

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GABA-A receptor; alpha-2/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50332377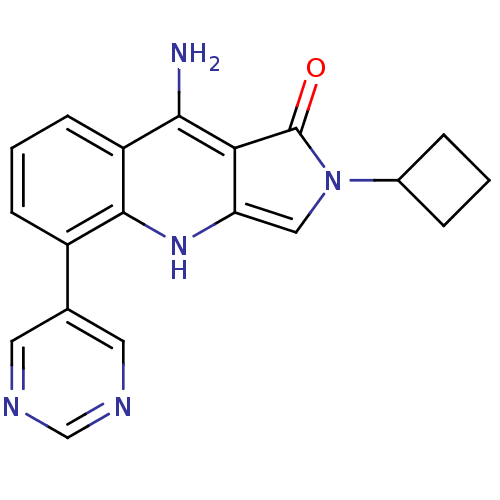 (9-Amino-2-cyclobutyl-5-(pyrimidin-5-yl)-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]flunitrazepam from human alpha2beta3gamma2 GABA receptor in Sf9 membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 8374-82 (2010) Article DOI: 10.1016/j.bmc.2010.09.058 BindingDB Entry DOI: 10.7270/Q2FB536X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50005429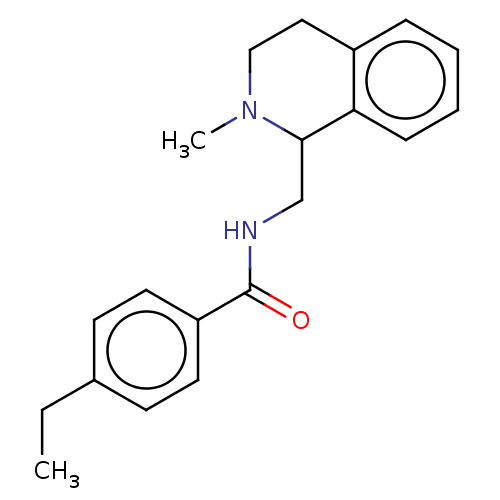 (CHEMBL4070288) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50007985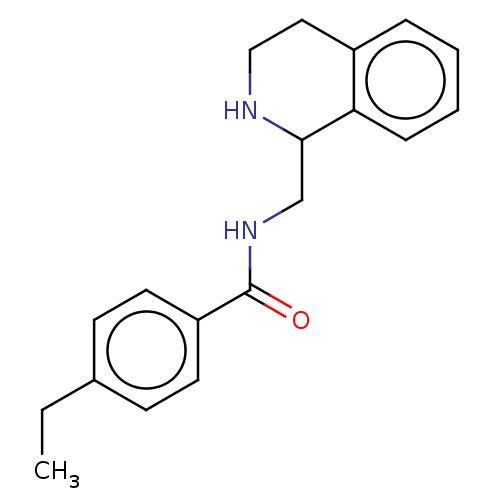 (CHEMBL4097865) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human MOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50042064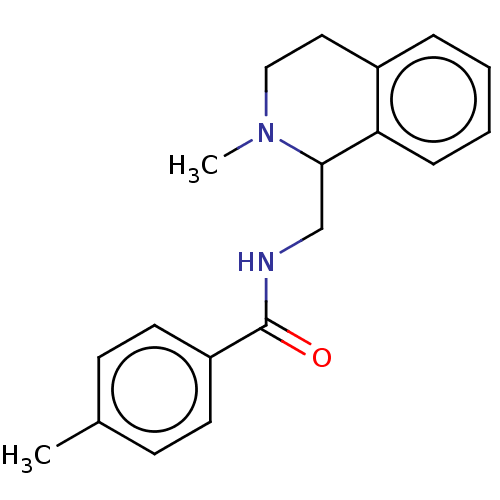 (CHEMBL4097466) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-2/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50332378 (4-Amino-7-fluoro-N-propyl-8-(pyrimidin-2-yl)cinnol...