

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458634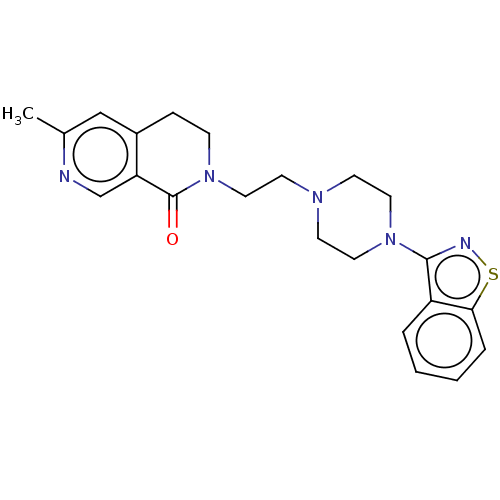 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458528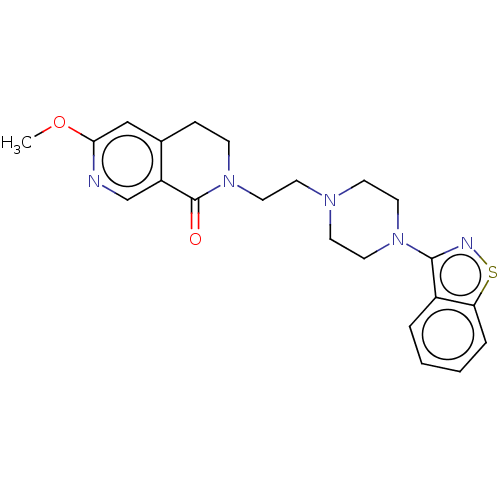 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458557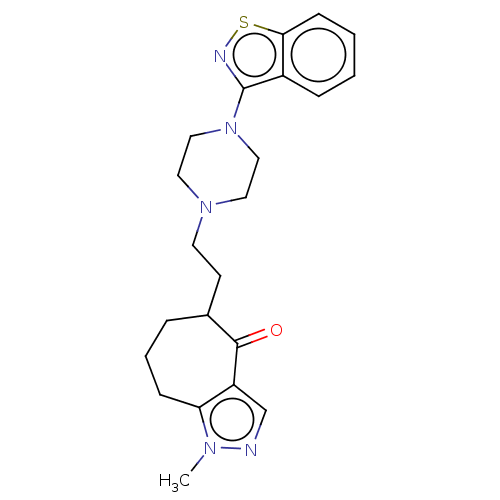 (US10745401, Example 31 | US11466007, Example 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458557 (US10745401, Example 31 | US11466007, Example 31) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458528 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458528 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM466559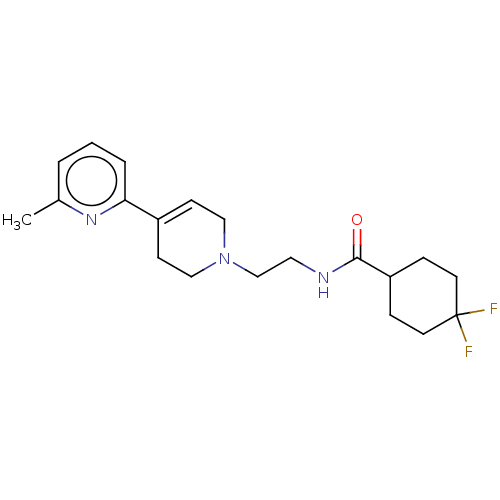 (US10800755, Example 14 | US11014905, Example 14) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description The binding affinity of the present compounds to human 5-HT1A receptor, human D4 receptor, and human D2 receptor was measured in a manner mentioned b... | US Patent US11014905 (2021) BindingDB Entry DOI: 10.7270/Q2C53PZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458588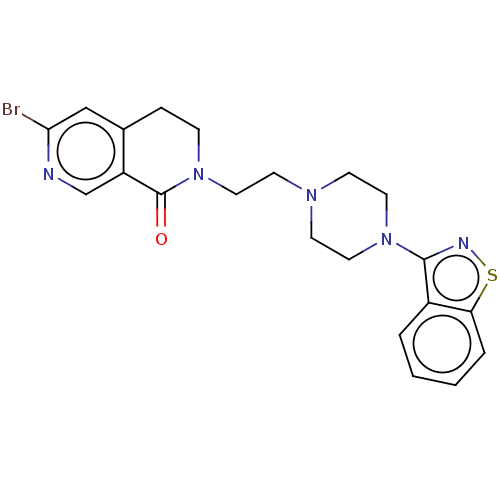 (US10745401, Example 62 | US11466007, Example 62) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458588 (US10745401, Example 62 | US11466007, Example 62) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458582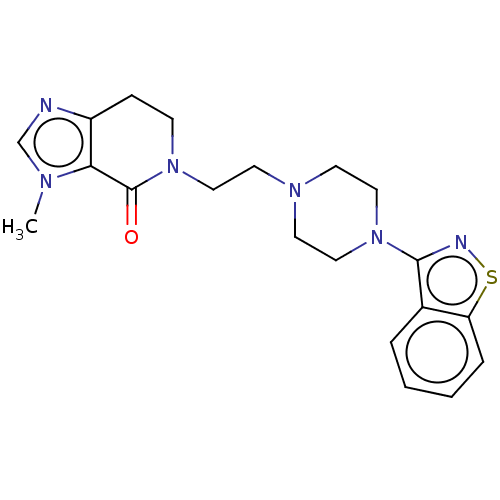 (US10745401, Example 56 | US11466007, Example 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458582 (US10745401, Example 56 | US11466007, Example 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM22416 ((3S,4R)-3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-f...