

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235631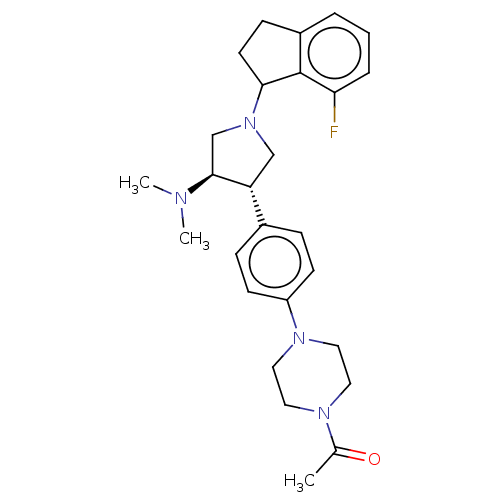 (CHEMBL4060827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to EED (unknown origin) by TR-FRET based binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50241662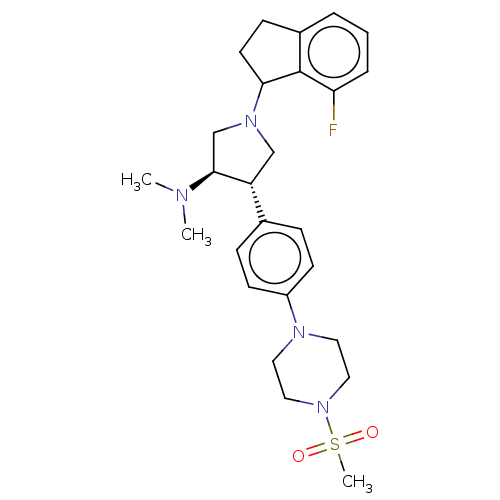 (CHEMBL4104741) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of OG(488) labeled probe from GST-tagged EED (unknown origin) incubated for 1 hr by lanthaScreen TR-FRET method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50562606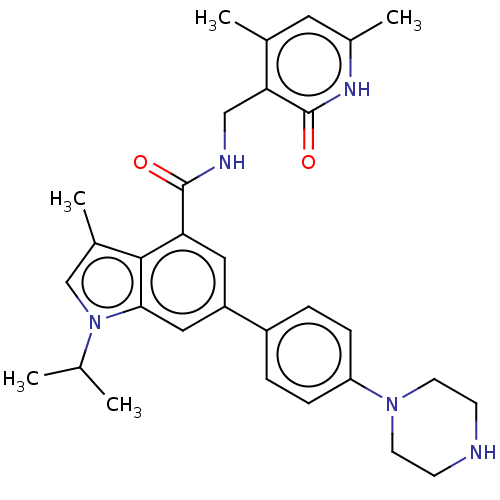 (CHEMBL4792256) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLAG-tagged EZH2 using H3K27me as substrate in presence of [3H]-SAM incubated for 30 mins by Cheng-Prusoff analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50535399 (CHEMBL4473857) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50283992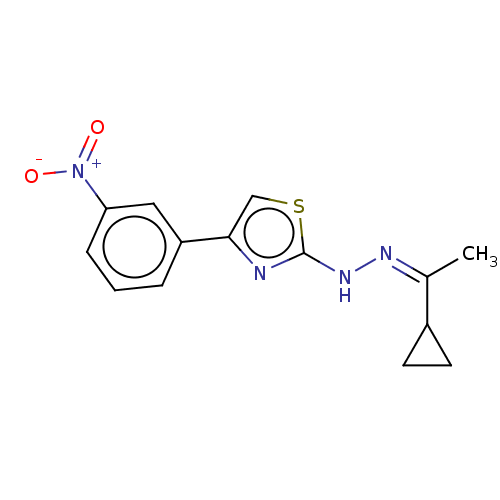 (CHEMBL4174116) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
"G. D'Annunzio" University of Chieti-Pescara Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human MAO-B using kynuramine as substrate pre-incubated for 15 mins followed by 2 fold compound dilution for 24... | Eur J Med Chem 143: 1543-1552 (2018) Article DOI: 10.1016/j.ejmech.2017.10.050 BindingDB Entry DOI: 10.7270/Q27H1N3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50535398 (CHEMBL4434948) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50562608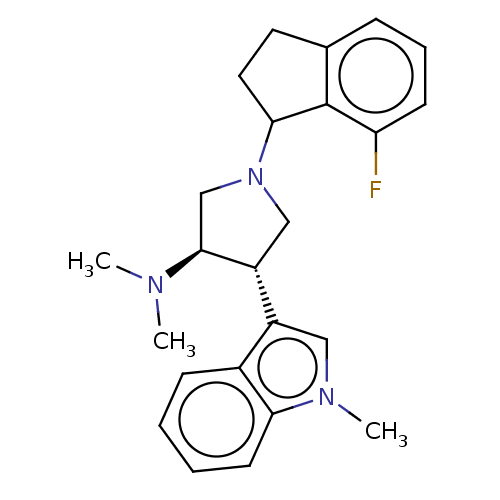 (CHEMBL4795893) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to EED (unknown origin) by TR-FRET based binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223985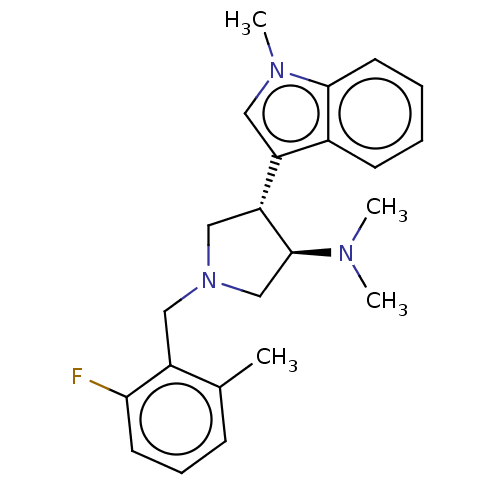 (rac-(3R,4S)-1-(2-fluoro-6-methylbenzyl)-N,N-dimeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to EED (unknown origin) by TR-FRET based binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50540615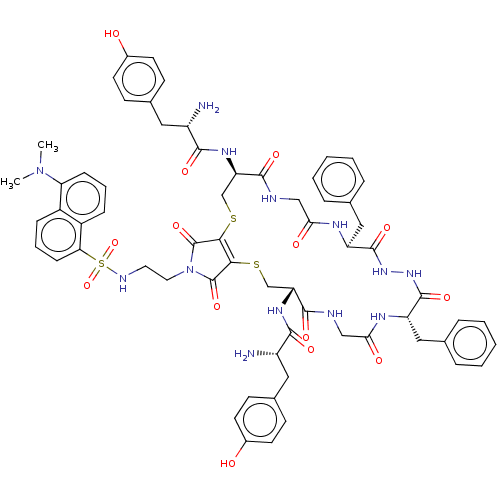 (CHEMBL4638703) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human delta opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075051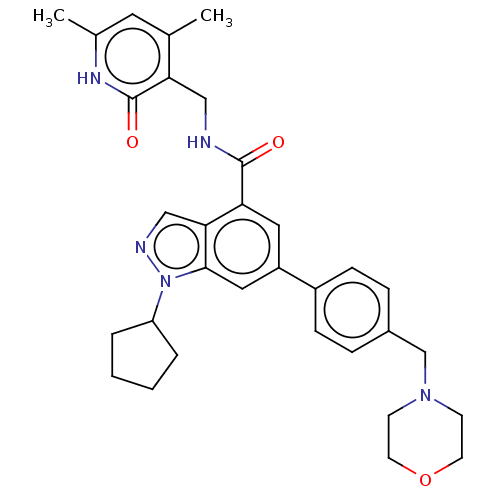 (CHEMBL3360855 | EPZ005687 | US10273223, Compound C...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of wild type EZH2 in human PRC2 complex using S-adenosylmethionine as substrate by Michaelis-Menten plot analysis | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human MOR expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation coun... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50535399 (CHEMBL4473857) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation coun... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human delta opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50535398 (CHEMBL4434948) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human mu opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation coun... