 Found 317 hits with Last Name = 'brandstetter' and Initial = 'h'
Found 317 hits with Last Name = 'brandstetter' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Furin
(Homo sapiens (Human)) | BDBM50552678

(CHEMBL4759036)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)Cc1ccc(CNC(N)=N)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00651
BindingDB Entry DOI: 10.7270/Q2JD51DD |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50552679

(CHEMBL4762678)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)Cc1ccc(CNC(N)=N)cc1)C(=O)N[C@@H](CCCCN)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00651
BindingDB Entry DOI: 10.7270/Q2JD51DD |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50552672
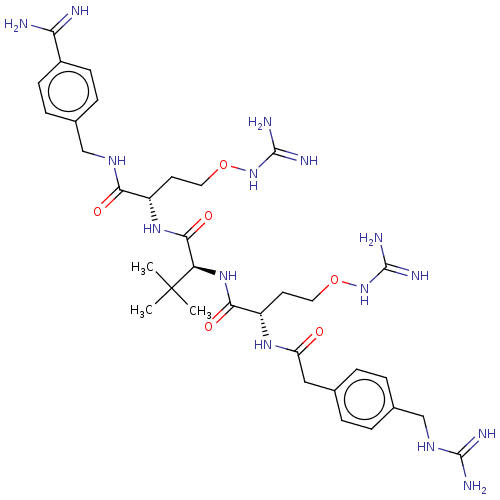
(CHEMBL4790628)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCONC(N)=N)NC(=O)Cc1ccc(CNC(N)=N)cc1)C(=O)N[C@@H](CCONC(N)=N)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00651
BindingDB Entry DOI: 10.7270/Q2JD51DD |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50552676
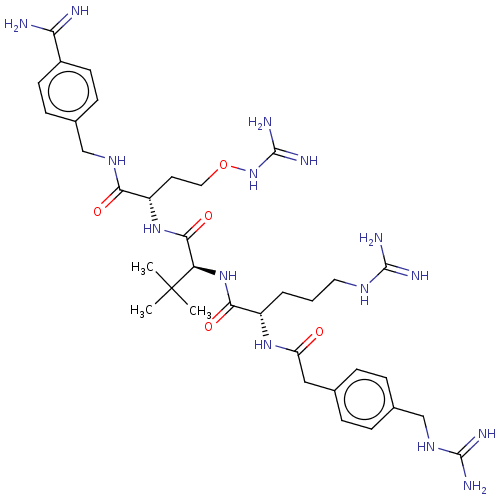
(CHEMBL4794635)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)Cc1ccc(CNC(N)=N)cc1)C(=O)N[C@@H](CCONC(N)=N)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00651
BindingDB Entry DOI: 10.7270/Q2JD51DD |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50552673
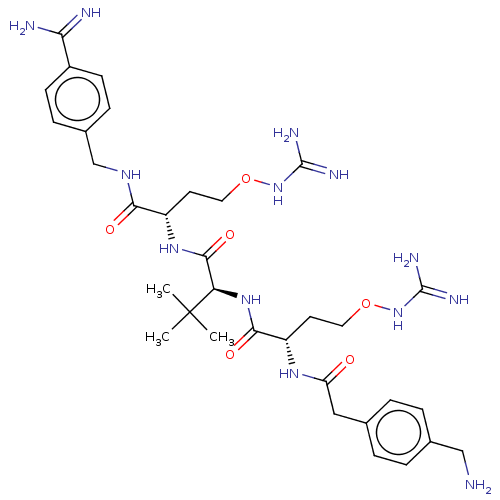
(CHEMBL4750900)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCONC(N)=N)NC(=O)Cc1ccc(CN)cc1)C(=O)N[C@@H](CCONC(N)=N)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00651
BindingDB Entry DOI: 10.7270/Q2JD51DD |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50552674

