 Found 6366 hits with Last Name = 'mclean' and Initial = 'h'
Found 6366 hits with Last Name = 'mclean' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50194747
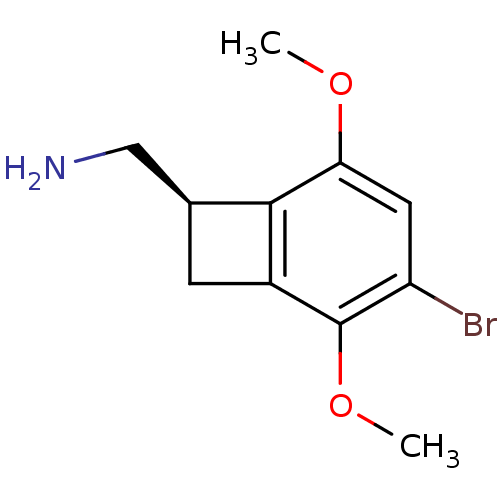
(((R)-4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)met...)Show InChI InChI=1S/C11H14BrNO2/c1-14-9-4-8(12)11(15-2)7-3-6(5-13)10(7)9/h4,6H,3,5,13H2,1-2H3/t6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from rat cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50194747

(((R)-4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)met...)Show InChI InChI=1S/C11H14BrNO2/c1-14-9-4-8(12)11(15-2)7-3-6(5-13)10(7)9/h4,6H,3,5,13H2,1-2H3/t6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from human cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50005267
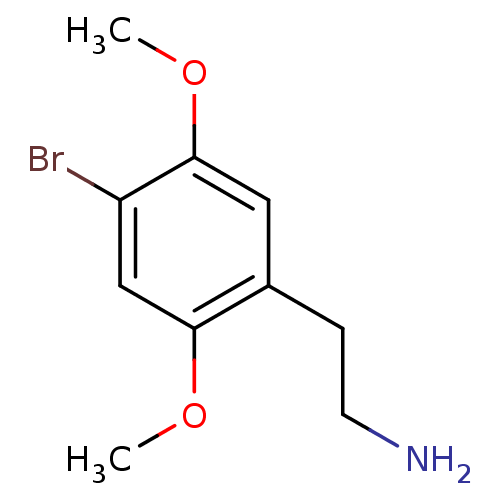
(2,5-dimethoxy-4-bromophenethylamine | 2-(4-Bromo-2...)Show InChI InChI=1S/C10H14BrNO2/c1-13-9-6-8(11)10(14-2)5-7(9)3-4-12/h5-6H,3-4,12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from human cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50194752
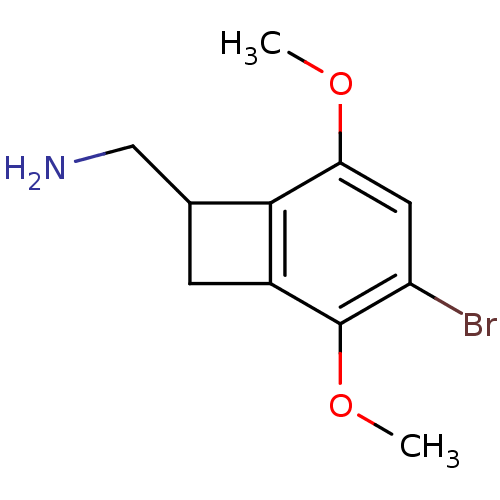
((+/-)-(4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)m...)Show InChI InChI=1S/C11H14BrNO2/c1-14-9-4-8(12)11(15-2)7-3-6(5-13)10(7)9/h4,6H,3,5,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from human cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50194752

((+/-)-(4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)m...)Show InChI InChI=1S/C11H14BrNO2/c1-14-9-4-8(12)11(15-2)7-3-6(5-13)10(7)9/h4,6H,3,5,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from rat cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50005267

(2,5-dimethoxy-4-bromophenethylamine | 2-(4-Bromo-2...)Show InChI InChI=1S/C10H14BrNO2/c1-13-9-6-8(11)10(14-2)5-7(9)3-4-12/h5-6H,3-4,12H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from rat cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM21342
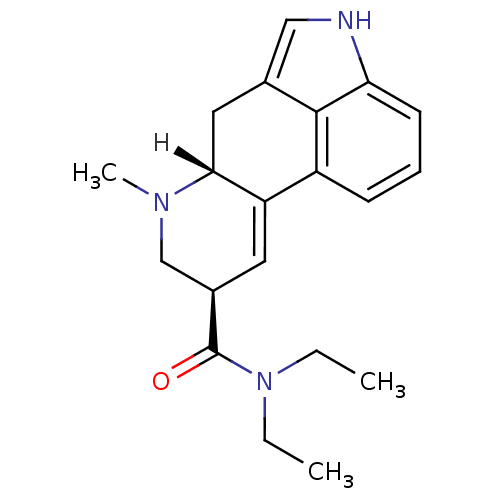
((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor |
J Med Chem 49: 4269-74 (2006)
Article DOI: 10.1021/jm060272y
BindingDB Entry DOI: 10.7270/Q2PC320K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM21342