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM326072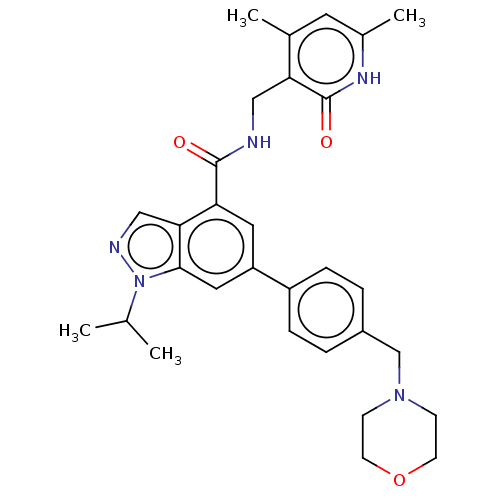 (US10273223, Compound C-5 | US9637472, Compound C-5) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type EZH2 in human PRC2 complex using S-adenosylmethionine and biotinylated histone peptides as substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50540615 (CHEMBL4638703) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human MOR expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50540613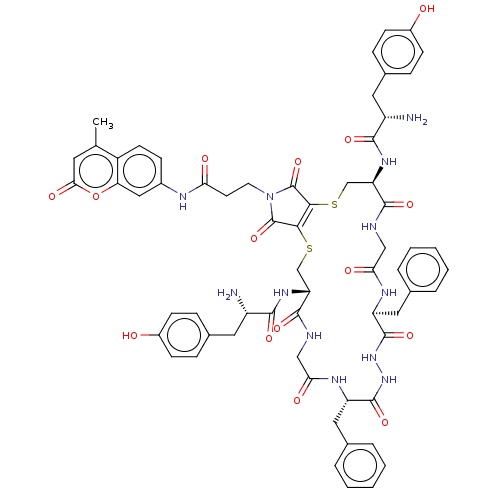 (CHEMBL4641588) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human delta opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50540614 (CHEMBL4636807) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human delta opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000788 ((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human delta opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human kappa opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM190179 (EPZ005030 | US10273223, Compound A-2 | US9175331, ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type EZH2 in human PRC2 complex using S-adenosylmethionine and biotinylated histone peptides as substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50075053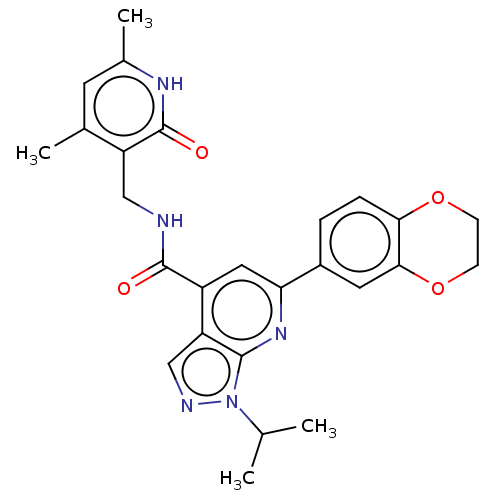 (CHEMBL3414618) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type EZH2 in human PRC2 complex using S-adenosylmethionine and biotinylated histone peptides as substrate | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235637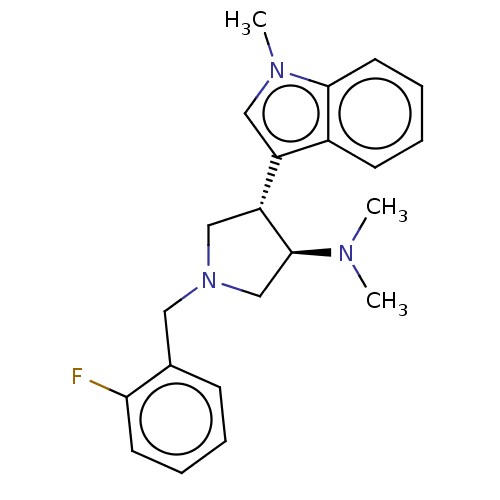 (CHEMBL4103354) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to EED (unknown origin) by TR-FRET based binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50535398 (CHEMBL4434948) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 495 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50540613 (CHEMBL4641588) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human MOR expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM223984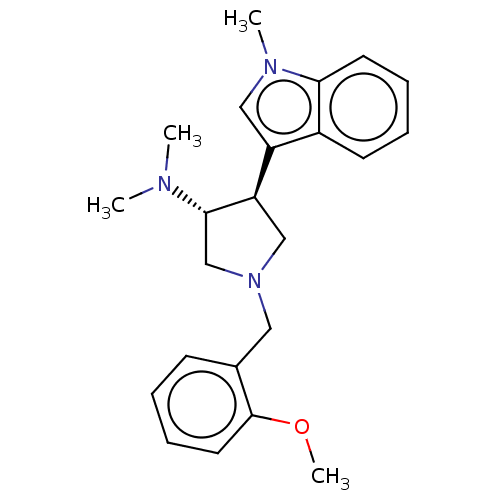 (rac-(3R,4S)-1-(2-methoxybenzyl)-N,N-dimethyl-4-(1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to EED (unknown origin) by TR-FRET based binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50535399 (CHEMBL4473857) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 686 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]-diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by micro beta2 scintillation c... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50400783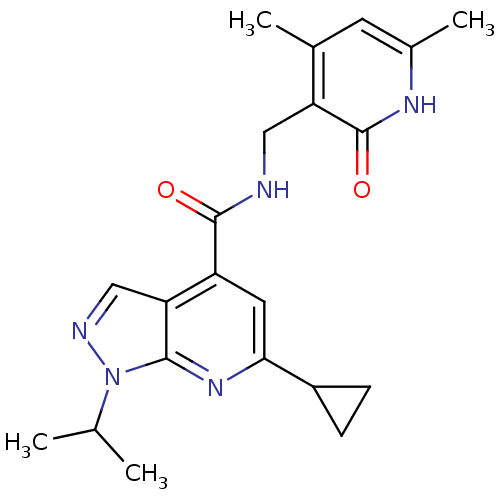 (CHEMBL1608462) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human FLAG-tev-fused EZH2 expressed in baculovirus infected Sf9 insect cells using H3K27 peptide as substrate in presence of [3H]-SAM b... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50540614 (CHEMBL4636807) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human MOR expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50562607 (CHEMBL4748918) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of OG(488) labeled probe from GST-tagged