(CHEMBL4742184)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCONC(N)=N)NC(=O)Cc1ccc(CNC(N)=N)cc1)C(=O)N[C@@H](CCCCN)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00651
BindingDB Entry DOI: 10.7270/Q2JD51DD |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50552677
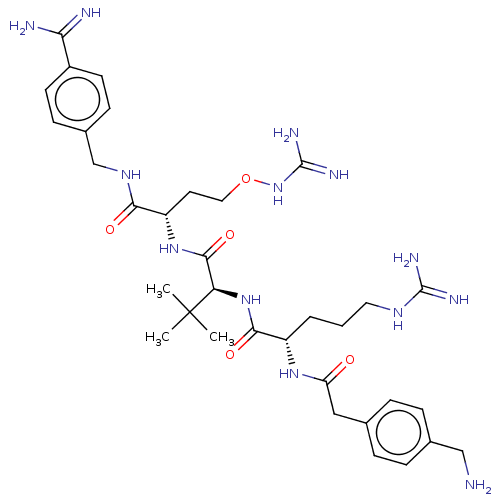
(CHEMBL4779067)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)Cc1ccc(CN)cc1)C(=O)N[C@@H](CCONC(N)=N)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00651
BindingDB Entry DOI: 10.7270/Q2JD51DD |
More data for this
Ligand-Target Pair | |
Furin
(Homo sapiens (Human)) | BDBM50552675
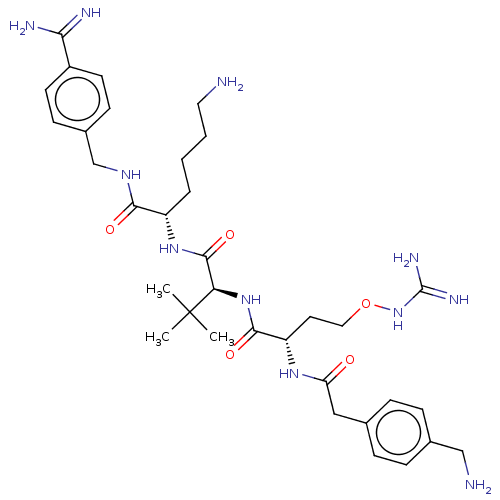
(CHEMBL4747919)Show SMILES CC(C)(C)[C@H](NC(=O)[C@H](CCONC(N)=N)NC(=O)Cc1ccc(CN)cc1)C(=O)N[C@@H](CCCCN)C(=O)NCc1ccc(cc1)C(N)=N |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.114 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant soluble human furin using Phac-Arg-Val-Arg-Arg-AMC fluorogenic substrate |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00651
BindingDB Entry DOI: 10.7270/Q2JD51DD |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50589973
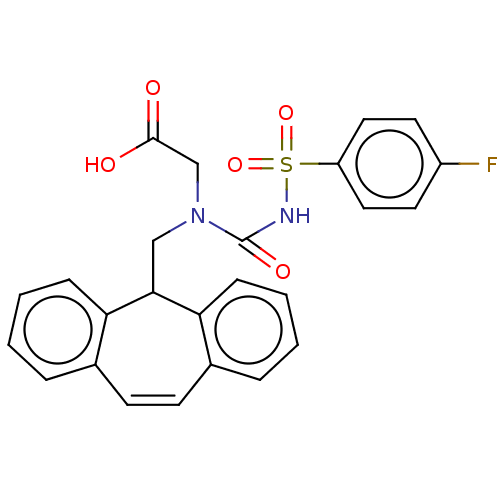
(CHEMBL5184569)Show SMILES OC(=O)CN(CC1c2ccccc2C=Cc2ccccc12)C(=O)NS(=O)(=O)c1ccc(F)cc1 |c:14| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50589973