((4R,7R)-N,N-diethyl-6-methyl-6,11-diazatetracyclo[...)Show SMILES [H][C@@]12Cc3c[nH]c4cccc(C1=C[C@H](CN2C)C(=O)N(CC)CC)c34 |c:12| Show InChI InChI=1S/C20H25N3O/c1-4-23(5-2)20(24)14-9-16-15-7-6-8-17-19(15)13(11-21-17)10-18(16)22(3)12-14/h6-9,11,14,18,21H,4-5,10,12H2,1-3H3/t14-,18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2C receptor |
J Med Chem 49: 4269-74 (2006)
Article DOI: 10.1021/jm060272y
BindingDB Entry DOI: 10.7270/Q2PC320K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50194750
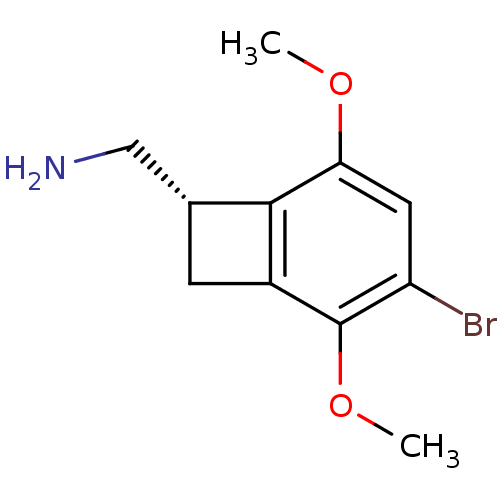
(((S)-4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)met...)Show InChI InChI=1S/C11H14BrNO2/c1-14-9-4-8(12)11(15-2)7-3-6(5-13)10(7)9/h4,6H,3,5,13H2,1-2H3/t6-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from human cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50194750

(((S)-4-bromo-3,6-dimethoxybenzocyclobuten-1-yl)met...)Show InChI InChI=1S/C11H14BrNO2/c1-14-9-4-8(12)11(15-2)7-3-6(5-13)10(7)9/h4,6H,3,5,13H2,1-2H3/t6-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from rat cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50194751
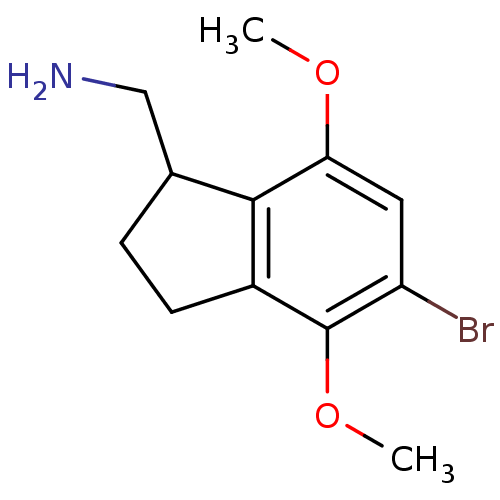
(C-(5-bromo-4,7-dimethoxyindan-1-yl)methylamine | C...)Show InChI InChI=1S/C12H16BrNO2/c1-15-10-5-9(13)12(16-2)8-4-3-7(6-14)11(8)10/h5,7H,3-4,6,14H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from rat cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50194751

(C-(5-bromo-4,7-dimethoxyindan-1-yl)methylamine | C...)Show InChI InChI=1S/C12H16BrNO2/c1-15-10-5-9(13)12(16-2)8-4-3-7(6-14)11(8)10/h5,7H,3-4,6,14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from human cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50190613
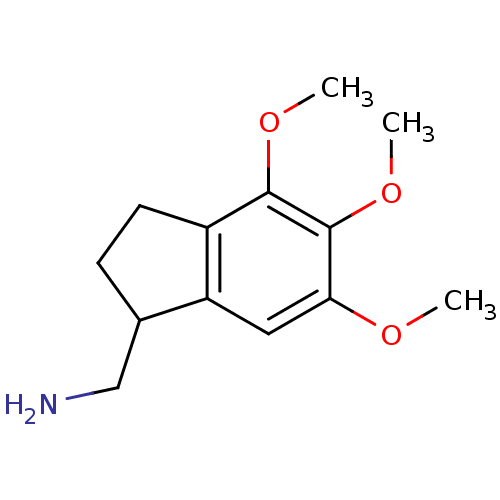
((+/-)-(2,3-dihydro-4,5,6-trimethoxy-1H-inden-1-yl)...)Show InChI InChI=1S/C13H19NO3/c1-15-11-6-10-8(7-14)4-5-9(10)12(16-2)13(11)17-3/h6,8H,4-5,7,14H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2C receptor |
J Med Chem 49: 4269-74 (2006)
Article DOI: 10.1021/jm060272y
BindingDB Entry DOI: 10.7270/Q2PC320K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50190615
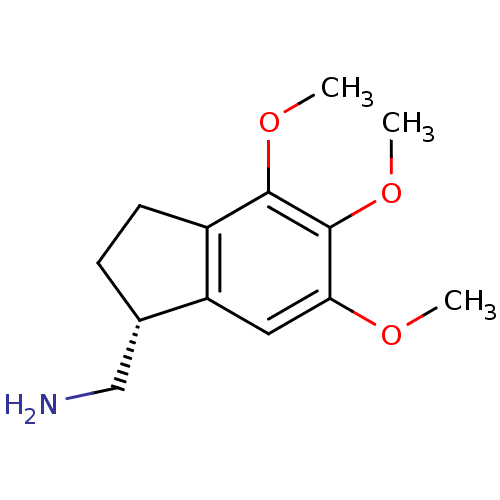
((R)-(+)-(4,5,6-trimethoxyindan-1-yl)methanamine | ...)Show InChI InChI=1S/C13H19NO3/c1-15-11-6-10-8(7-14)4-5-9(10)12(16-2)13(11)17-3/h6,8H,4-5,7,14H2,1-3H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor |
J Med Chem 49: 4269-74 (2006)
Article DOI: 10.1021/jm060272y
BindingDB Entry DOI: 10.7270/Q2PC320K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50194749
