

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095261 ((3-Chloro-phenyl)-(6,7-diethoxy-quinazolin-4-yl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3556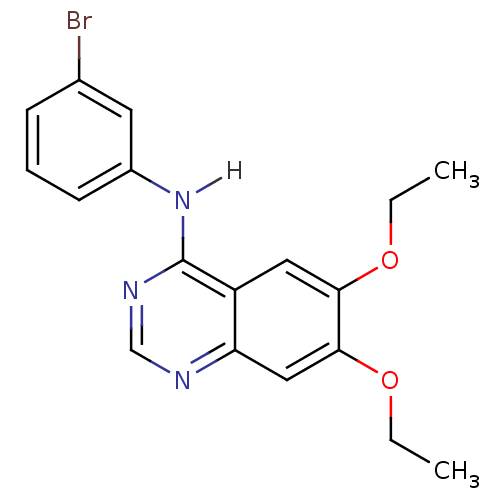 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095273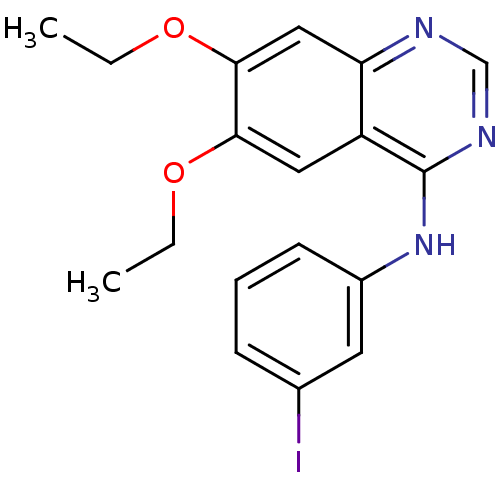 ((6,7-Diethoxy-quinazolin-4-yl)-(3-iodo-phenyl)-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3532 (CHEMBL540068 | CHEMBL7917 | N-(3-chlorophenyl)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3533 (CHEMBL1204305 | CHEMBL96065 | N-(3-iodophenyl)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095261 ((3-Chloro-phenyl)-(6,7-diethoxy-quinazolin-4-yl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3532 (CHEMBL540068 | CHEMBL7917 | N-(3-chlorophenyl)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095273 ((6,7-Diethoxy-quinazolin-4-yl)-(3-iodo-phenyl)-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3531 (CHEMBL541586 | CHEMBL94431 | N-(3-fluorophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3533 (CHEMBL1204305 | CHEMBL96065 | N-(3-iodophenyl)-6,7...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095277 ((6,7-Diethoxy-quinazolin-4-yl)-(3-fluoro-phenyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370935 (CHEMBL1203937) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50095277 ((6,7-Diethoxy-quinazolin-4-yl)-(3-fluoro-phenyl)-a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.17 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370943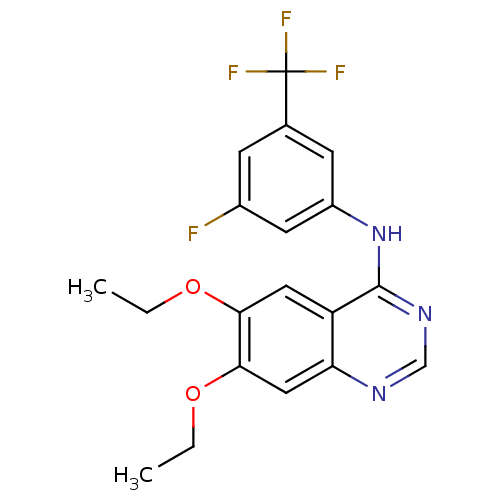 (CHEMBL1203935) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.95 