(CHEMBL5184569)Show SMILES OC(=O)CN(CC1c2ccccc2C=Cc2ccccc12)C(=O)NS(=O)(=O)c1ccc(F)cc1 |c:14| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104706
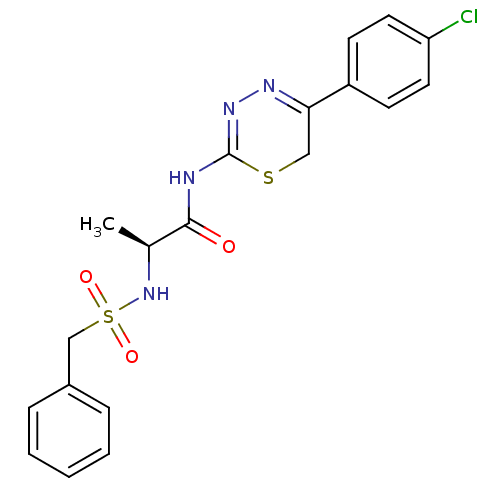
(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104718

((R)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Elastase
(Pseudomonas aeruginosa) | BDBM50542712
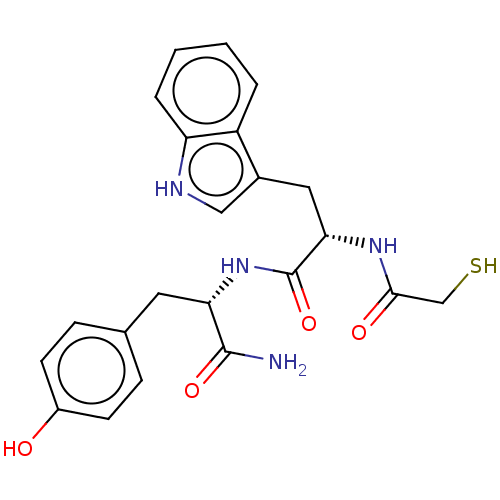
(CHEMBL4647152)Show SMILES NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)CS |r| Show InChI InChI=1S/C22H24N4O4S/c23-21(29)18(9-13-5-7-15(27)8-6-13)26-22(30)19(25-20(28)12-31)10-14-11-24-17-4-2-1-3-16(14)17/h1-8,11,18-19,24,27,31H,9-10,12H2,(H2,23,29)(H,25,28)(H,26,30)/t18-,19-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Helmholtz Institute for Pharmaceutical Research Saarland (HIPS) - Helmholtz Centre for Infection Research (HZI)
Curated by ChEMBL
| Assay Description
Inhibition of Pseudomonas aeruginosa LasB using aminobenzoyl-Ala-Gly-Leu-Ala-p-nitro-benzyl-amide as fluorogenic substrate by fluorimetric assay |
J Med Chem 63: 8359-8368 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00584
BindingDB Entry DOI: 10.7270/Q20005N6 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104717
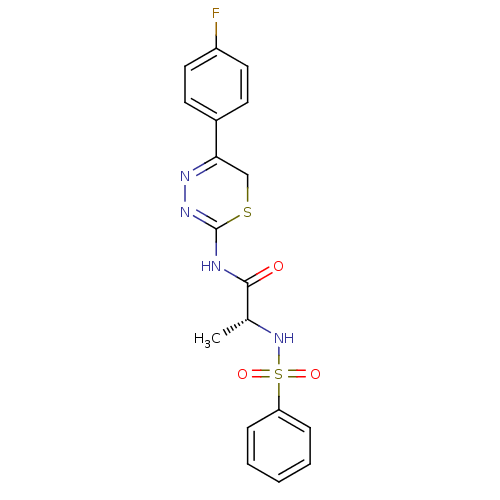
((R)-N-(5-(4-fluorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(F)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17FN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104710
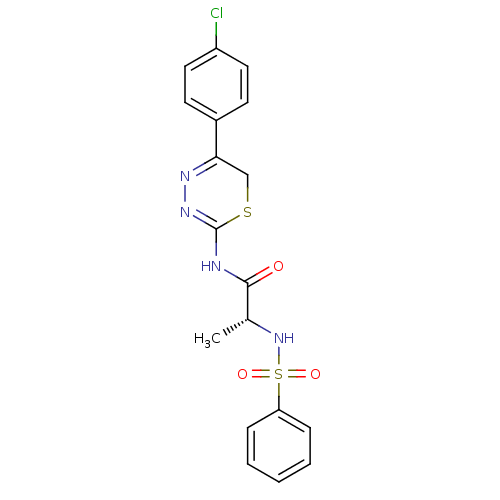
((R)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104721
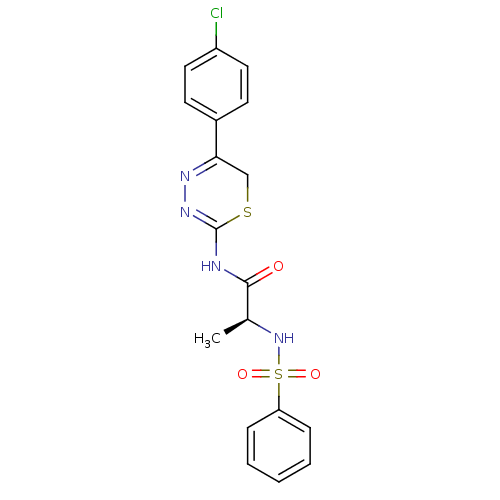
((S)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104714
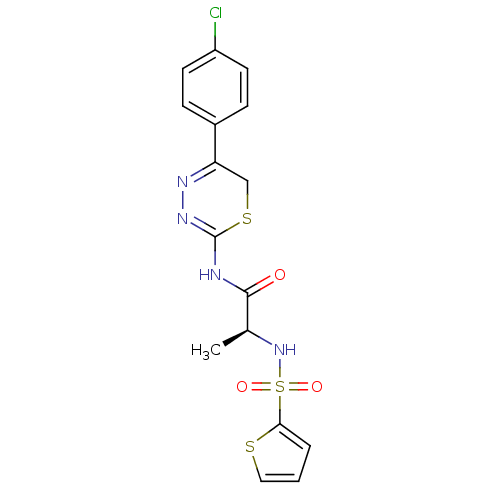
(CHEMBL109861 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)c1cccs1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:17,t:15| Show InChI InChI=1S/C16H15ClN4O3S3/c1-10(21-27(23,24)14-3-2-8-25-14)15(22)18-16-20-19-13(9-26-16)11-4-6-12(17)7-5-11/h2-8,10,21H,9H2,1H3,(H,18,20,22)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104710