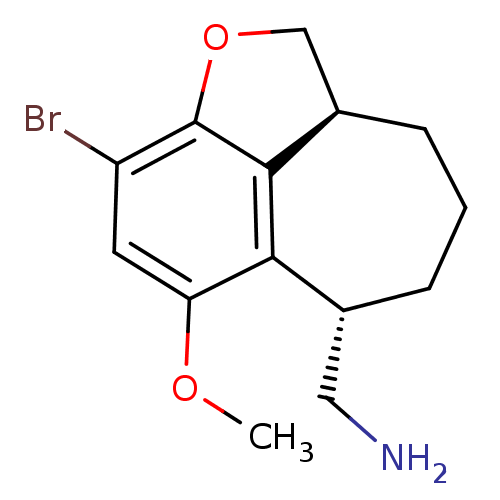
(C-(3-bromo-5-methoxy-1,6,7,8,9,9a-hexahydro-2-oxab...)Show InChI InChI=1S/C14H18BrNO2/c1-17-11-5-10(15)14-13-9(7-18-14)4-2-3-8(6-16)12(11)13/h5,8-9H,2-4,6-7,16H2,1H3/t8-,9-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from rat cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50190613

((+/-)-(2,3-dihydro-4,5,6-trimethoxy-1H-inden-1-yl)...)Show InChI InChI=1S/C13H19NO3/c1-15-11-6-10-8(7-14)4-5-9(10)12(16-2)13(11)17-3/h6,8H,4-5,7,14H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor |
J Med Chem 49: 4269-74 (2006)
Article DOI: 10.1021/jm060272y
BindingDB Entry DOI: 10.7270/Q2PC320K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50194749

(C-(3-bromo-5-methoxy-1,6,7,8,9,9a-hexahydro-2-oxab...)Show InChI InChI=1S/C14H18BrNO2/c1-17-11-5-10(15)14-13-9(7-18-14)4-2-3-8(6-16)12(11)13/h5,8-9H,2-4,6-7,16H2,1H3/t8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from human cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50194748
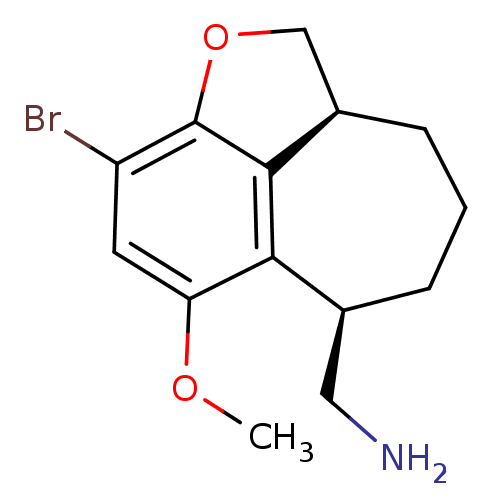
(C-(3-bromo-5-methoxy-1,6,7,8,9,9a-hexahydro-2-oxab...)Show InChI InChI=1S/C14H18BrNO2/c1-17-11-5-10(15)14-13-9(7-18-14)4-2-3-8(6-16)12(11)13/h5,8-9H,2-4,6-7,16H2,1H3/t8-,9+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from rat cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50194748

(C-(3-bromo-5-methoxy-1,6,7,8,9,9a-hexahydro-2-oxab...)Show InChI InChI=1S/C14H18BrNO2/c1-17-11-5-10(15)14-13-9(7-18-14)4-2-3-8(6-16)12(11)13/h5,8-9H,2-4,6-7,16H2,1H3/t8-,9+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of (+/-)-[125I]DOI from human cloned 5HT2A receptor |
J Med Chem 49: 5794-803 (2006)
Article DOI: 10.1021/jm060656o
BindingDB Entry DOI: 10.7270/Q2NS0TH3 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50059891
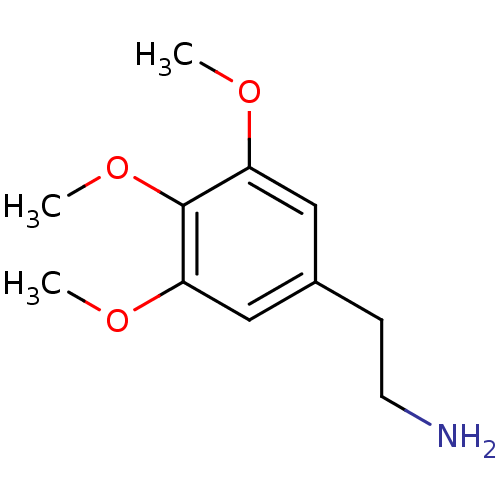
(1-amino-2-(3,4,5-trimethoxyphenyl)ethane | 2-(3,4,...)Show InChI InChI=1S/C11H17NO3/c1-13-9-6-8(4-5-12)7-10(14-2)11(9)15-3/h6-7H,4-5,12H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor |
J Med Chem 49: 4269-74 (2006)
Article DOI: 10.1021/jm060272y
BindingDB Entry DOI: 10.7270/Q2PC320K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Rattus norvegicus (Rat)) | BDBM50059891

(1-amino-2-(3,4,5-trimethoxyphenyl)ethane | 2-(3,4,...)Show InChI InChI=1S/C11H17NO3/c1-13-9-6-8(4-5-12)7-10(14-2)11(9)15-3/h6-7H,4-5,12H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2C receptor |
