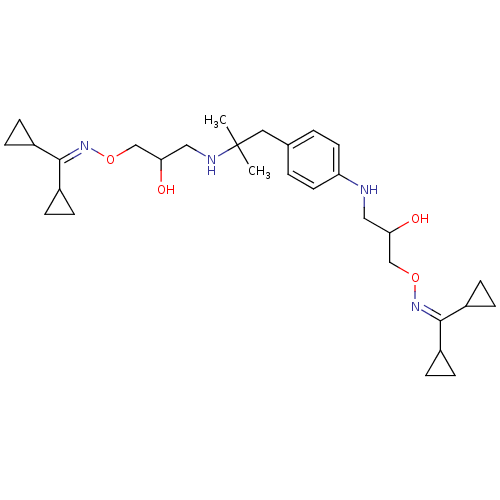 (CHEMBL41628 | Dicyclopropyl-methanone O-(3-{2-[4-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Grenoble Curated by ChEMBL | Assay Description Inhibition of beta-2 adrenergic receptor isolated from rat lung homogenates | J Med Chem 41: 1613-8 (1998) Article DOI: 10.1021/jm970338c BindingDB Entry DOI: 10.7270/Q2416W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228240 (CHEMBL284056) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Ability to inhibit [3H]yohimbine binding to alpha-2 adrenergic receptor of rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228236 (CHEMBL3350238) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228236 (CHEMBL3350238) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228236 (CHEMBL3350238) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228243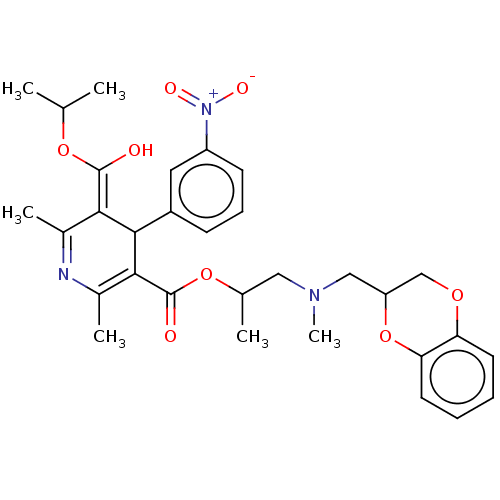 (CHEMBL542814) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-2-adrenolytic activity was assessed from the ability to inhibit [3H]yohimbine binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228239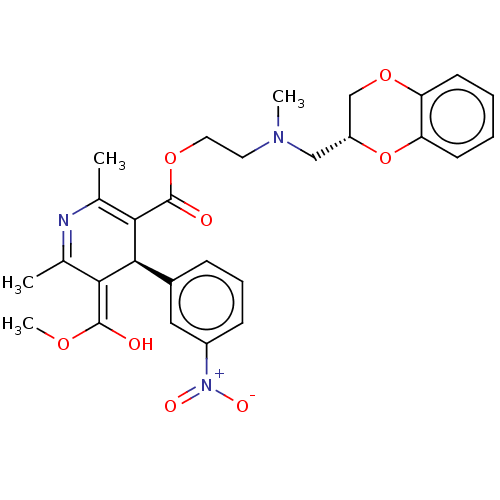 (CHEMBL2093965) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50064233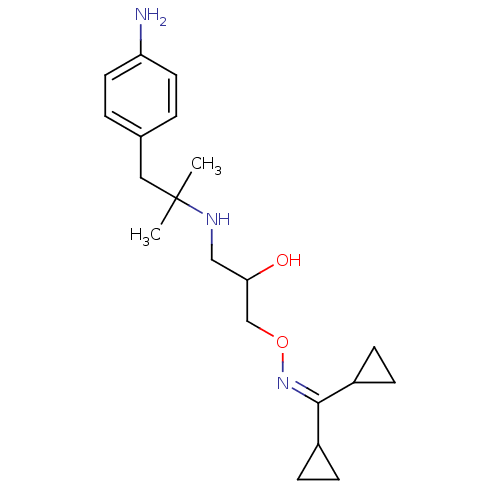 (CHEMBL41149 | Dicyclopropyl-methanone O-{3-[2-(4-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Grenoble Curated by ChEMBL | Assay Description Inhibition of beta-2 adrenergic receptor isolated from rat lung homogenates | J Med Chem 41: 1613-8 (1998) Article DOI: 10.1021/jm970338c BindingDB Entry DOI: 10.7270/Q2416W5G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50228241 (CHEMBL540511) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50101815 (CHEBI:7550 | Nicardipine) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Pharmacologie (UA 589 CNRS) Curated by ChEMBL | Assay Description Alpha-1-adrenolytic activity was assessed from the ability to inhibit [3H]prazosin binding to rat cerebral cortex preparation | J Med Chem 32: 1402-7 (1989) BindingDB Entry DOI: 10.7270/Q2SQ92M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 182 total ) | Next | Last >> |