((R)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104723
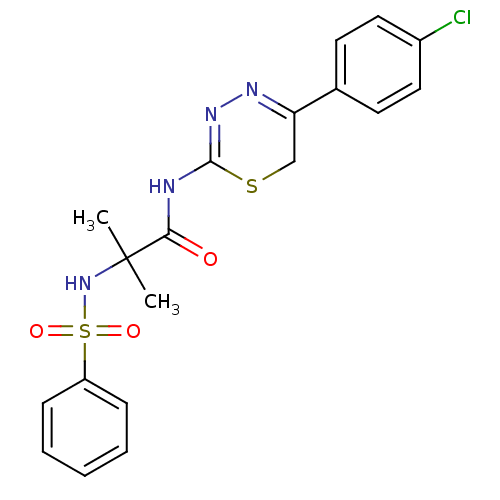
(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of the human matrix metalloprotease-2 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104709
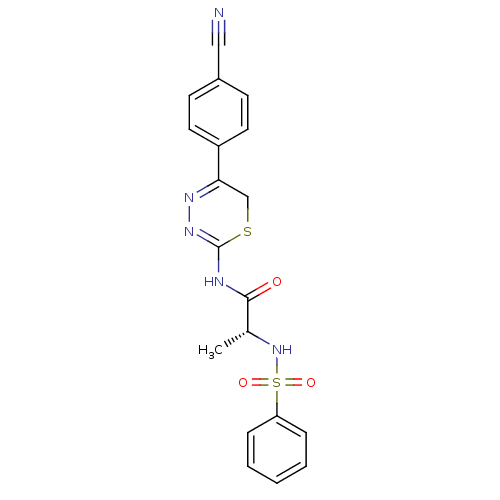
((R)-N-(5-(4-cyanophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(cc1)C#N |c:18,t:16| Show InChI InChI=1S/C19H17N5O3S2/c1-13(24-29(26,27)16-5-3-2-4-6-16)18(25)21-19-23-22-17(12-28-19)15-9-7-14(11-20)8-10-15/h2-10,13,24H,12H2,1H3,(H,21,23,25)/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104707
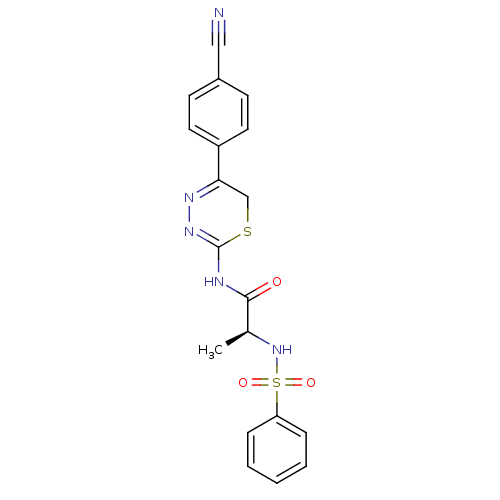
((S)-N-(5-(4-cyanophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(cc1)C#N |c:18,t:16| Show InChI InChI=1S/C19H17N5O3S2/c1-13(24-29(26,27)16-5-3-2-4-6-16)18(25)21-19-23-22-17(12-28-19)15-9-7-14(11-20)8-10-15/h2-10,13,24H,12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104706