J Med Chem 49: 4269-74 (2006)
Article DOI: 10.1021/jm060272y
BindingDB Entry DOI: 10.7270/Q2PC320K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM50190614
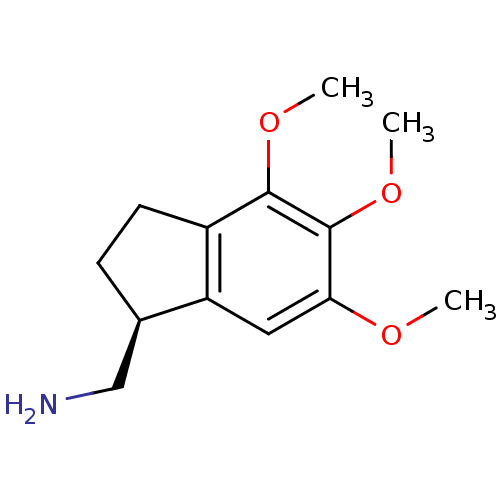
((S)-(-)-(4,5,6-trimethoxyindan-1-yl)methanamine | ...)Show InChI InChI=1S/C13H19NO3/c1-15-11-6-10-8(7-14)4-5-9(10)12(16-2)13(11)17-3/h6,8H,4-5,7,14H2,1-3H3/t8-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from rat 5HT2A receptor |
J Med Chem 49: 4269-74 (2006)
Article DOI: 10.1021/jm060272y
BindingDB Entry DOI: 10.7270/Q2PC320K |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50467024

(CHEMBL4280697)Show SMILES CCC\N=C1/S\C(=C/c2ccc(C(=O)OC)c(Cl)c2)C(=O)N1c1ccccc1C Show InChI InChI=1S/C22H21ClN2O3S/c1-4-11-24-22-25(18-8-6-5-7-14(18)2)20(26)19(29-22)13-15-9-10-16(17(23)12-15)21(27)28-3/h5-10,12-13H,4,11H2,1-3H3/b19-13-,24-22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of S1PR1 (unknown origin) expressed in CHOK1 cells after 90 mins by beta-arresting recuitment assay |
Bioorg Med Chem Lett 28: 3255-3259 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.044
BindingDB Entry DOI: 10.7270/Q2BG2RPC |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50316768
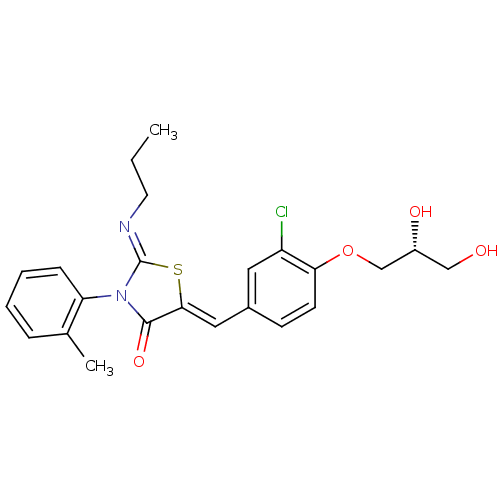
((Z,Z)-5-[3-Chloro-4-((2S)-2,3-dihydroxy-propoxy)-b...)Show SMILES CCC\N=C1/S\C(=C/c2ccc(OC[C@H](O)CO)c(Cl)c2)C(=O)N1c1ccccc1C |r| Show InChI InChI=1S/C23H25ClN2O4S/c1-3-10-25-23-26(19-7-5-4-6-15(19)2)22(29)21(31-23)12-16-8-9-20(18(24)11-16)30-14-17(28)13-27/h4-9,11-12,17,27-28H,3,10,13-14H2,1-2H3/b21-12-,25-23-/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of S1PR1 (unknown origin) expressed in CHOK1 cells after 90 mins by beta-arresting recuitment assay |
Bioorg Med Chem Lett 28: 3255-3259 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.044
BindingDB Entry DOI: 10.7270/Q2BG2RPC |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553299
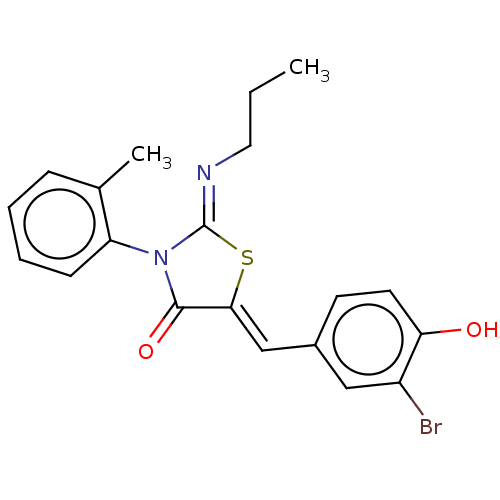
(CHEMBL4790404)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)c(Br)c2)C(=O)N1c1ccccc1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553304
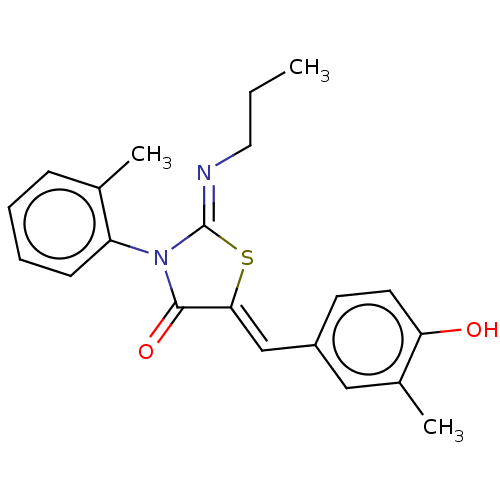
(CHEMBL4748743)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)c(C)c2)C(=O)N1c1ccccc1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553297