EED (unknown origin) incubated for 1 hr by lanthaScreen TR-FRET method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50540614 (CHEMBL4636807) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human kappa opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50540613 (CHEMBL4641588) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human kappa opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50540615 (CHEMBL4638703) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >3.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine human kappa opioid receptor expressed in CHO cell membranes after 1 hr by micro beta2 scintillation counting method | ACS Med Chem Lett 11: 720-726 (2020) Article DOI: 10.1021/acsmedchemlett.9b00569 BindingDB Entry DOI: 10.7270/Q2FR015W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50169493 ((S)-3,3-dimethyl-1-(4-(4-(trifluoromethyl)phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0251 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50169495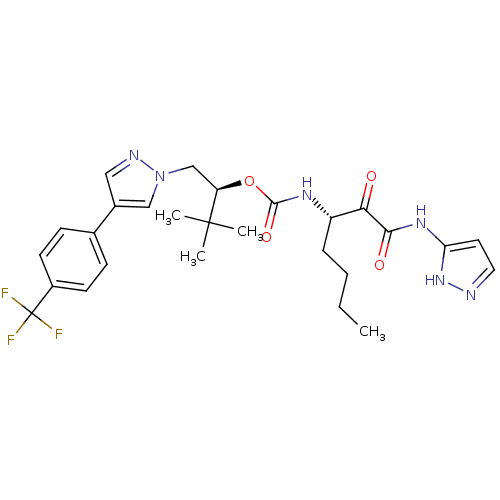 ((S)-3,3-dimethyl-1-(4-(4-(trifluoromethyl)phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0257 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50169482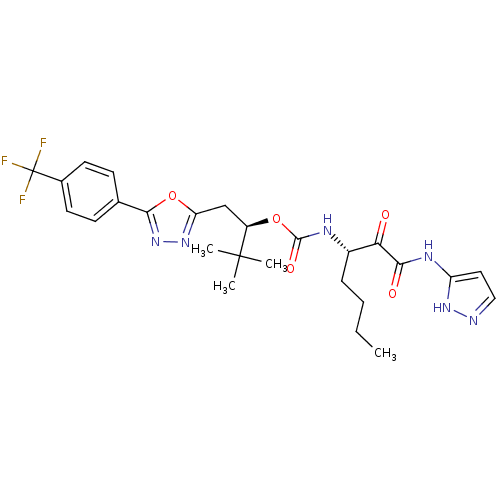 ((R)-3,3-dimethyl-1-(5-(4-(trifluoromethyl)phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0288 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM19783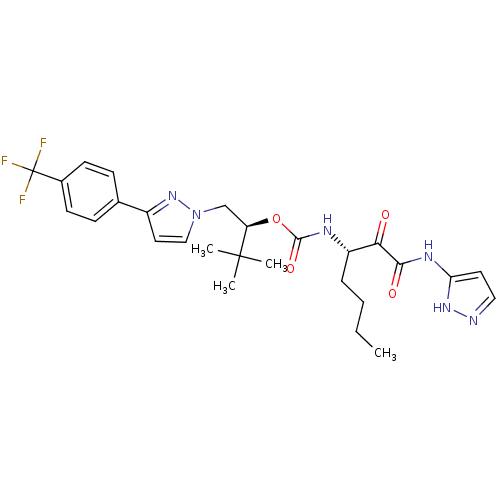 ((2S)-3,3-dimethyl-1-{3-[4-(trifluoromethyl)phenyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0724 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50169488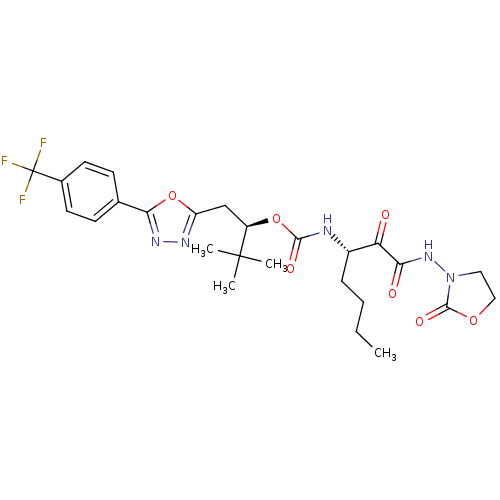 ((1R)-2,2-DIMETHYL-1-({5-[4-(TRIFLUOROMETHYL)PHENYL...