(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of the human matrix metalloprotease-2 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50104716
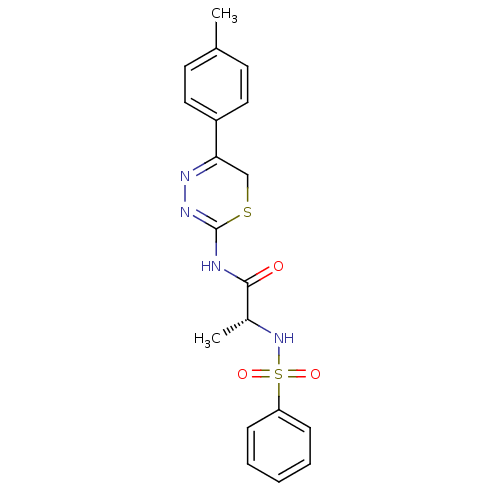
((R)-2-(phenylsulfonamido)-N-(5-p-tolyl-6H-1,3,4-th...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(C)cc1 |c:18,t:16| Show InChI InChI=1S/C19H20N4O3S2/c1-13-8-10-15(11-9-13)17-12-27-19(22-21-17)20-18(24)14(2)23-28(25,26)16-6-4-3-5-7-16/h3-11,14,23H,12H2,1-2H3,(H,20,22,24)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-12 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104723

(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104721

((S)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of human matrix metalloprotease-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM11859
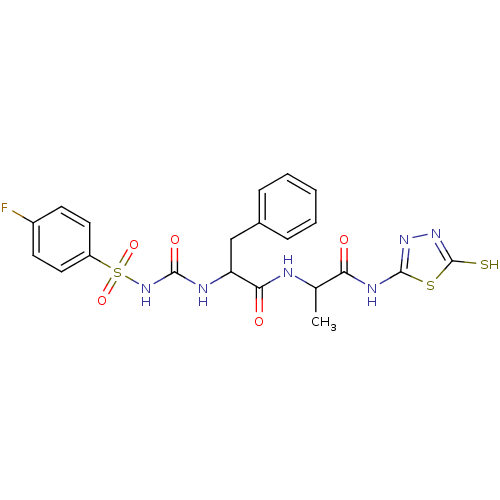
(2-({[(4-fluorobenzene)sulfonyl]carbamoyl}amino)-3-...)Show SMILES CC(NC(=O)C(Cc1ccccc1)NC(=O)NS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1nnc(S)s1 Show InChI InChI=1S/C21H21FN6O5S3/c1-12(17(29)25-20-26-27-21(34)35-20)23-18(30)16(11-13-5-3-2-4-6-13)24-19(31)28-36(32,33)15-9-7-14(22)8-10-15/h2-10,12,16H,11H2,1H3,(H,23,30)(H,27,34)(H2,24,28,31)(H,25,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Collagenase ColQ1
(Bacillus cereus (strain Q1)) | BDBM50589971