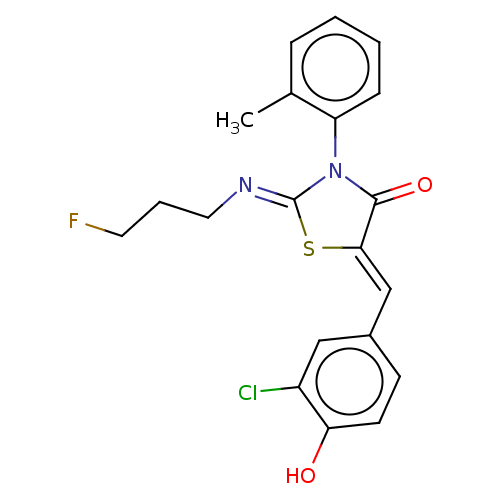
(CHEMBL4747234)Show SMILES Cc1ccccc1N1C(=O)\C(S\C1=N/CCCF)=C\c1ccc(O)c(Cl)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553300
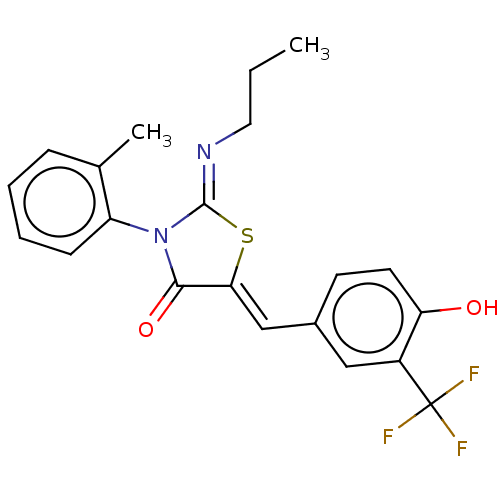
(CHEMBL4796729)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)c(c2)C(F)(F)F)C(=O)N1c1ccccc1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553289
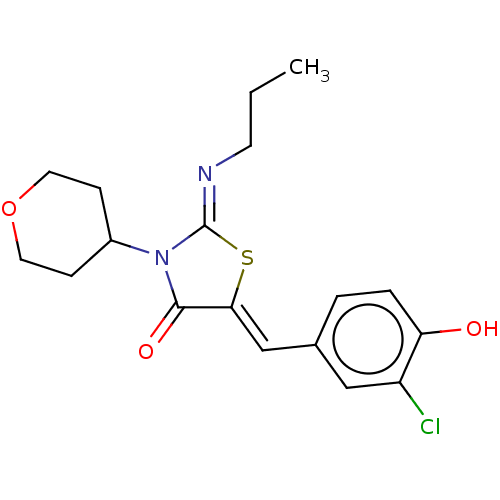
(CHEMBL4763318)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)c(Cl)c2)C(=O)N1C1CCOCC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50316771
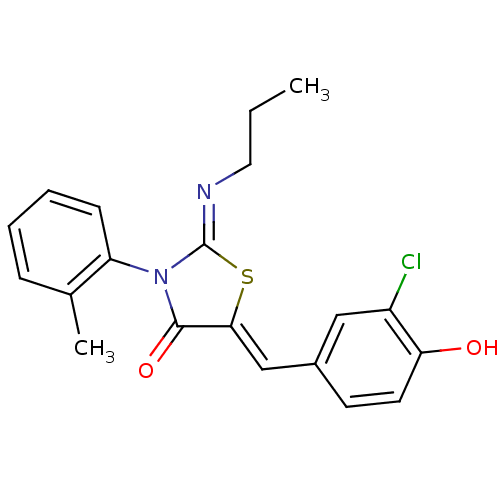
((Z,Z)-5-(3-Chloro-4-hydroxy-benzylidene)-2-propyli...)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)c(Cl)c2)C(=O)N1c1ccccc1C Show InChI InChI=1S/C20H19ClN2O2S/c1-3-10-22-20-23(16-7-5-4-6-13(16)2)19(25)18(26-20)12-14-8-9-17(24)15(21)11-14/h4-9,11-12,24H,3,10H2,1-2H3/b18-12-,22-20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553294
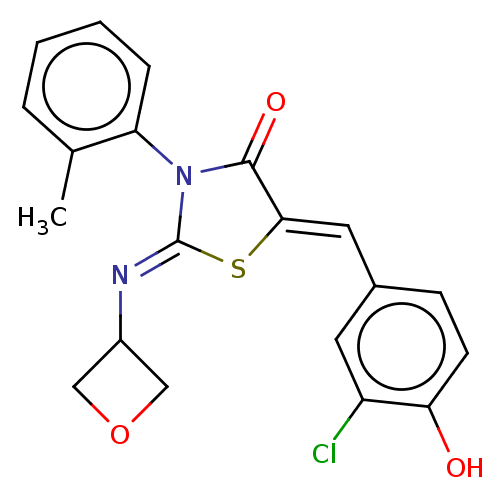
(CHEMBL4759157)Show SMILES Cc1ccccc1N1C(=O)\C(S\C1=N/C1COC1)=C\c1ccc(O)c(Cl)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553287
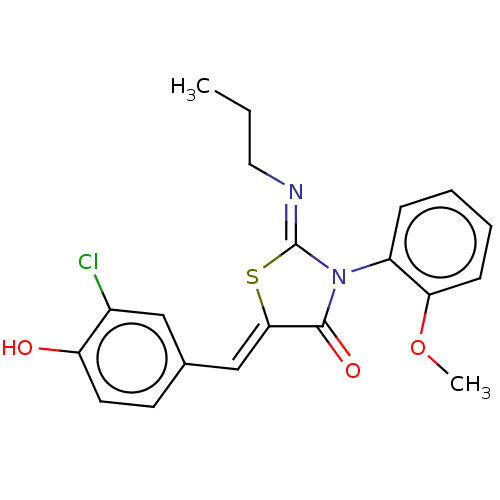
(CHEMBL4745979)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)c(Cl)c2)C(=O)N1c1ccccc1OC | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50164336

(2-(2-Chloro-4,6-dimethoxy-phenyl)-1-methyl-ethylam...)Show InChI InChI=1S/C11H16ClNO2/c1-7(13)4-9-10(12)5-8(14-2)6-11(9)15-3/h5-7H,4,13H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Chile
Curated by ChEMBL
| Assay Description
Inhibitory concentration against monoamine oxidase A in rat brain mitochondrial suspension |
J Med Chem 48: 2407-19 (2005)
Article DOI: 10.1021/jm0493109
BindingDB Entry DOI: 10.7270/Q27080XX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553303
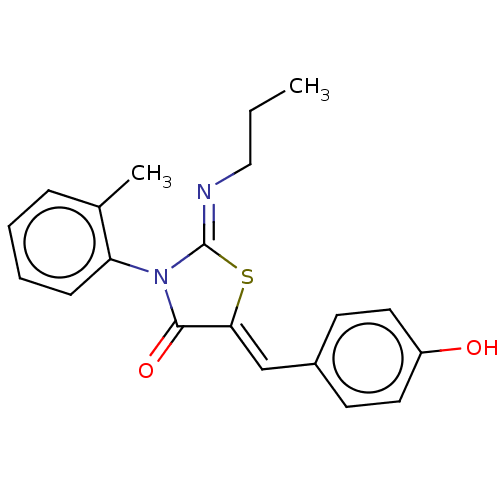
(CHEMBL4748038)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)cc2)C(=O)N1c1ccccc1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50164326
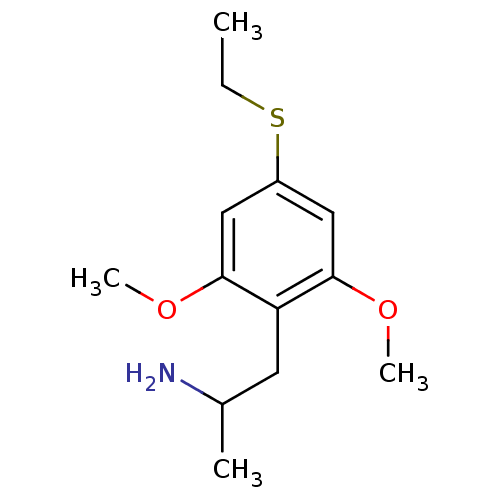
(2-(4-Ethylsulfanyl-2,6-dimethoxy-phenyl)-1-methyl-...)Show InChI InChI=1S/C13H21NO2S/c1-5-17-10-7-12(15-3)11(6-9(2)14)13(8-10)16-4/h7-9H,5-6,14H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Chile
Curated by ChEMBL
| Assay Description
Inhibitory concentration against monoamine oxidase A in rat brain mitochondrial suspension |