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50169496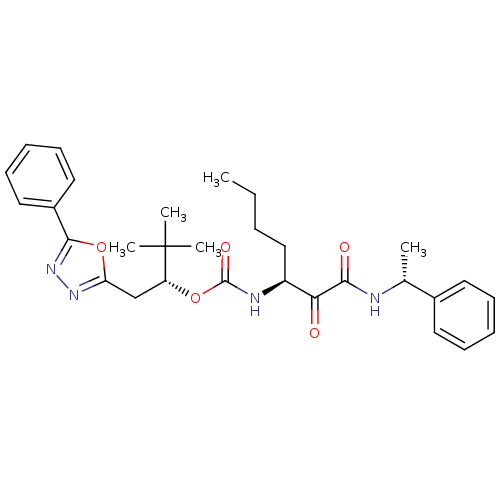 ((R)-3,3-dimethyl-1-(5-phenyl-1,3,4-oxadiazol-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50169490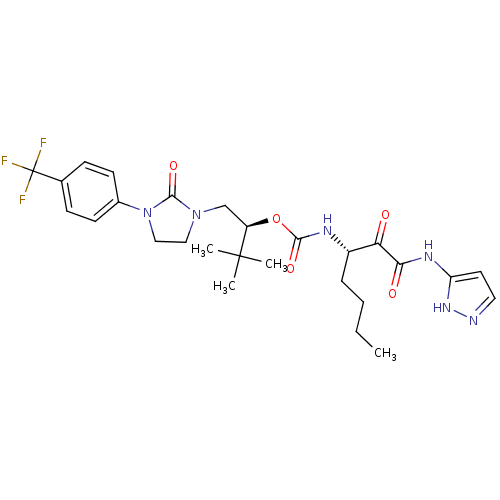 ((S)-3,3-dimethyl-1-(2-oxo-3-(4-(trifluoromethyl)ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.339 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50535399 (CHEMBL4473857) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cell membranes assessed as reduction in SNC80-induced [35S]GTPgammaS binding incu... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50169483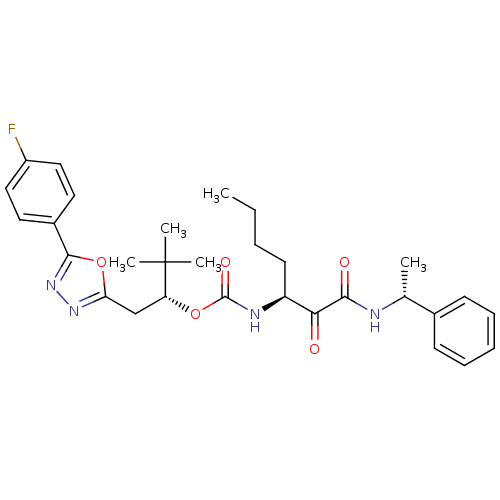 ((R)-1-(5-(4-fluorophenyl)-1,3,4-oxadiazol-2-yl)-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.372 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50169492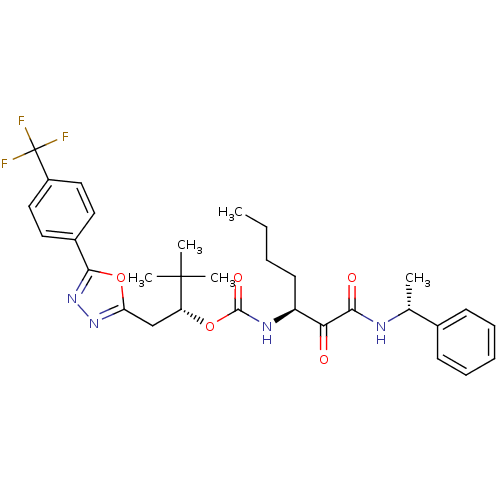 ((R)-3,3-dimethyl-1-(5-(4-(trifluoromethyl)phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50415754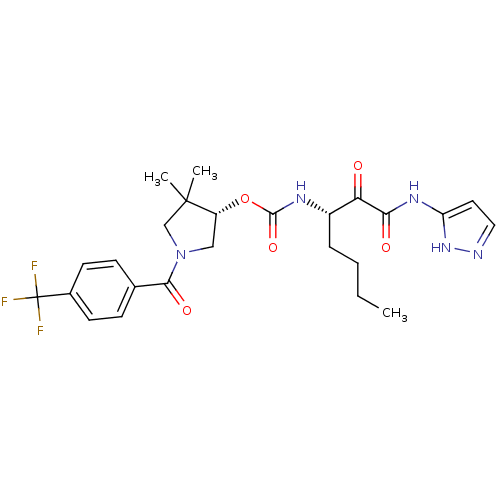 (CHEMBL1092804) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.417 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50237601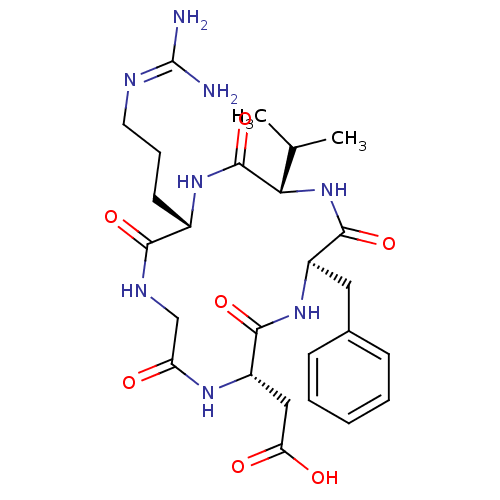 (CHEMBL411941 | CycloRGDfV | [(2S,5R,8S,11S)-5-Benz...