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]flunitrazepam from human alpha2beta3gamma2 GABA receptor in Sf9 membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 8374-82 (2010) Article DOI: 10.1016/j.bmc.2010.09.058 BindingDB Entry DOI: 10.7270/Q2FB536X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015426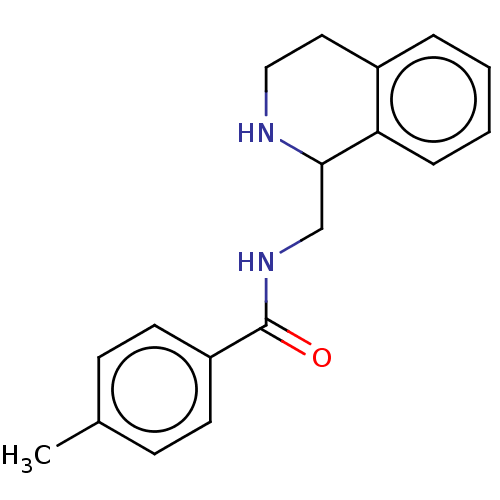 (CHEMBL4070750) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50009239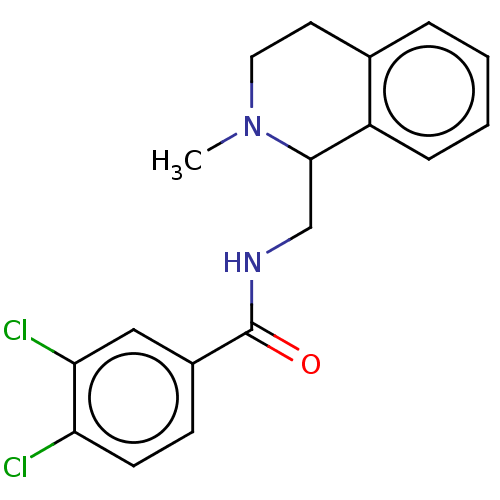 (CHEMBL4092125) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-2/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50332374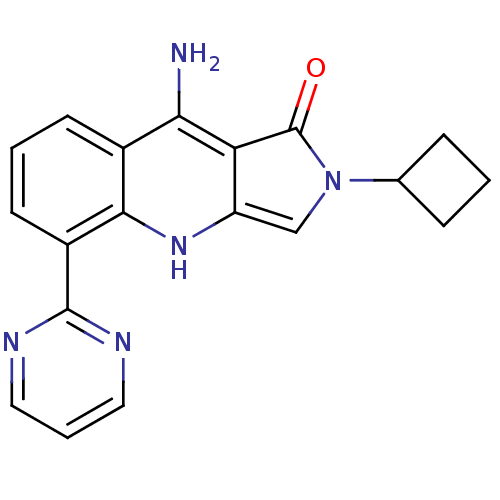 (9-Amino-2-cyclobutyl-5-(pyrimidin-2-yl)-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]flunitrazepam from human alpha2beta3gamma2 GABA receptor in Sf9 membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 8374-82 (2010) Article DOI: 10.1016/j.bmc.2010.09.058 BindingDB Entry DOI: 10.7270/Q2FB536X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50332377 (9-Amino-2-cyclobutyl-5-(pyrimidin-5-yl)-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]flunitrazepam from human alpha5beta3gamma2 GABA receptor in Sf9 membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 8374-82 (2010) Article DOI: 10.1016/j.bmc.2010.09.058 BindingDB Entry DOI: 10.7270/Q2FB536X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50332374 (9-Amino-2-cyclobutyl-5-(pyrimidin-2-yl)-2,3-dihydr...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]flunitrazepam from human alpha5beta3gamma2 GABA receptor in Sf9 membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 8374-82 (2010) Article DOI: 10.1016/j.bmc.2010.09.058 BindingDB Entry DOI: 10.7270/Q2FB536X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008523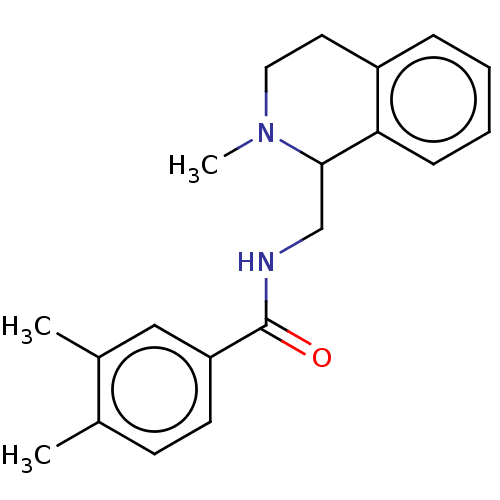 (CHEMBL4071332) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50015433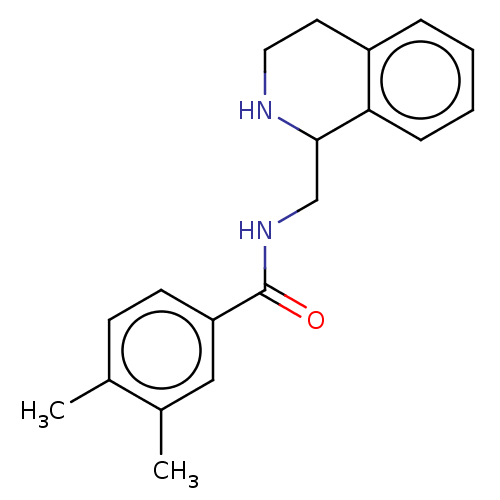 (CHEMBL4105599) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50008003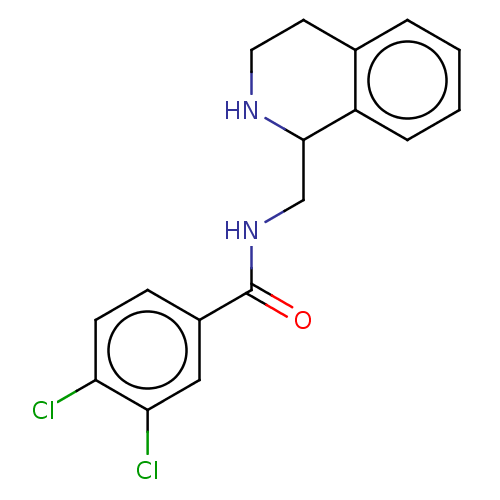 (CHEMBL4065924) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Displacement