J Med Chem 48: 2407-19 (2005)
Article DOI: 10.1021/jm0493109
BindingDB Entry DOI: 10.7270/Q27080XX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50164327
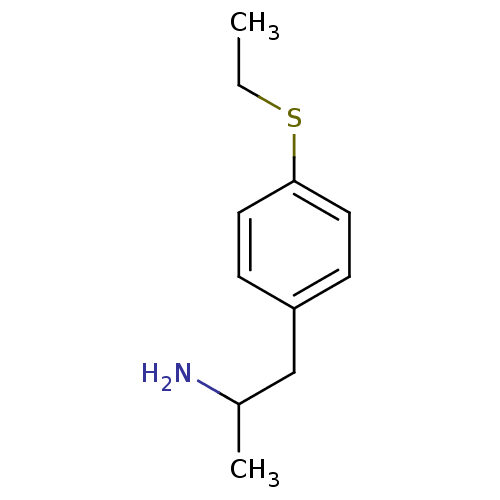
(2-(4-Ethylsulfanyl-phenyl)-1-methyl-ethylamine | 2...)Show InChI InChI=1S/C11H17NS/c1-3-13-11-6-4-10(5-7-11)8-9(2)12/h4-7,9H,3,8,12H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Chile
Curated by ChEMBL
| Assay Description
Inhibitory concentration against monoamine oxidase A in rat brain mitochondrial suspension |
J Med Chem 48: 2407-19 (2005)
Article DOI: 10.1021/jm0493109
BindingDB Entry DOI: 10.7270/Q27080XX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553302
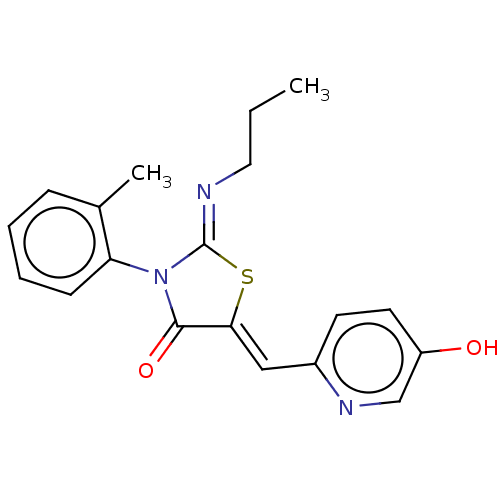
(CHEMBL4751870)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)cn2)C(=O)N1c1ccccc1C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50467023
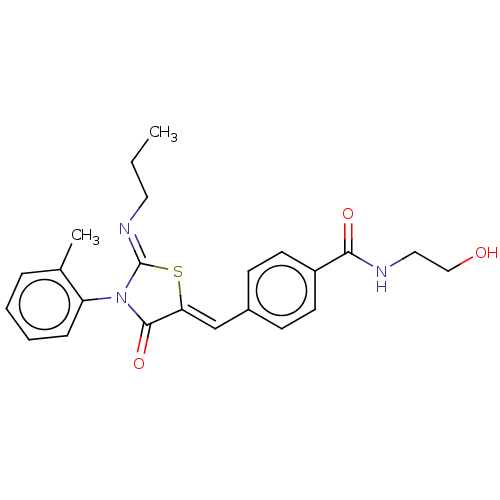
(CHEMBL4287514)Show SMILES CCC\N=C1/S\C(=C/c2ccc(cc2)C(=O)NCCO)C(=O)N1c1ccccc1C Show InChI InChI=1S/C23H25N3O3S/c1-3-12-25-23-26(19-7-5-4-6-16(19)2)22(29)20(30-23)15-17-8-10-18(11-9-17)21(28)24-13-14-27/h4-11,15,27H,3,12-14H2,1-2H3,(H,24,28)/b20-15-,25-23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of S1PR1 (unknown origin) expressed in CHOK1 cells after 90 mins by beta-arresting recuitment assay |
Bioorg Med Chem Lett 28: 3255-3259 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.044
BindingDB Entry DOI: 10.7270/Q2BG2RPC |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50467022
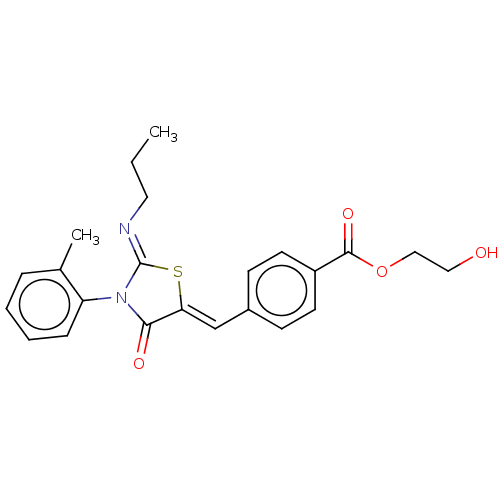
(CHEMBL4292022)Show SMILES CCC\N=C1/S\C(=C/c2ccc(cc2)C(=O)OCCO)C(=O)N1c1ccccc1C Show InChI InChI=1S/C23H24N2O4S/c1-3-12-24-23-25(19-7-5-4-6-16(19)2)21(27)20(30-23)15-17-8-10-18(11-9-17)22(28)29-14-13-26/h4-11,15,26H,3,12-14H2,1-2H3/b20-15-,24-23- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of S1PR1 (unknown origin) expressed in CHOK1 cells after 90 mins by beta-arresting recuitment assay |
Bioorg Med Chem Lett 28: 3255-3259 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.044
BindingDB Entry DOI: 10.7270/Q2BG2RPC |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553288
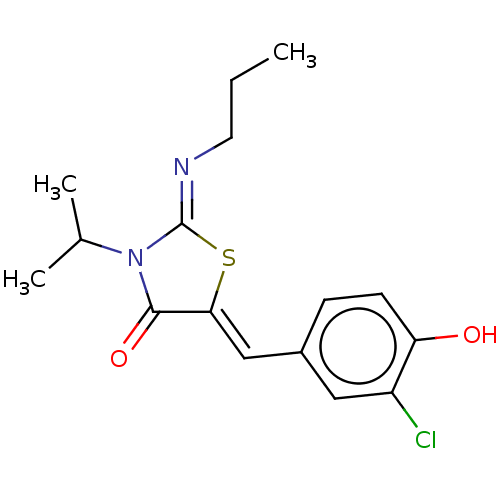
(CHEMBL4755800)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)c(Cl)c2)C(=O)N1C(C)C | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553290