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.487 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human alpha-v-beta-3 integrin receptor by ELISA | Bioorg Med Chem Lett 16: 6178-80 (2006) Article DOI: 10.1016/j.bmcl.2006.09.042 BindingDB Entry DOI: 10.7270/Q2C25069 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110356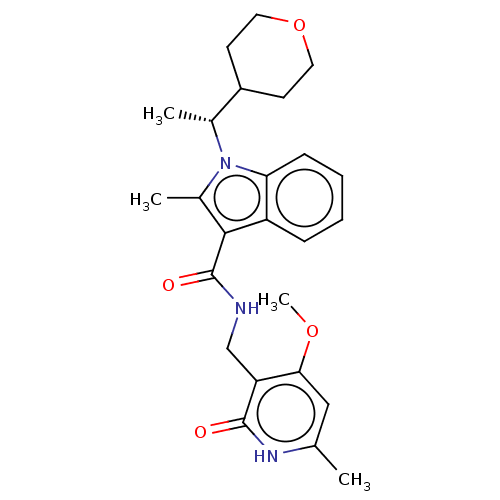 (CHEMBL3605456) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of wild type EZH2 (unknown origin) using histone H3 peptide (17 to 38 residues) by radiometric method | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01344 BindingDB Entry DOI: 10.7270/Q23R0XMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50370067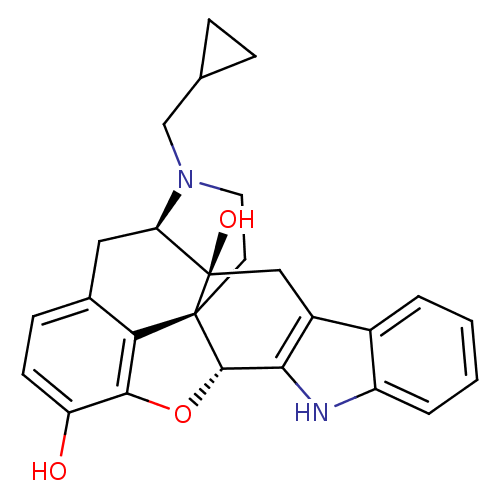 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Chieti-Pescara "G. d'Annunzio" Curated by ChEMBL | Assay Description Antagonist activity at human delta opioid receptor expressed in CHO cells assessed as reduction in SNC80-induced inhibition of forskolin stimulated c... | ACS Med Chem Lett 10: 450-456 (2019) Article DOI: 10.1021/acsmedchemlett.8b00495 BindingDB Entry DOI: 10.7270/Q2N58QWM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50415755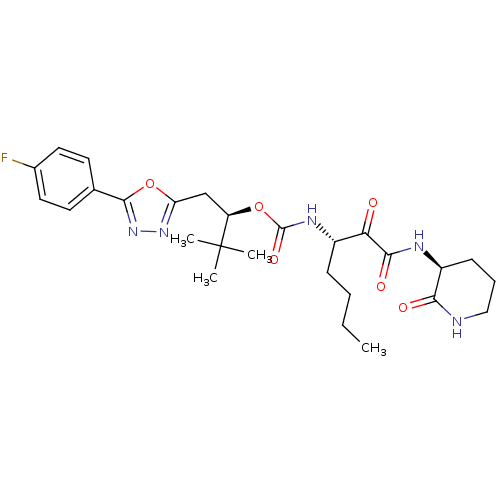 (CHEMBL1087934) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.646 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human cathepsin K | Eur J Med Chem 45: 667-81 (2010) Article DOI: 10.1016/j.ejmech.2009.11.010 BindingDB Entry DOI: 10.7270/Q29C6ZP1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 224 total ) | Next | Last >> |