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from human SERT expressed in HEK293 cell membranes after 1 hr by liquid scintillation counting method | Bioorg Med Chem 25: 293-304 (2017) Article DOI: 10.1016/j.bmc.2016.10.034 BindingDB Entry DOI: 10.7270/Q2571F0B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458613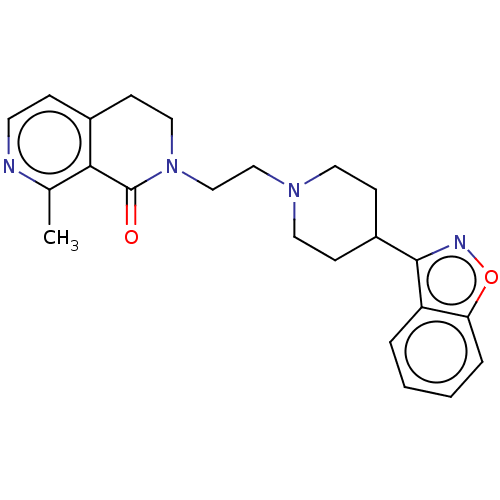 (US10745401, Example 87 | US11466007, Example 87) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458613 (US10745401, Example 87 | US11466007, Example 87) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458694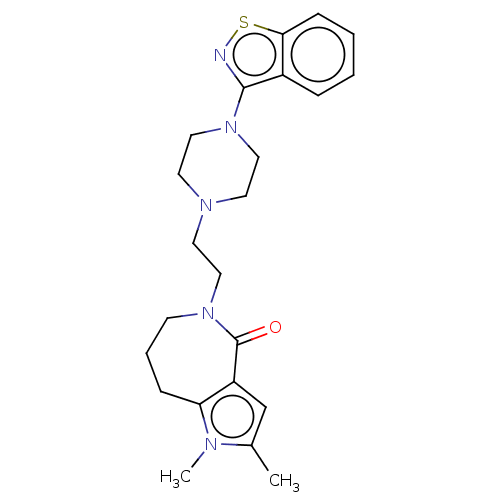 (US10745401, Example 167 | US11466007, Example 167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458694 (US10745401, Example 167 | US11466007, Example 167) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458591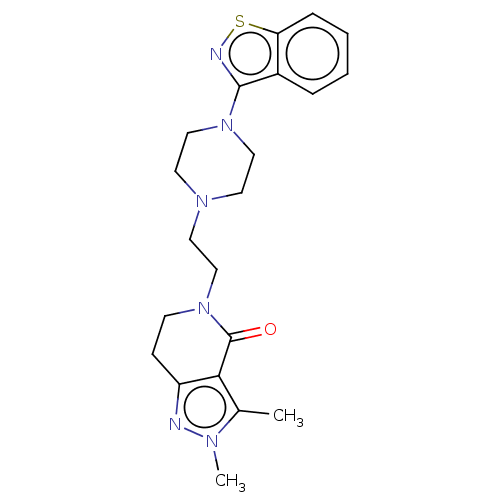 (US10745401, Example 65 | US11466007, Example 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458540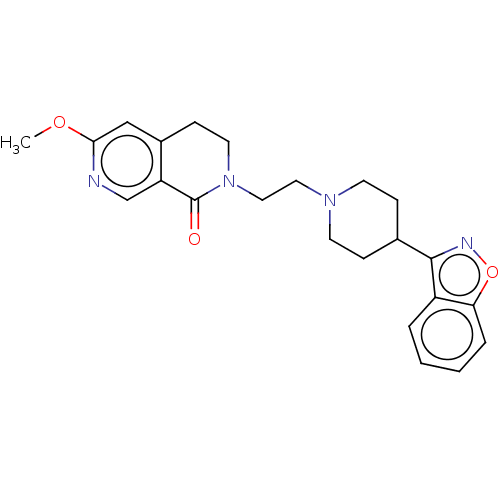 (US10745401, Example 14 | US11466007, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458591 (US10745401, Example 65 | US11466007, Example 65) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458540 (US10745401, Example 14 | US11466007, Example 20) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458634 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458634 (2-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458693 (US10745401, Example 166 | US11466007, Example 166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458693 (US10745401, Example 166 | US11466007, Example 166) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458695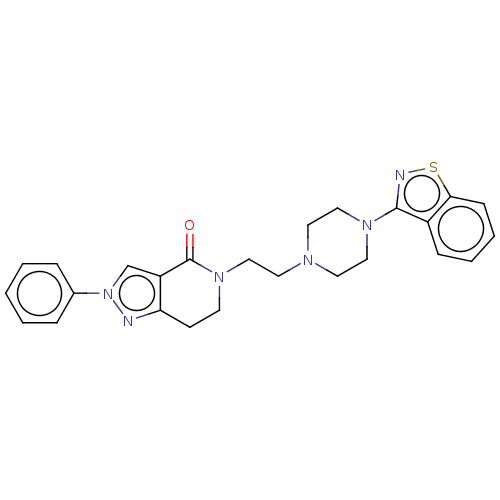 (US10745401, Example 168 | US11466007, Example 