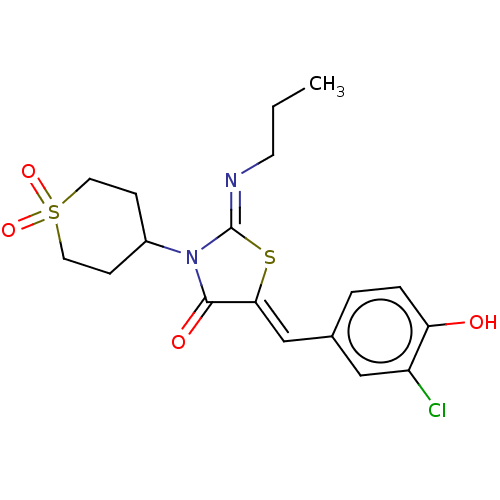
(CHEMBL4740803)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)c(Cl)c2)C(=O)N1C1CCS(=O)(=O)CC1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50005270
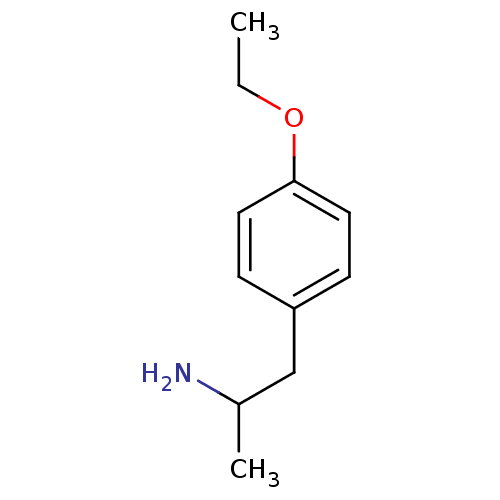
(1-(4-ethoxyphenyl)propan-2-amine | 2-(4-Ethoxy-phe...)Show InChI InChI=1S/C11H17NO/c1-3-13-11-6-4-10(5-7-11)8-9(2)12/h4-7,9H,3,8,12H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Chile
Curated by ChEMBL
| Assay Description
Inhibitory concentration against monoamine oxidase A in rat brain mitochondrial suspension |
J Med Chem 48: 2407-19 (2005)
Article DOI: 10.1021/jm0493109
BindingDB Entry DOI: 10.7270/Q27080XX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50063544
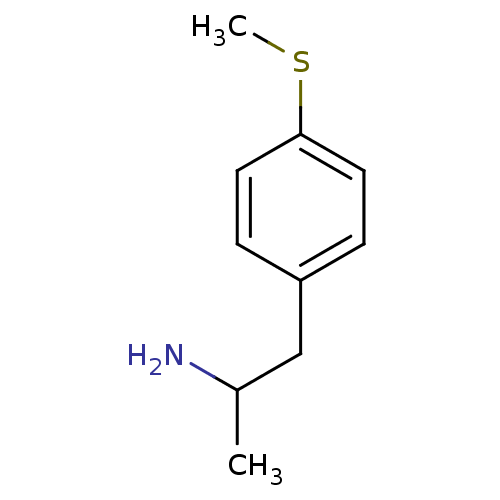
(1-(6-(methylthio)naphthalen-2-yl)propan-2-amine | ...)Show InChI InChI=1S/C10H15NS/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6,8H,7,11H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Chile
Curated by ChEMBL
| Assay Description
Inhibitory concentration against monoamine oxidase A in rat brain mitochondrial suspension |
J Med Chem 48: 2407-19 (2005)
Article DOI: 10.1021/jm0493109
BindingDB Entry DOI: 10.7270/Q27080XX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553295
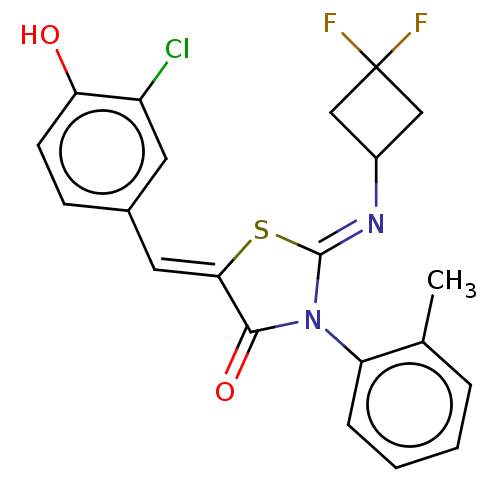
(CHEMBL4758919)Show SMILES Cc1ccccc1N1C(=O)\C(S\C1=N/C1CC(F)(F)C1)=C\c1ccc(O)c(Cl)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50553293
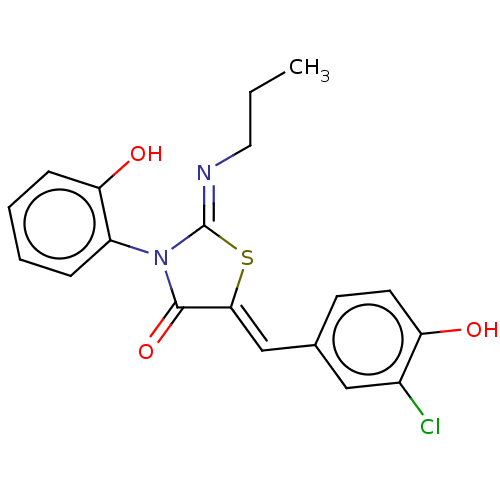
(CHEMBL4757599)Show SMILES CCC\N=C1/S\C(=C/c2ccc(O)c(Cl)c2)C(=O)N1c1ccccc1O | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Immunomodulatory activity at human S1PR1 expressed in CHO-K1 EDG1 beta-arrestin cells assessed as stimulation of beta-arrestin recruitment incubated ... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.8b00616
BindingDB Entry DOI: 10.7270/Q20868ZH |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50164324

(2-(2,6-Dimethoxy-4-methylsulfanyl-phenyl)-1-methyl...)Show InChI InChI=1S/C12H19NO2S/c1-8(13)5-10-11(14-2)6-9(16-4)7-12(10)15-3/h6-8H,5,13H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Chile
Curated by ChEMBL
| Assay Description
Inhibitory concentration against monoamine oxidase A in rat brain mitochondrial suspension |
J Med Chem 48: 2407-19 (2005)
Article DOI: 10.1021/jm0493109