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370938 (CHEMBL1203939) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.31 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50177114 (CHEMBL1203938 | CHEMBL374810 | cid_1474879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4619 (Anilinoquinazoline deriv. 2 | CHEMBL1204360 | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370938 (CHEMBL1203939) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370934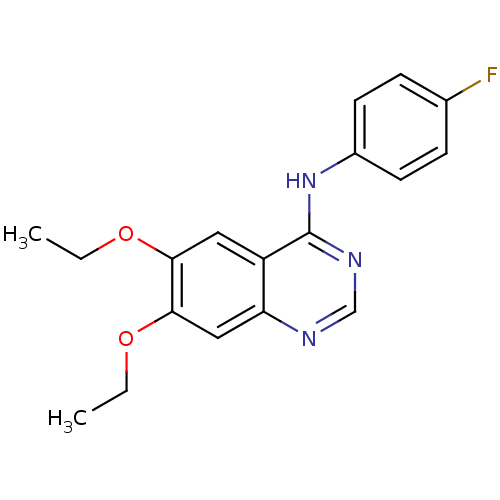 (CHEMBL1203940) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370935 (CHEMBL1203937) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370944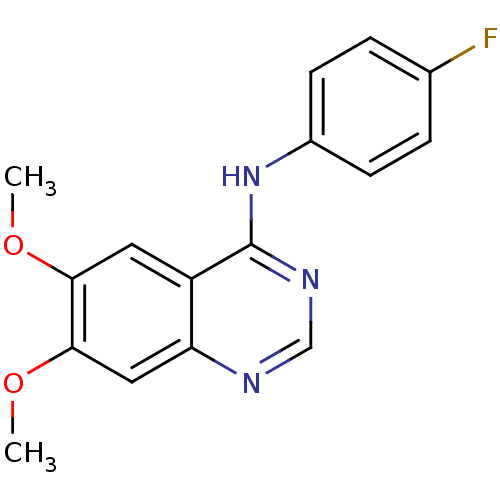 (CHEMBL1203934) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370942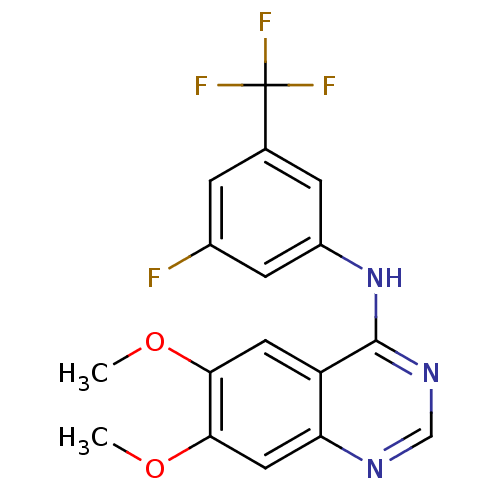 (CHEMBL1203936) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370942 (CHEMBL1203936) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370934 (CHEMBL1203940) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM3531 (CHEMBL541586 | CHEMBL94431 | N-(3-fluorophenyl)-6,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM4619 (Anilinoquinazoline deriv. 2 | CHEMBL1204360 | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50177114 (CHEMBL1203938 | CHEMBL374810 | cid_1474879) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB4 tyrosine kinase phosphorylation expressed in human CEM/4 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370943 (CHEMBL1203935) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB4 tyrosine kinase phosphorylation