(CHEMBL5187038)Show SMILES CC(C)CC(C(=O)NO)C(=O)NCCn1cc(COc2ccccc2NC(C)=O)nn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00785
BindingDB Entry DOI: 10.7270/Q2HD80NZ |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104723

(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the ectodomain of the human matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50104712
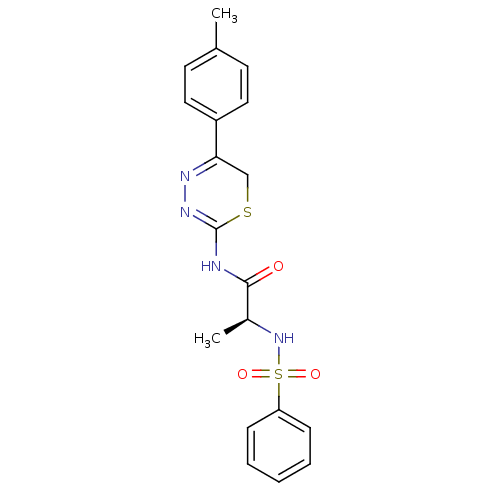
((S)-2-(phenylsulfonamido)-N-(5-p-tolyl-6H-1,3,4-th...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(C)cc1 |c:18,t:16| Show InChI InChI=1S/C19H20N4O3S2/c1-13-8-10-15(11-9-13)17-12-27-19(22-21-17)20-18(24)14(2)23-28(25,26)16-6-4-3-5-7-16/h3-11,14,23H,12H2,1-2H3,(H,20,22,24)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-12 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104722
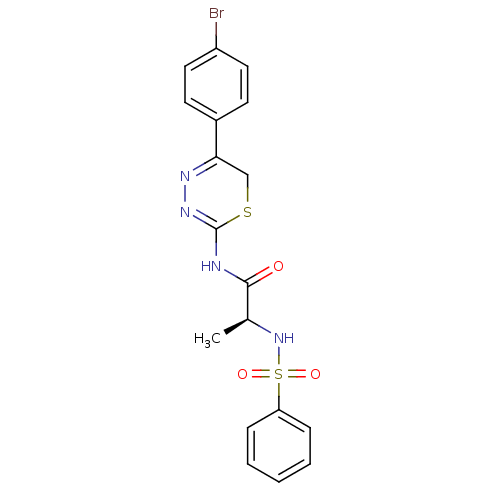
((S)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104706

(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104718

((R)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104713
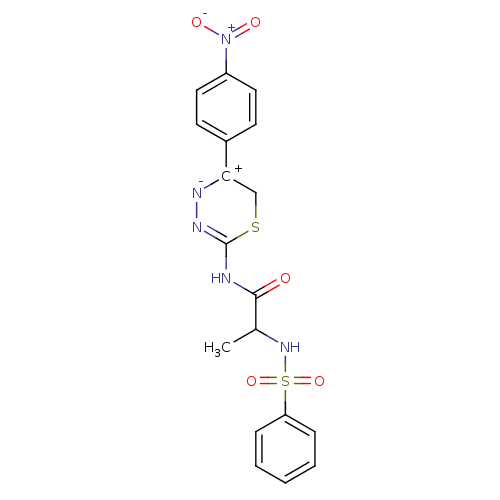
((S)-N-(5-(4-nitrophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES CC(NS(=O)(=O)c1ccccc1)C(=O)NC1=N[N-][C+](CS1)c1ccc(cc1)[N+]([O-])=O |t:16| Show InChI InChI=1S/C18H17N5O5S2/c1-12(22-30(27,28)15-5-3-2-4-6-15)17(24)19-18-21-20-16(11-29-18)13-7-9-14(10-8-13)23(25)26/h2-10,12,22H,11H2,1H3,(H,19,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104710

