

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030754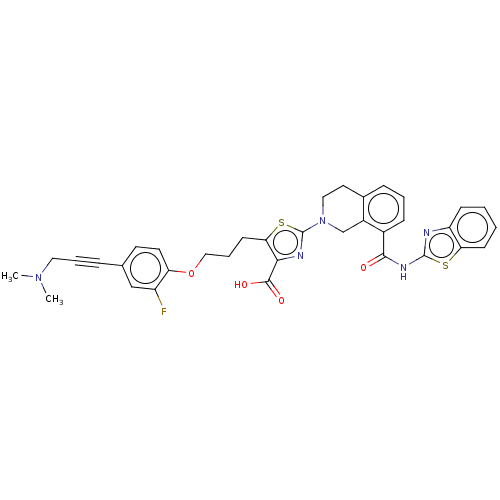 (CHEMBL3342332) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030758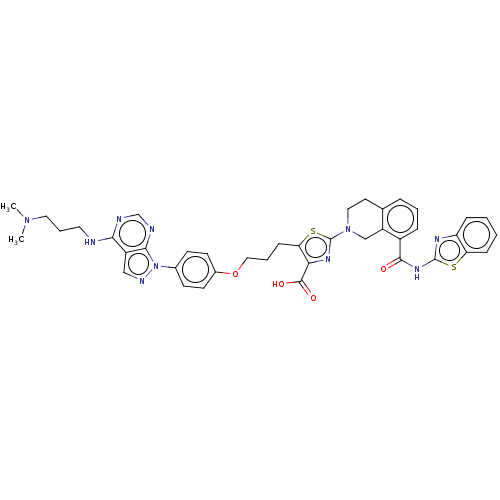 (CHEMBL3342195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030757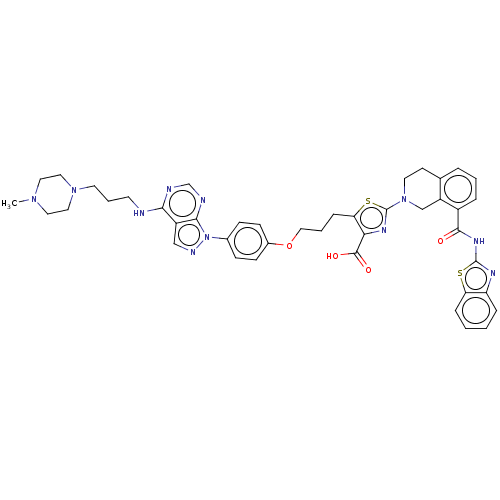 (CHEMBL3342196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030759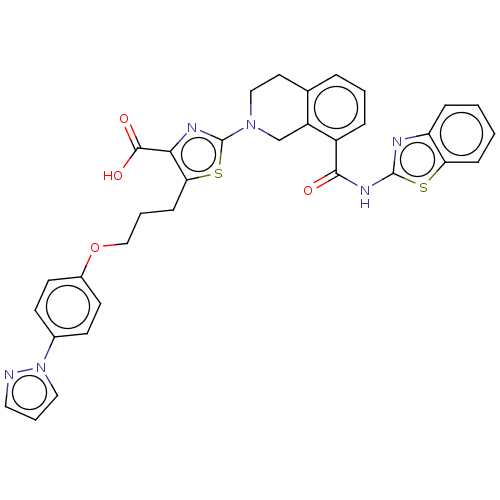 (CHEMBL3342194) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030752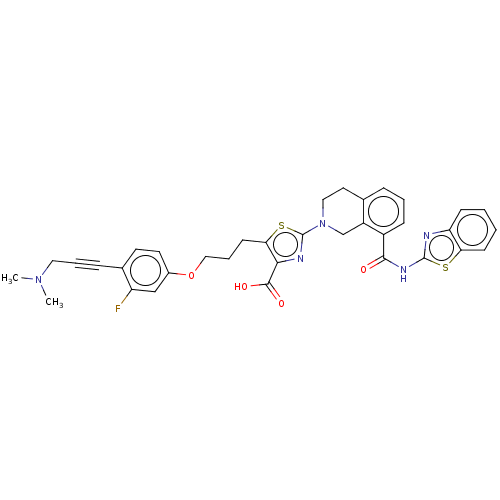 (CHEMBL3342333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030756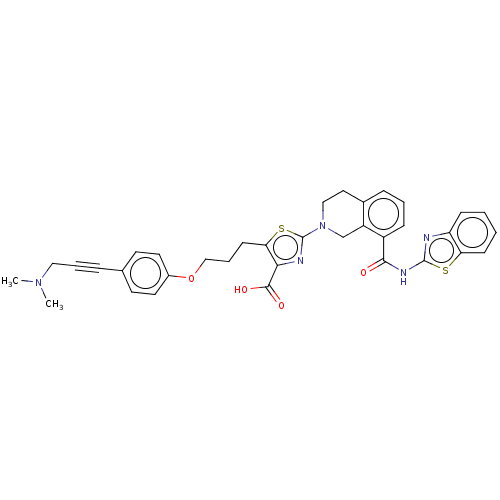 (CHEMBL3342197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563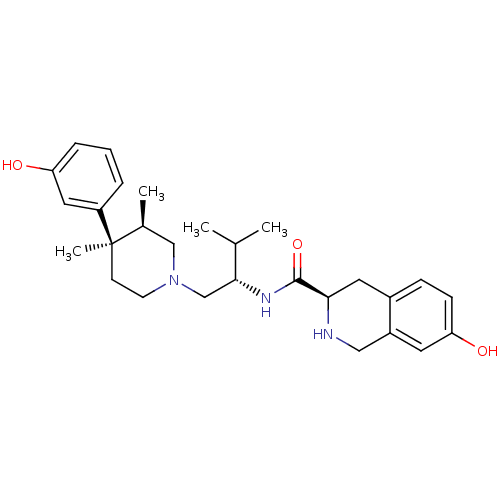 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at kappa opioid receptor in guinea pig caudate assessed as inhibition of U69593-stimulated [35S]-GTP[gammaS] binding | Bioorg Med Chem Lett 24: 2021-32 (2014) Article DOI: 10.1016/j.bmcl.2014.03.040 BindingDB Entry DOI: 10.7270/Q2222WBX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030751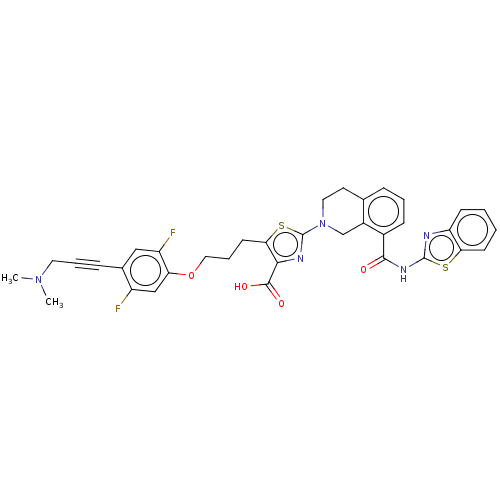 (CHEMBL3342334) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030757 (CHEMBL3342196) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030755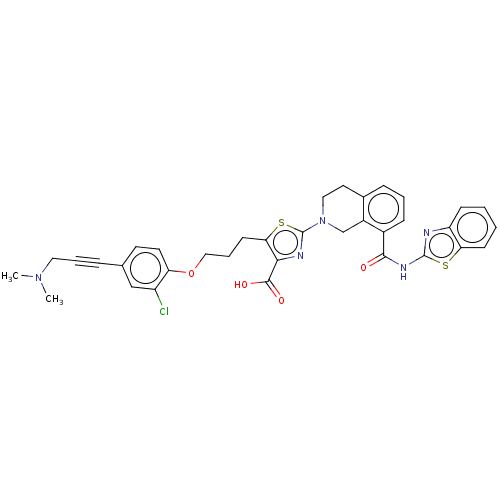 (CHEMBL3342198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030761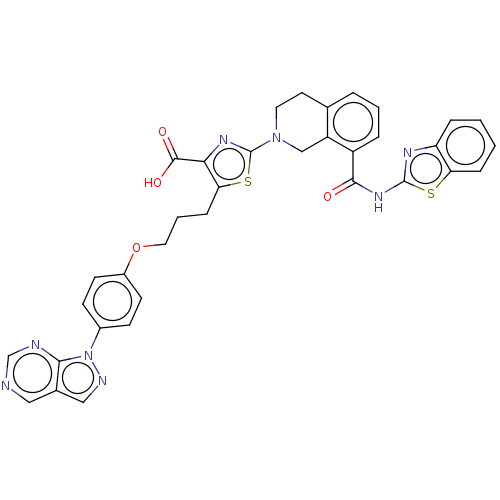 (CHEMBL3342192) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030758 (CHEMBL3342195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030754 (CHEMBL3342332) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50030760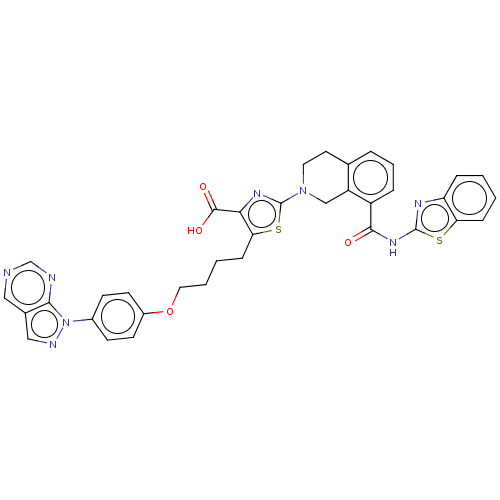 (CHEMBL3342193) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-2 (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D] (Homo sapiens (Human)) | BDBM15131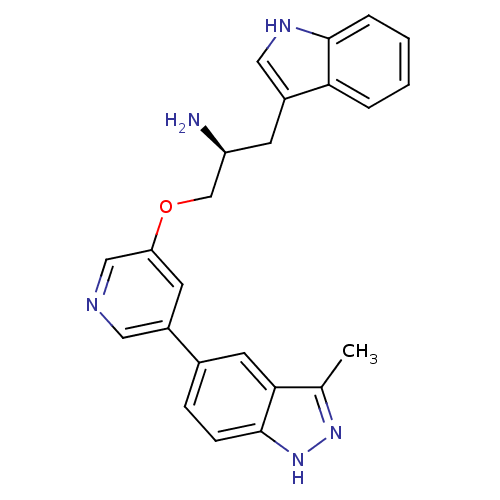 (5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories | Assay Description The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... | Bioorg Med Chem Lett 16: 3740-4 (2006) Article DOI: 10.1016/j.bmcl.2006.04.046 BindingDB Entry DOI: 10.7270/Q2ZP44C9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078161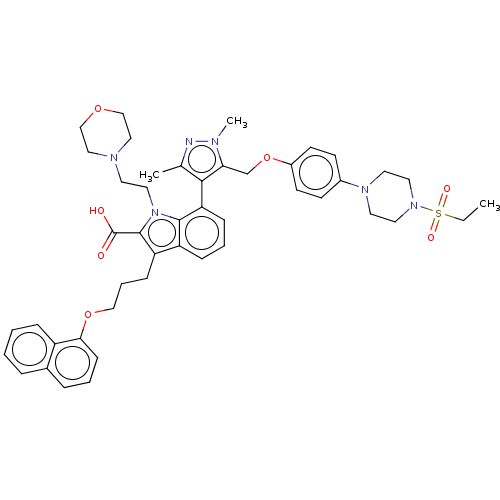 (CHEMBL3417702) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078159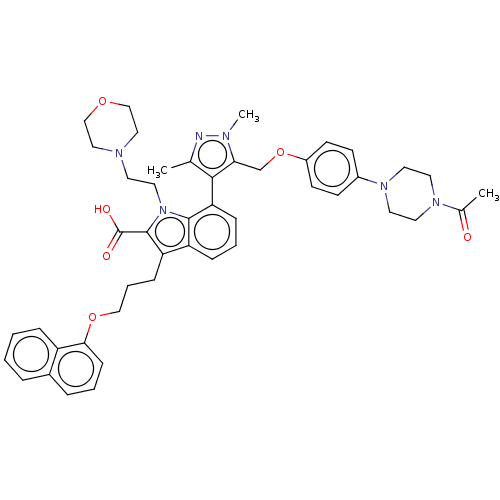 (CHEMBL3417700) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030756 (CHEMBL3342197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078162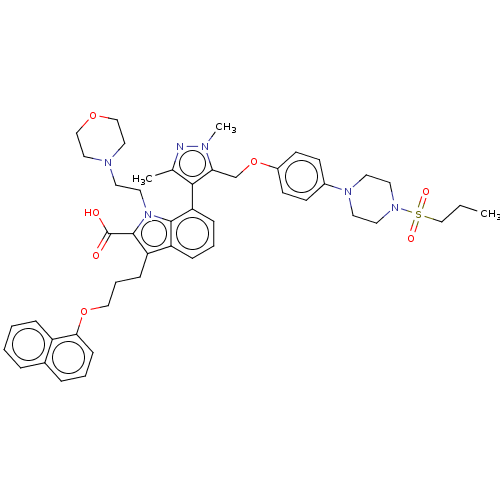 (CHEMBL3417703) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030751 (CHEMBL3342334) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030749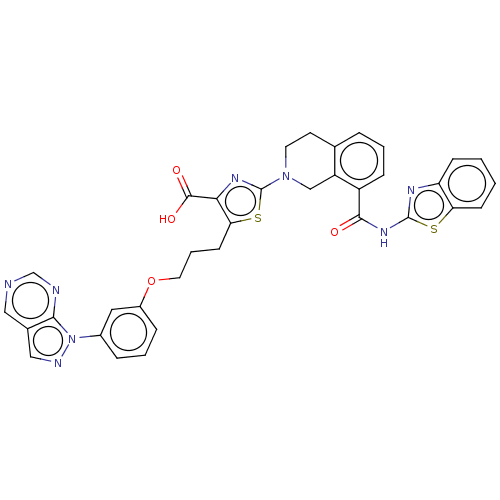 (CHEMBL3342190) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030761 (CHEMBL3342192) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM35048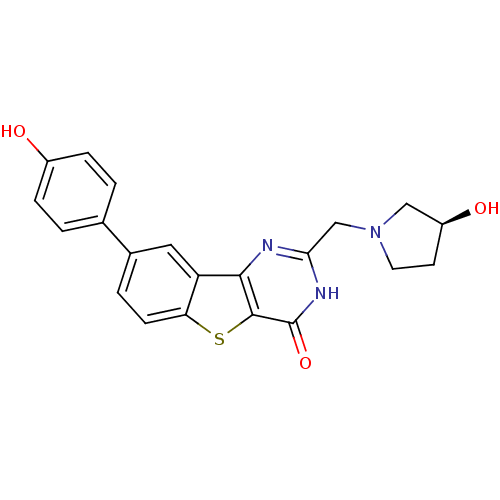 (benzothienopyrimidinone deriv., 20c) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.400 | -53.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Abbott Laboratories | Assay Description In 384-well v-bottom polypropylene plates, compound (2% DMSO) was mixed with Pim kinase and peptide substrate, followed by immediate initiation with ... | J Med Chem 52: 6621-36 (2009) Article DOI: 10.1021/jm900943h BindingDB Entry DOI: 10.7270/Q27P8WQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50030755 (CHEMBL3342198) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie, Inc. Curated by ChEMBL | Assay Description Inhibition of BCL-XL (unknown origin) incubated for 1 hr in presence of 1% human serum by TR-FRET assay | ACS Med Chem Lett 5: 1088-93 (2014) Article DOI: 10.1021/ml5001867 BindingDB Entry DOI: 10.7270/Q2VX0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078160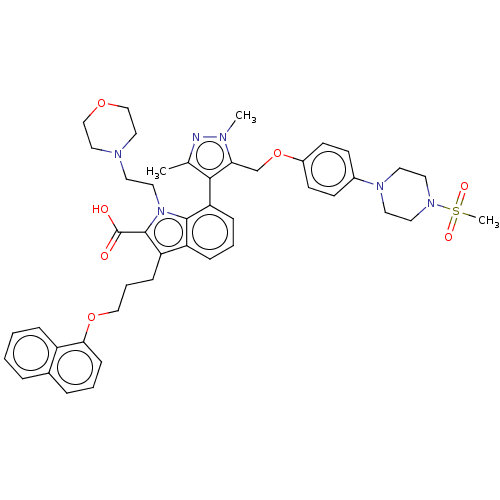 (CHEMBL3417701) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078163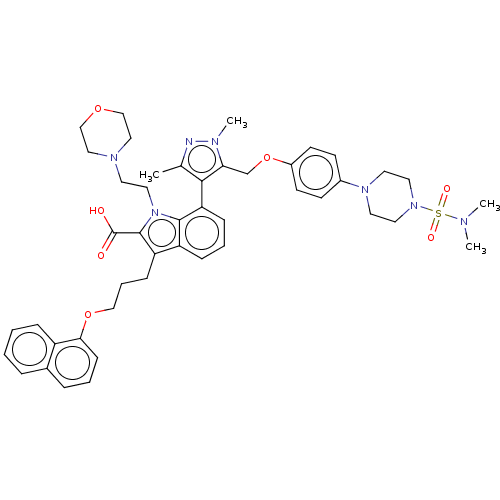 (CHEMBL3417704) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50078164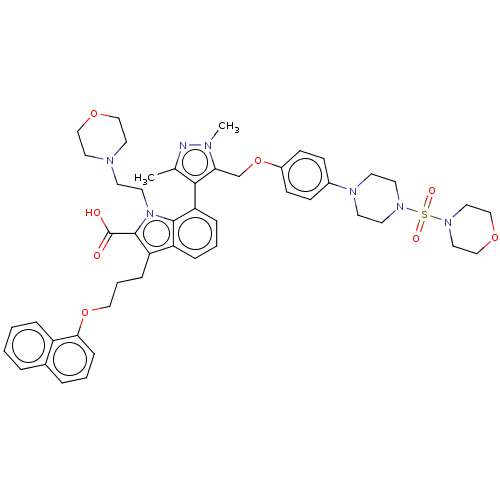 (CHEMBL3417705) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Displacement of F-Bak (GQVGRQLAIIGDK(6-FAM)INR-amide) from MCL1 (unknown origin) after 1 hr by TR-FRET assay | J Med Chem 58: 2180-94 (2015) Article DOI: 10.1021/jm501258m BindingDB Entry DOI: 10.7270/Q2HX1FC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21441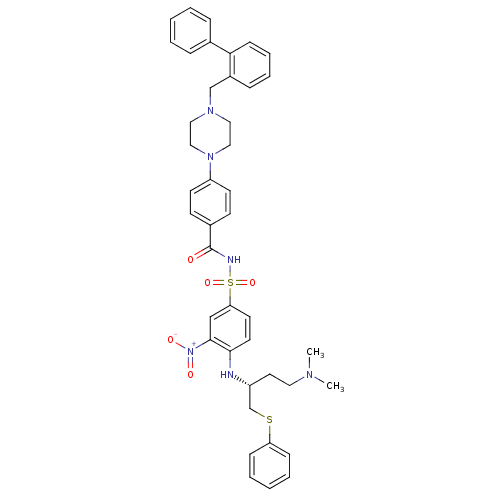 (N-Benylpiperazine derivative, 23i | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 18 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21439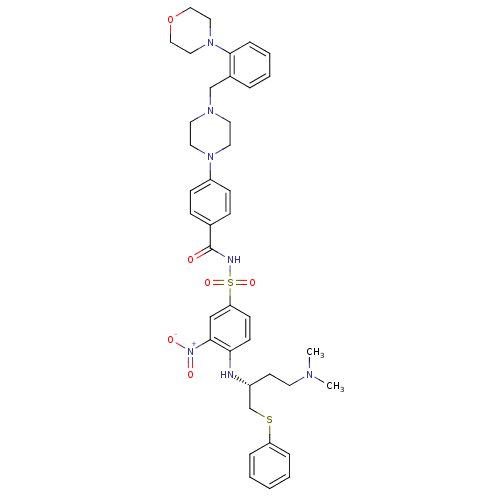 (N-Benylpiperazine derivative, 23g | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 430 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21436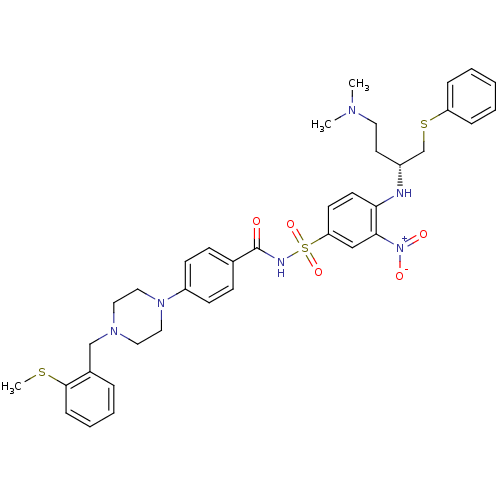 (N-Benylpiperazine derivative, 23d | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 95 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21421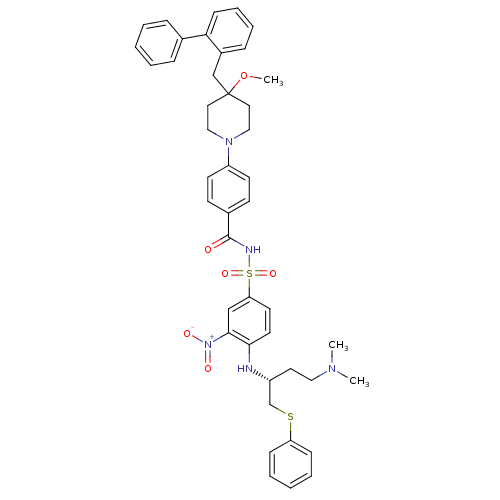 (4-Alkyl-4-methoxypiperidine derivative, 8m | N-[(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | <60 | n/a | 35 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21450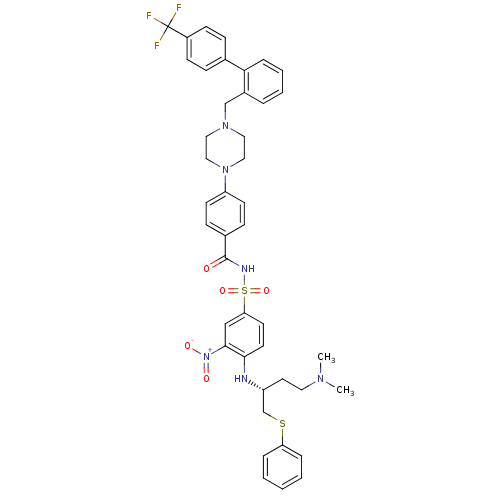 (N-Benylpiperazine derivative, 23r | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 40 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21449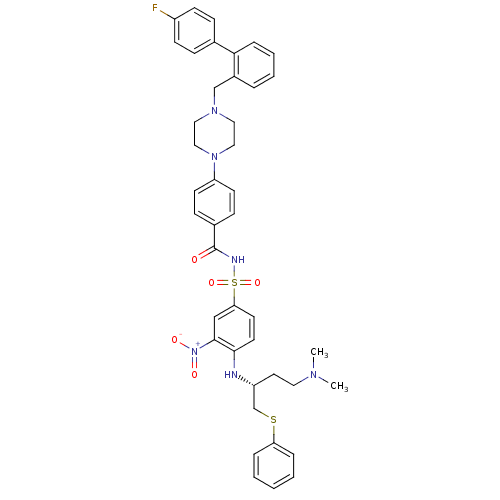 (N-Benylpiperazine derivative, 23q | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 130 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21447 (4-(4-{[2-(4-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 30 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21445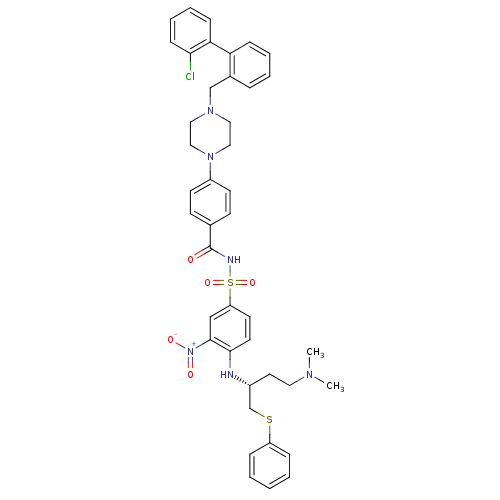 (4-(4-{[2-(2-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 