expressed in human CEM/4 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50370944 (CHEMBL1203934) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranes | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50095273 ((6,7-Diethoxy-quinazolin-4-yl)-(3-iodo-phenyl)-ami...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB4 tyrosine kinase phosphorylation expressed in human CEM/4 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50095273 ((6,7-Diethoxy-quinazolin-4-yl)-(3-iodo-phenyl)-ami...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB2 tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-4 (Homo sapiens (Human)) | BDBM50370935 (CHEMBL1203937) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB4 tyrosine kinase phosphorylation expressed in human CEM/4 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM3032 (CHEMBL1204168 | CHEMBL29197 | N-(3-bromophenyl)-6,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB2 tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM3556 (4-[(3-Bromophenyl)amino]-6,7-diethoxyquinazoline |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 215 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB2 tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor tyrosine-protein kinase erbB-2 (Homo sapiens (Human)) | BDBM50370935 (CHEMBL1203937) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 231 | n/a | n/a | n/a | n/a | n/a | n/a |
Lawrence Berkeley National Laboratory Curated by ChEMBL | Assay Description Inhibition of human ErbB2 tyrosine kinase phosphorylation expressed in mouse BaF3 cells | J Med Chem 48: 7445-56 (2005) Article DOI: 10.1021/jm050607w BindingDB Entry DOI: 10.7270/Q26D5TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088185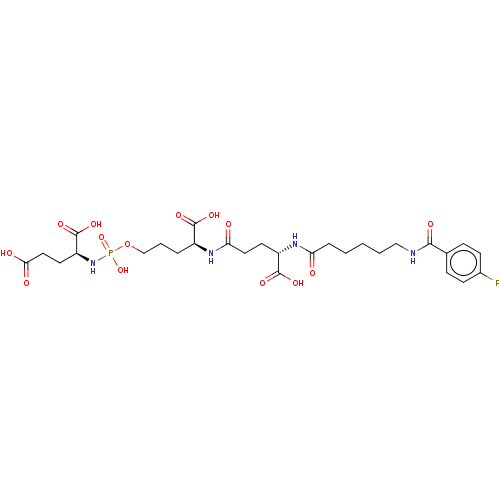 (CHEMBL3427435) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088192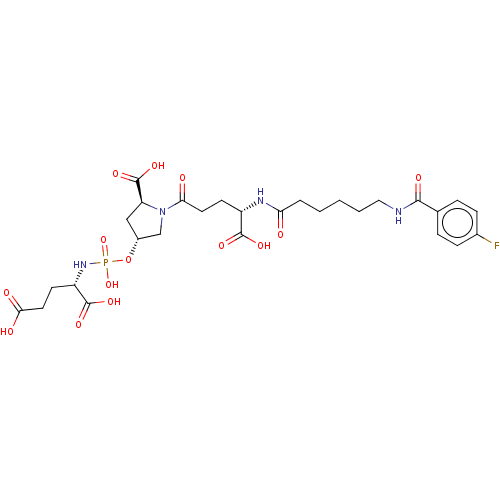 (CHEMBL3427442) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088182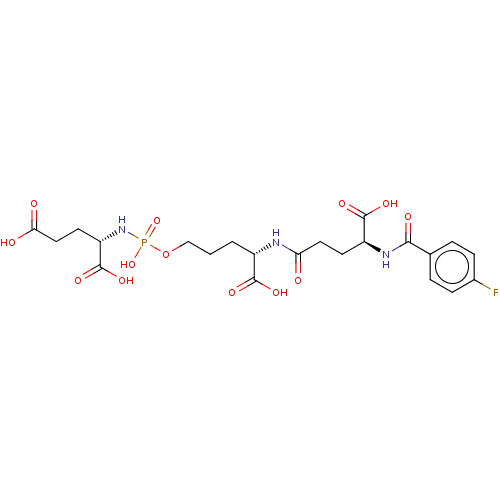 (CHEMBL3427434) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088190 (CHEMBL3427440) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088191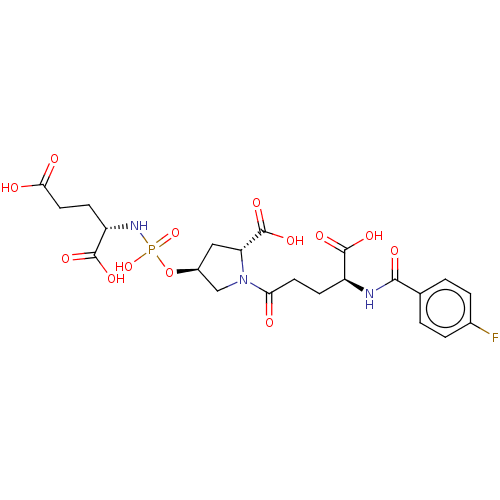 (CHEMBL3427441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088193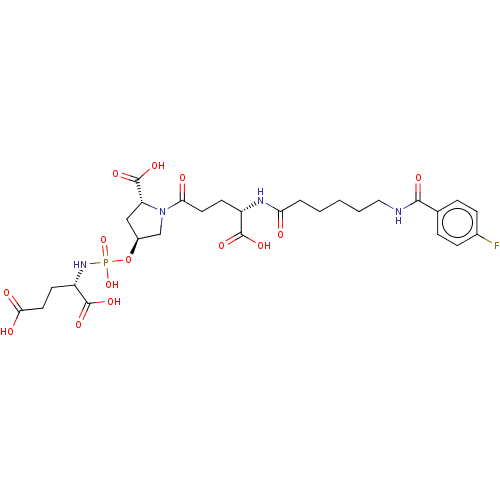 (CHEMBL3427443) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088183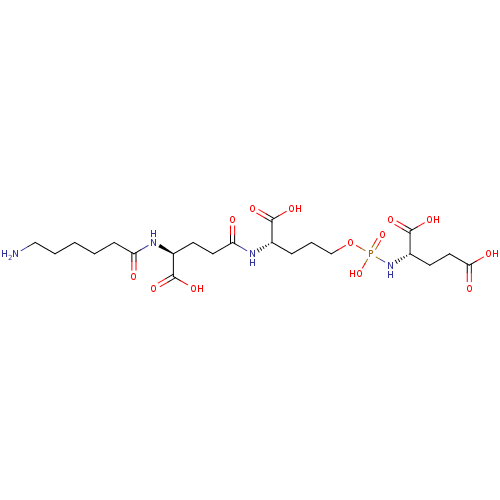 (CHEMBL3427433) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088184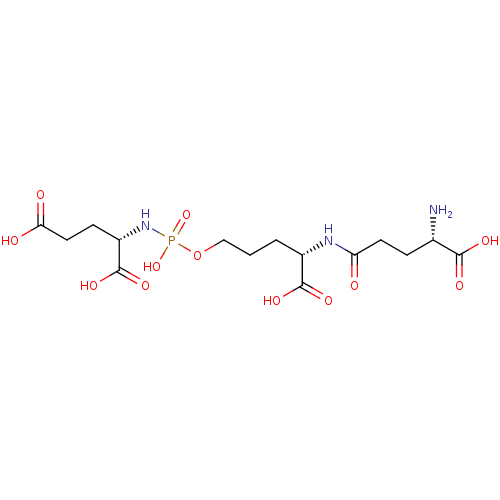 (CHEMBL3427432) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088186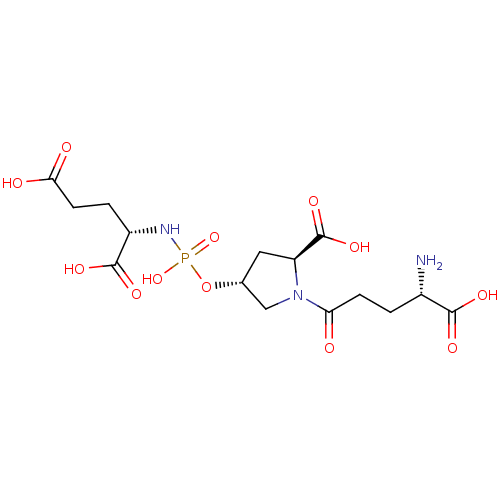 (CHEMBL3427436) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50088188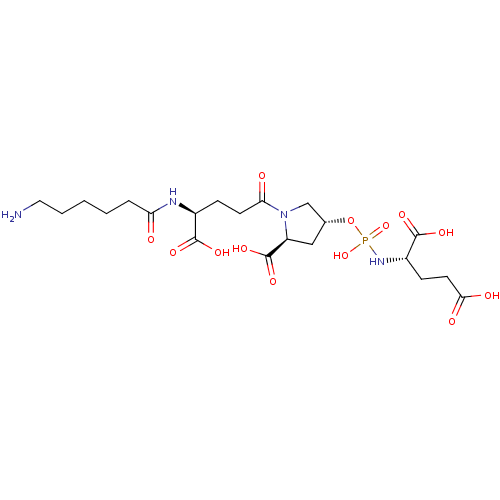 (CHEMBL3427438) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University Curated by ChEMBL | Assay Description Inhibition of PSMA (unknown origin) incubated for 15 mins using PABGgG substrate by HPLC method | Bioorg Med Chem Lett 25: 2536-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.047 BindingDB Entry DOI: 10.7270/Q29G5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 52 total ) | Next | Last >> |