168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458695 (US10745401, Example 168 | US11466007, Example 168) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458605 (US10745401, Example 79 | US11466007, Example 79) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458551 (US10745401, Example 25 | US11466007, Example 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458605 (US10745401, Example 79 | US11466007, Example 79) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458551 (US10745401, Example 25 | US11466007, Example 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM466556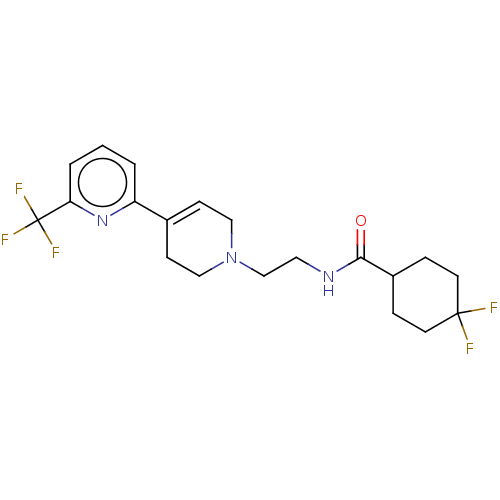 (4,4-Difluoro-N-{2-[6-(trifluoromethyl)-3′,6&...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description The binding affinity of the present compounds to human 5-HT1A receptor, human D4 receptor, and human D2 receptor was measured in a manner mentioned b... | US Patent US11014905 (2021) BindingDB Entry DOI: 10.7270/Q2C53PZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM84745 (CAS_136434-34-9 | DULOXETINE | LY-248686 | LY24868...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Curated by PDSP Ki Database | Neuropsychopharmacology 8: 23-33 (1993) Article DOI: 10.1038/npp.1993.4 BindingDB Entry DOI: 10.7270/Q2XS5SXF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458570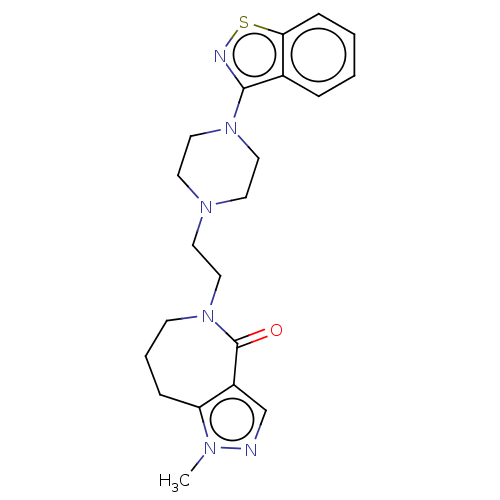 (US10745401, Example 44 | US11466007, Example 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458570 (US10745401, Example 44 | US11466007, Example 44) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458577 (US10745401, Example 51 | US11466007, Example 51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458587 (US10745401, Example 61 | US11466007, Example 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458555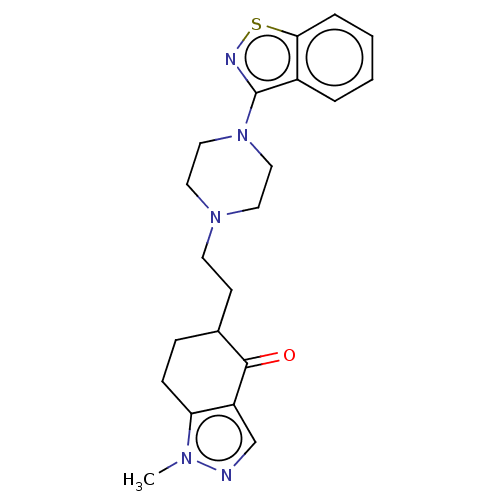 (US10745401, Example 29 | US11466007, Example 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458587 (US10745401, Example 61 | US11466007, Example 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458577 (US10745401, Example 51 | US11466007, Example 51) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458555 (US10745401, Example 29 | US11466007, Example 29) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458603 (US10745401, Example 77 | US11466007, Example 77) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458603 (US10745401, Example 77 | US11466007, Example 77) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458628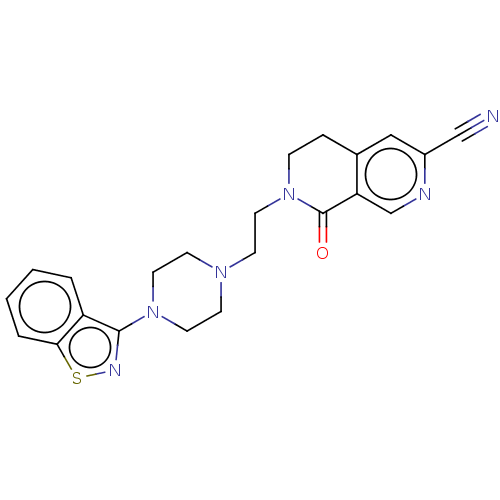 (7-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM458628 (7-{2-[4-(1,2-Benzoisothiazol-3-yl)piperazin-1-yl]e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM126698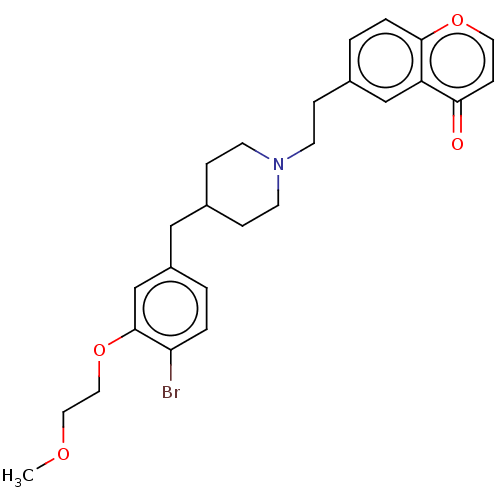 (US8778970, 5-6) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.270 | -54.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description [3H] citalopram binding was assayed according to the method of Owens et al. [Owens M. J. et al., J. Pharm. Exp. Ther., 283, 1305-1322 (1997)]. Specif... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458530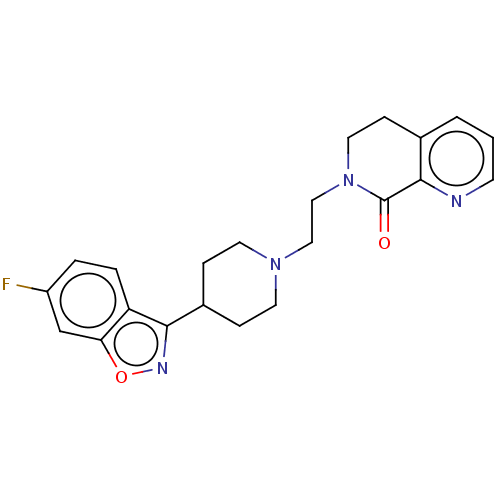 (US10745401, Example 125 | US10745401, Example 4 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following procedures. CHO cell membrane ... | Citation and Details BindingDB Entry DOI: 10.7270/Q22V2KB4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM458530 (US10745401, Example 125 | US10745401, Example 4 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description Binding affinity of the present compound for human 5-HT1A receptor, human 5-HT2A receptor, and human D2 receptor was measured by the following proced... | US Patent US10745401 (2020) BindingDB Entry DOI: 10.7270/Q2D221P5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM126701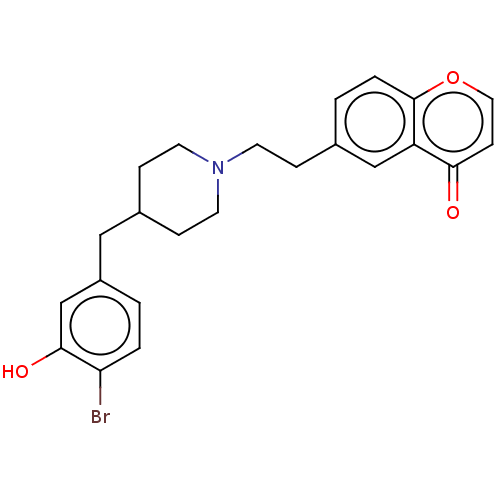 (US8778970, 5-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.270 | -54.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description The experiment was carried out according to the method of Yabuuchi et al. [Yabuuchi K. et al., Biogenic Amines, 18, 319-328 (2004)]. 50 ul of [3H] 8-... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50180206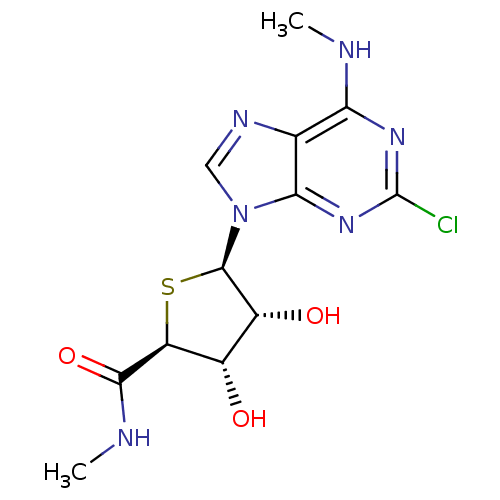 ((2S,3S,4R,5R)-5-(2-chloro-6-(methylamino)-9H-purin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ewha Womans University Curated by ChEMBL | Assay Description Displacement of [125I]AB-MECA from human adenosine A3 receptor transfected in CHO cells | J Med Chem 49: 273-81 (2006) Article DOI: 10.1021/jm050595e BindingDB Entry DOI: 10.7270/Q2736QG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM466577 (2,2-Dimethyl-N-{2-[6-(trifluoromethyl)-3′,6&...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SUMITOMO DAINIPPON PHARMA CO., LTD. US Patent | Assay Description The binding affinity of the present compounds to human 5-HT1A receptor, human D4 receptor, and human D2 receptor was measured in a manner mentioned b... | US Patent US11014905 (2021) BindingDB Entry DOI: 10.7270/Q2C53PZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5055 total ) | Next | Last >> |