BindingDB Entry DOI: 10.7270/Q27080XX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50164325
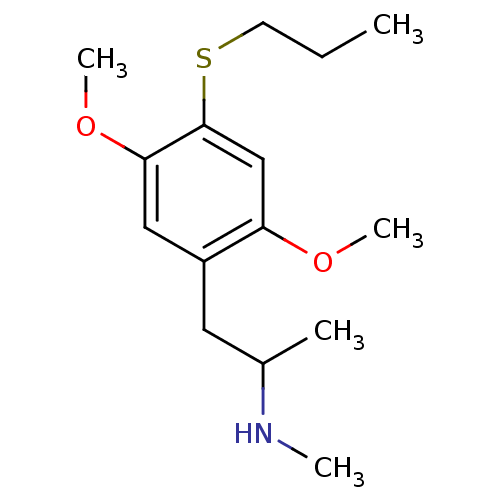
(CHEMBL189630 | [2-(2,5-Dimethoxy-4-propylsulfanyl-...)Show InChI InChI=1S/C15H25NO2S/c1-6-7-19-15-10-13(17-4)12(8-11(2)16-3)9-14(15)18-5/h9-11,16H,6-8H2,1-5H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Chile
Curated by ChEMBL
| Assay Description
Inhibitory concentration against monoamine oxidase A in rat brain mitochondrial suspension |
J Med Chem 48: 2407-19 (2005)
Article DOI: 10.1021/jm0493109
BindingDB Entry DOI: 10.7270/Q27080XX |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50024209
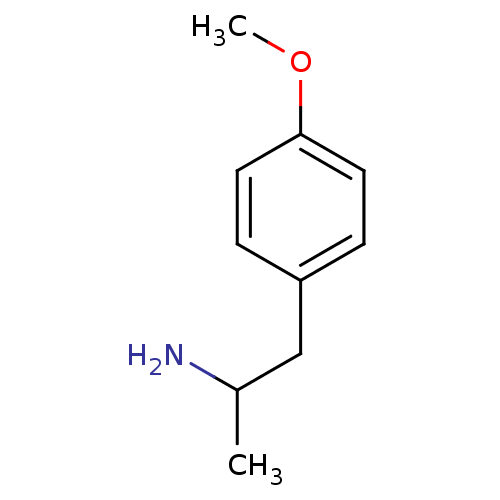
((+/-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine | (-...)Show InChI InChI=1S/C10H15NO/c1-8(11)7-9-3-5-10(12-2)6-4-9/h3-6,8H,7,11H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Chile
Curated by ChEMBL
| Assay Description
Inhibitory concentration against monoamine oxidase A in rat brain mitochondrial suspension |
J Med Chem 48: 2407-19 (2005)
Article DOI: 10.1021/jm0493109
BindingDB Entry DOI: 10.7270/Q27080XX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50467025
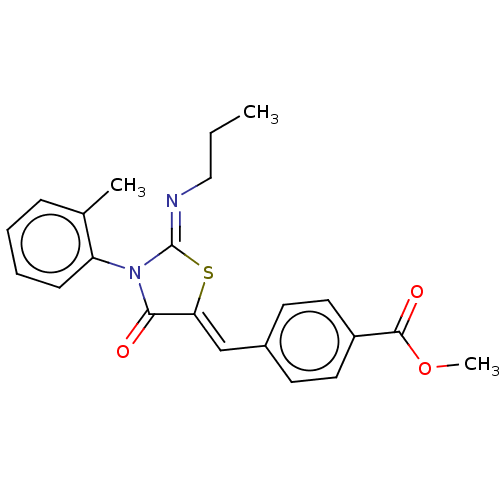
(CHEMBL4288608)Show SMILES CCC\N=C1/S\C(=C/c2ccc(cc2)C(=O)OC)C(=O)N1c1ccccc1C Show InChI InChI=1S/C22H22N2O3S/c1-4-13-23-22-24(18-8-6-5-7-15(18)2)20(25)19(28-22)14-16-9-11-17(12-10-16)21(26)27-3/h5-12,14H,4,13H2,1-3H3/b19-14-,23-22- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee
Curated by ChEMBL
| Assay Description
Inhibition of S1PR1 (unknown origin) expressed in CHOK1 cells after 90 mins by beta-arresting recuitment assay |
Bioorg Med Chem Lett 28: 3255-3259 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.044
BindingDB Entry DOI: 10.7270/Q2BG2RPC |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Rattus norvegicus (rat)) | BDBM50164320

(2-(4-Isobutylsulfanyl-phenyl)-1-methyl-ethylamine ...)Show InChI InChI=1S/C13H21NS/c1-10(2)9-15-13-6-4-12(5-7-13)8-11(3)14/h4-7,10-11H,8-9,14H2,1-3H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Chile
Curated by ChEMBL
| Assay Description
Inhibitory concentration against monoamine oxidase A in rat brain mitochondrial suspension |
J Med Chem 48: 2407-19 (2005)
Article DOI: 10.1021/jm0493109
BindingDB Entry DOI: 10.7270/Q27080XX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data