56 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21444 (N-Benylpiperazine derivative, 23m | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | <60 | n/a | 30 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21420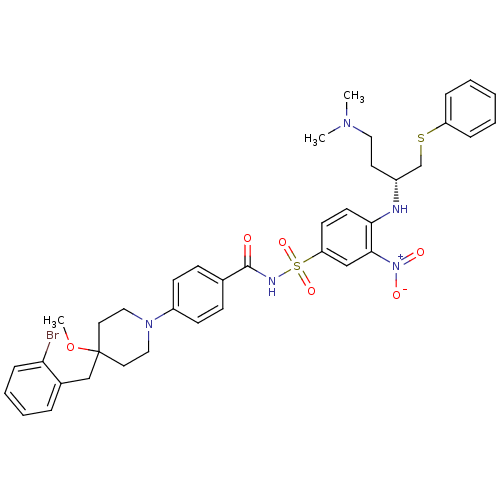 (4-Alkyl-4-methoxypiperidine derivative, 8l | 4-{4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | <60 | n/a | 100 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21440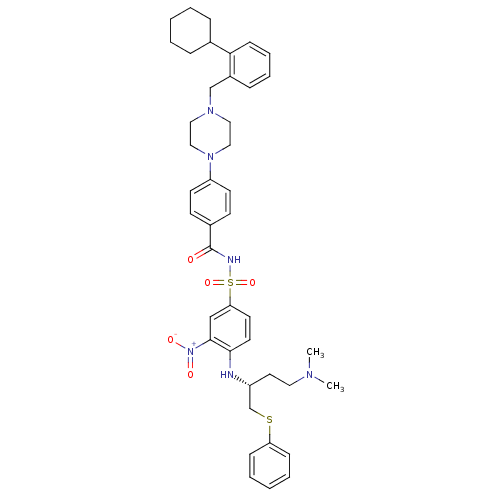 (4-{4-[(2-cyclohexylphenyl)methyl]piperazin-1-yl}-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 61 | n/a | 340 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21414 (4-Alkyl-4-methoxypiperidine derivative, 8f | 4-{4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 62 | n/a | 230 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21418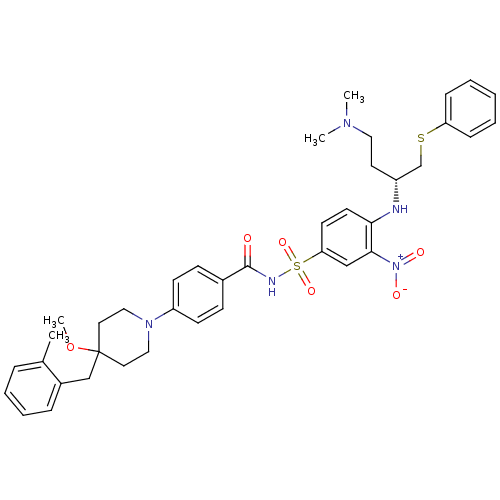 (4-Alkyl-4-methoxypiperidine derivative, 8j | N-[(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 71 | n/a | 150 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21435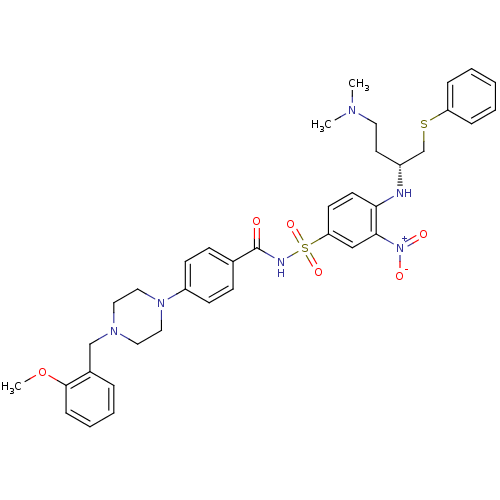 (N-Benylpiperazine derivative, 23c | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 80 | n/a | 390 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21442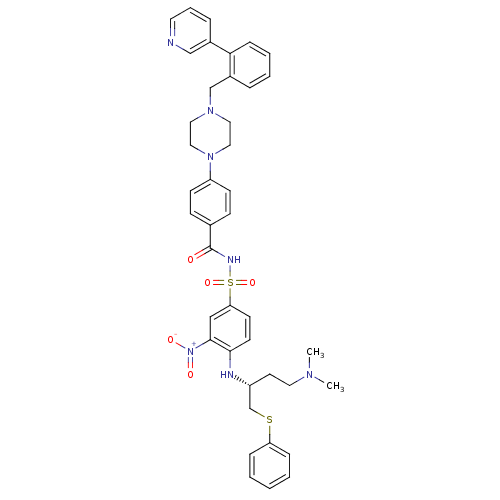 (N-Benylpiperazine derivative, 23k | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 81 | n/a | 800 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21432 (4-Piperidinebenzylidene derivative, 10k | N-[(4-{[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 83 | n/a | 160 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21434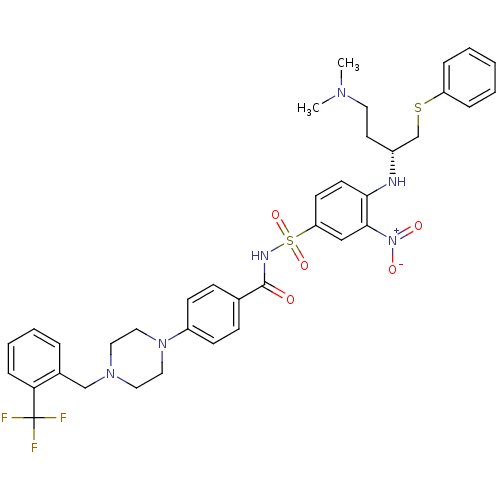 (N-Benylpiperazine derivative, 23j | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 85 | n/a | 390 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21446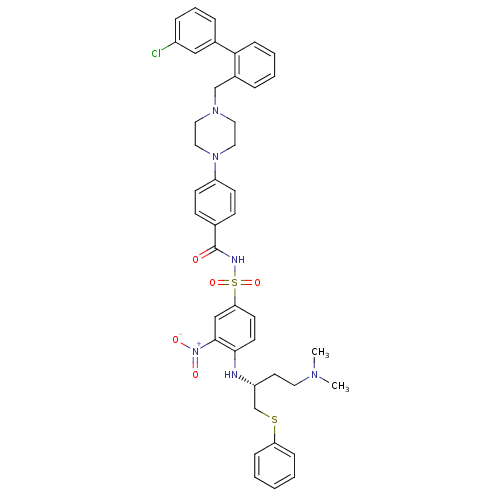 (4-(4-{[2-(3-chlorophenyl)phenyl]methyl}piperazin-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 140 | n/a | 180 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21415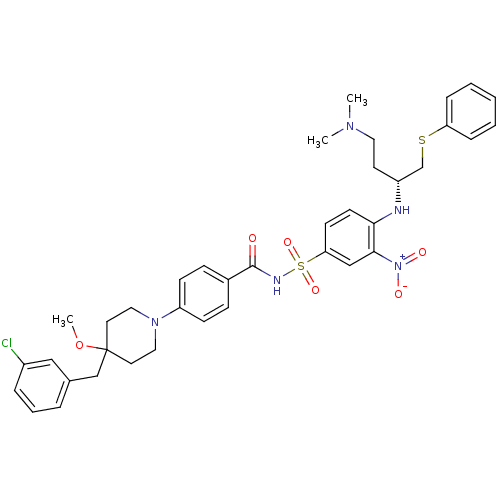 (4-Alkyl-4-methoxypiperidine derivative, 8g | 4-{4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 170 | n/a | 250 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21443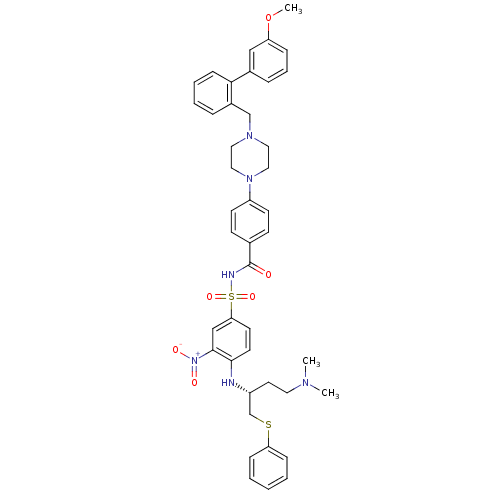 (N-Benylpiperazine derivative, 23l | N-[(4-{[(2R)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | n/a | 178 | n/a | 380 | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21426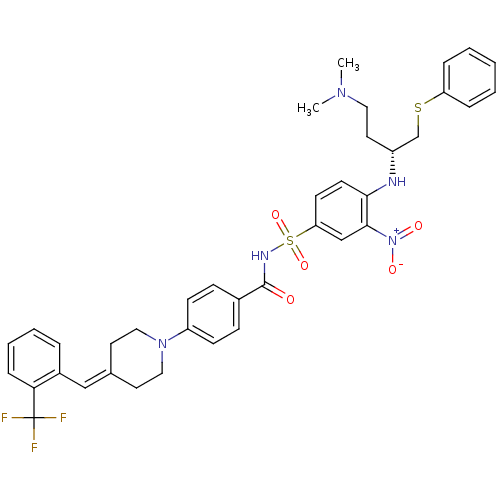 (4-Piperidinebenzylidene derivative, 10e | N-[(4-{[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 300 | n/a | 150 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21425 (4-Piperidinebenzylidene derivative, 10d | 4-{4-[(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 510 | n/a | 620 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM21429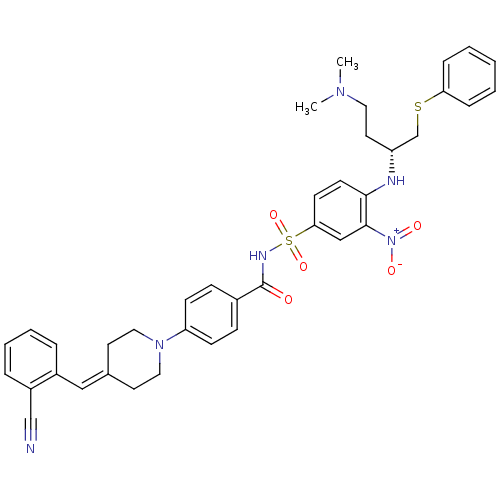 (4-Piperidinebenzylidene derivative, 10h | 4-{4-[(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <0.5 | <-53.1 | 520 | n/a | 1.10E+3 | n/a | n/a | 7.4 | 25 |
Abbott Laboratories | Assay Description The binding affinities (Ki) of compounds were determined using fluorescence polarization assays (FPA) that measure their ability to competitively dis... | J Med Chem 50: 641-62 (2007) Article DOI: 10.1021/jm061152t BindingDB Entry DOI: 10.7270/Q2V69GVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 7881 total ) | Next | Last >> |