((R)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104721

((S)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of the human matrix metalloprotease-2 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104716

((R)-2-(phenylsulfonamido)-N-(5-p-tolyl-6H-1,3,4-th...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(C)cc1 |c:18,t:16| Show InChI InChI=1S/C19H20N4O3S2/c1-13-8-10-15(11-9-13)17-12-27-19(22-21-17)20-18(24)14(2)23-28(25,26)16-6-4-3-5-7-16/h3-11,14,23H,12H2,1-2H3,(H,20,22,24)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the ectodomain of the human matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104710

((R)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50104718

((R)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of the human matrix metalloprotease-2 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104723

(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the catalytic domain of human matrix metalloprotease-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104722

((S)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104712

((S)-2-(phenylsulfonamido)-N-(5-p-tolyl-6H-1,3,4-th...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(C)cc1 |c:18,t:16| Show InChI InChI=1S/C19H20N4O3S2/c1-13-8-10-15(11-9-13)17-12-27-19(22-21-17)20-18(24)14(2)23-28(25,26)16-6-4-3-5-7-16/h3-11,14,23H,12H2,1-2H3,(H,20,22,24)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104704
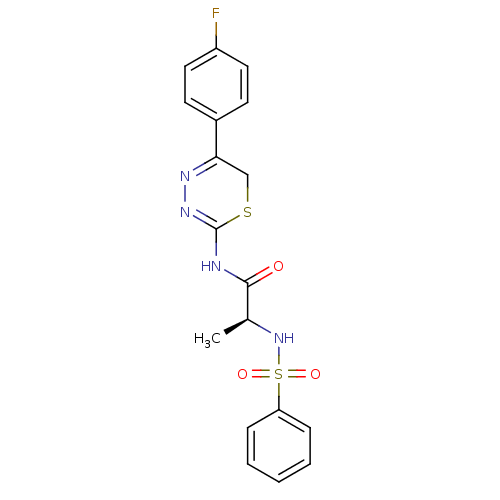
((S)-N-(5-(4-fluorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(F)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17FN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50104711
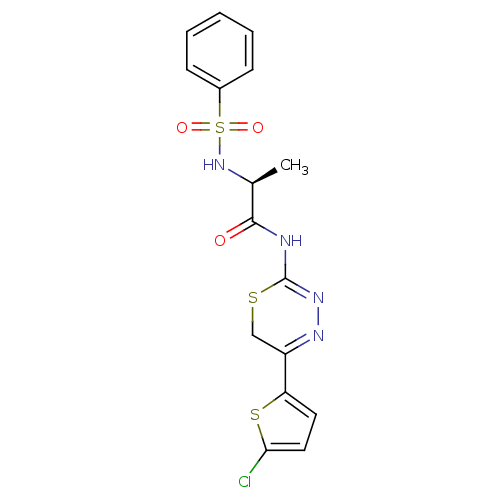
(2-Benzenesulfonylamino-N-[5-(5-chloro-thiophen-2-y...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)s1 |c:18,t:16| Show InChI InChI=1S/C16H15ClN4O3S3/c1-10(21-27(23,24)11-5-3-2-4-6-11)15(22)18-16-20-19-12(9-25-16)13-7-8-14(17)26-13/h2-8,10,21H,9H2,1H3,(H,18,20,22)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibition of human matrix metalloprotease-9 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104713

((S)-N-(5-(4-nitrophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES CC(NS(=O)(=O)c1ccccc1)C(=O)NC1=N[N-][C+](CS1)c1ccc(cc1)[N+]([O-])=O |t:16| Show InChI InChI=1S/C18H17N5O5S2/c1-12(22-30(27,28)15-5-3-2-4-6-15)17(24)19-18-21-20-16(11-29-18)13-7-9-14(10-8-13)23(25)26/h2-10,12,22H,11H2,1H3,(H,19,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104723

(2-Benzenesulfonylamino-N-[5-(4-chloro-phenyl)-6H-[...)Show SMILES CC(C)(NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-19(2,24-29(26,27)15-6-4-3-5-7-15)17(25)21-18-23-22-16(12-28-18)13-8-10-14(20)11-9-13/h3-11,24H,12H2,1-2H3,(H,21,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104711

(2-Benzenesulfonylamino-N-[5-(5-chloro-thiophen-2-y...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)s1 |c:18,t:16| Show InChI InChI=1S/C16H15ClN4O3S3/c1-10(21-27(23,24)11-5-3-2-4-6-11)15(22)18-16-20-19-12(9-25-16)13-7-8-14(17)26-13/h2-8,10,21H,9H2,1H3,(H,18,20,22)/t10-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50104721

((S)-N-(5-(4-chlorophenyl)-6H-1,3,4-thiadiazin-2-yl...)Show SMILES C[C@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17ClN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In Vitro inhibitory activity against the catalytic domain of the matrix metalloprotease-13 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50104708
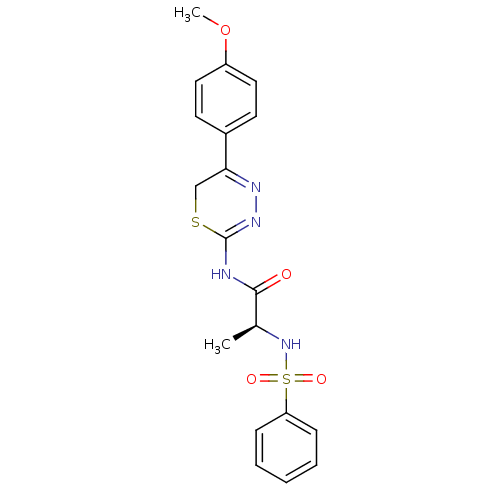
((S)-N-(5-(4-methoxyphenyl)-6H-1,3,4-thiadiazin-2-y...)Show SMILES COc1ccc(cc1)C1=NN=C(NC(=O)[C@H](C)NS(=O)(=O)c2ccccc2)SC1 |t:9,11| Show InChI InChI=1S/C19H20N4O4S2/c1-13(23-29(25,26)16-6-4-3-5-7-16)18(24)20-19-22-21-17(12-28-19)14-8-10-15(27-2)11-9-14/h3-11,13,23H,12H2,1-2H3,(H,20,22,24)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-8 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104718

((R)-N-(5-(4-bromophenyl)-6H-1,3,4-thiadiazin-2-yl)...)Show SMILES C[C@@H](NS(=O)(=O)c1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Br)cc1 |c:18,t:16| Show InChI InChI=1S/C18H17BrN4O3S2/c1-12(23-28(25,26)15-5-3-2-4-6-15)17(24)20-18-22-21-16(11-27-18)13-7-9-14(19)10-8-13/h2-10,12,23H,11H2,1H3,(H,20,22,24)/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the ectodomain of the human matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-14
(Homo sapiens (Human)) | BDBM50104706

(CHEMBL111179 | N-[5-(4-Chloro-phenyl)-6H-[1,3,4]th...)Show SMILES C[C@H](NS(=O)(=O)Cc1ccccc1)C(=O)NC1=NN=C(CS1)c1ccc(Cl)cc1 |c:19,t:17| Show InChI InChI=1S/C19H19ClN4O3S2/c1-13(24-29(26,27)12-14-5-3-2-4-6-14)18(25)21-19-23-22-17(11-28-19)15-7-9-16(20)10-8-15/h2-10,13,24H,11-12H2,1H3,(H,21,23,25)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Bielefeld
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against the ectodomain of the human matrix metalloproteinase-14 |
J Med Chem 44: 3231-43 (2001)
BindingDB Entry DOI: 10.7270/Q2RN3758 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data