of [3H]naloxone from recombinant human MOR expressed in HEK cell membranes incubated for 60 mins | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-2/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50332376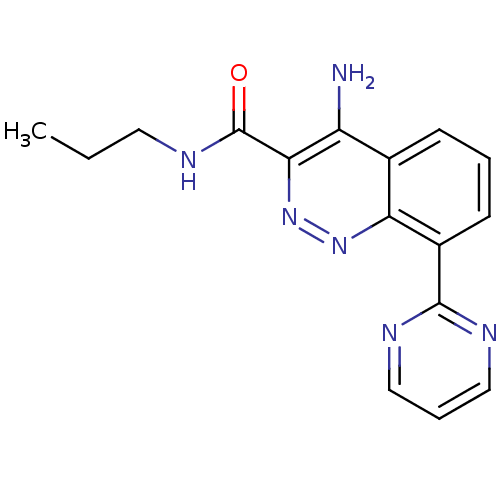 (4-Amino-N-propyl-8-(pyrimidin-2-yl)cinnoline-3-car...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]flunitrazepam from human alpha2beta3gamma2 GABA receptor in Sf9 membranes after 1 hr by liquid scintillation counting | Bioorg Med Chem 18: 8374-82 (2010) Article DOI: 10.1016/j.bmc.2010.09.058 BindingDB Entry DOI: 10.7270/Q2FB536X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50203422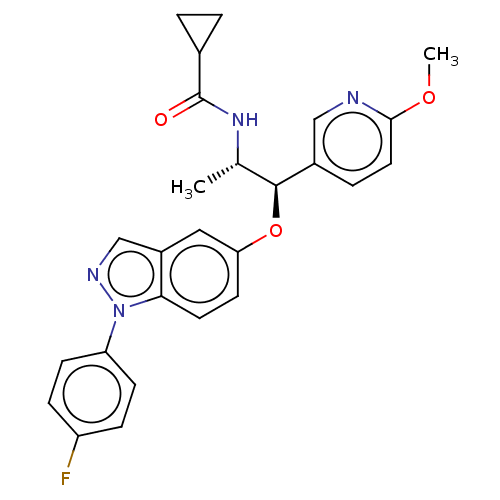 (CHEMBL3937635) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288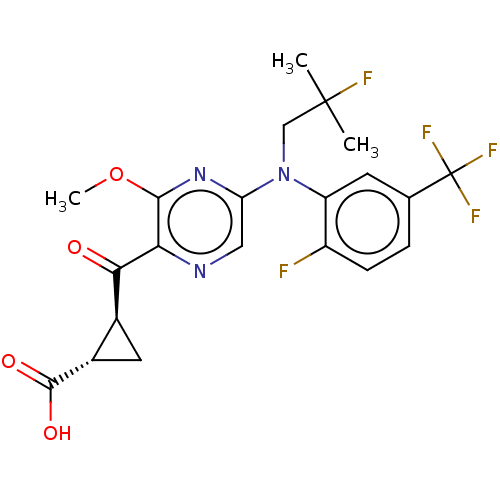 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287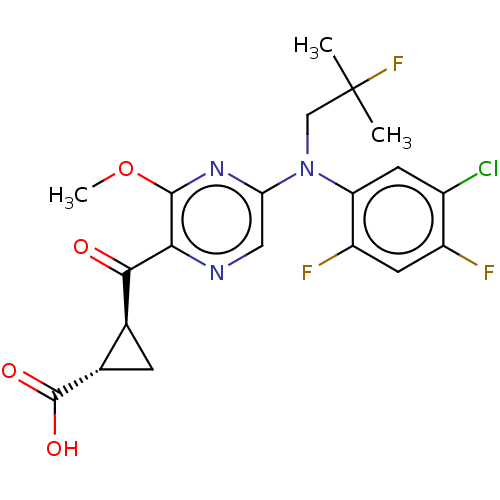 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519568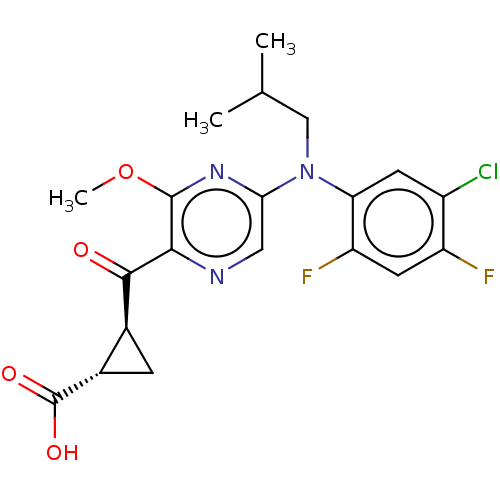 (CHEMBL4549822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008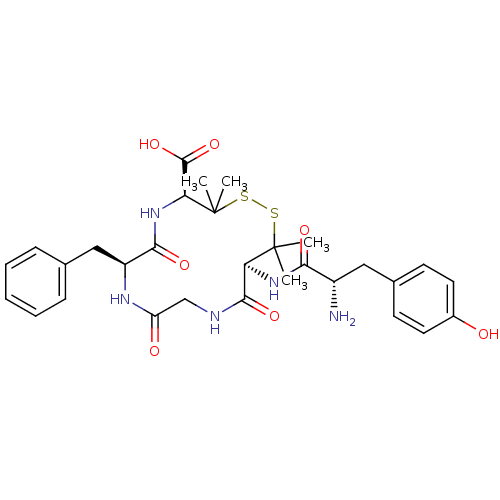 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human DOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457410 (CHEMBL4213191) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519570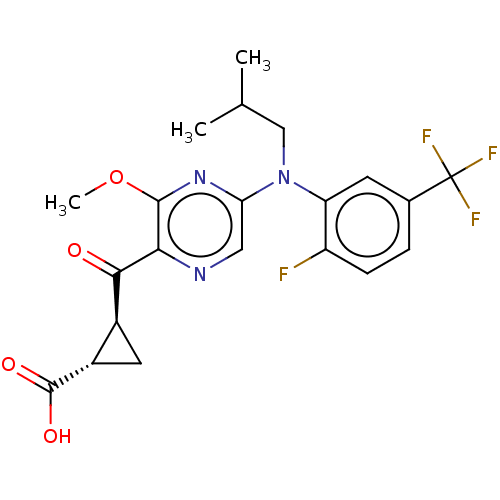 (CHEMBL4567083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519585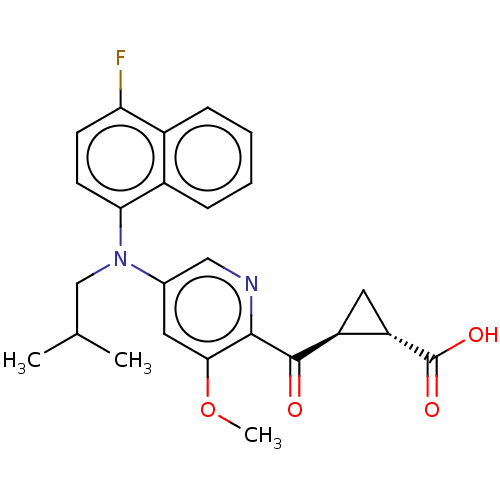 (CHEMBL4464773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.381 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457406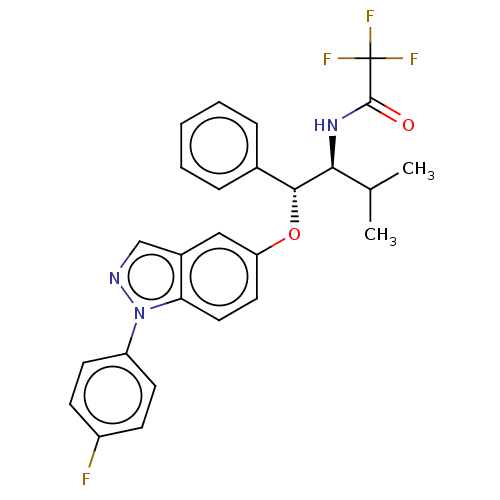 (CHEMBL4208262) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457403 (CHEMBL4208798) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519591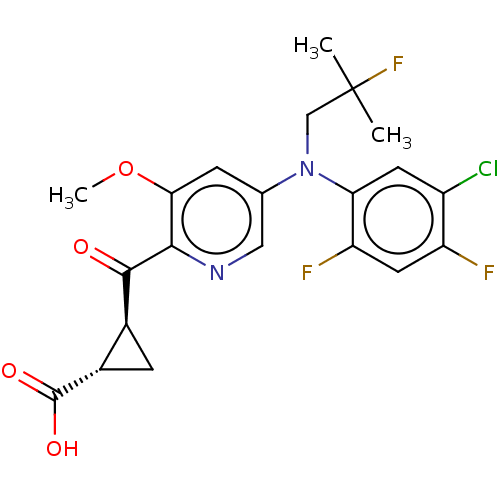 (CHEMBL4562583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519574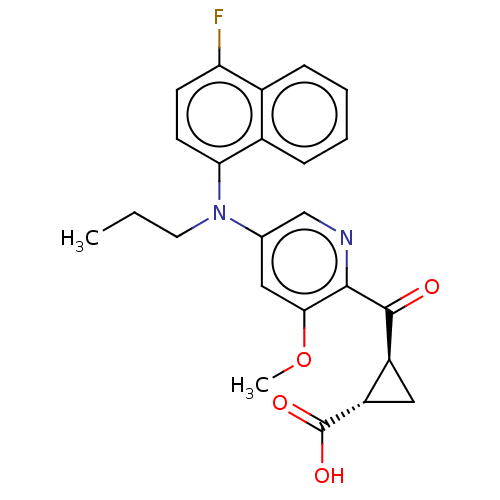 (CHEMBL4563433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.626 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519588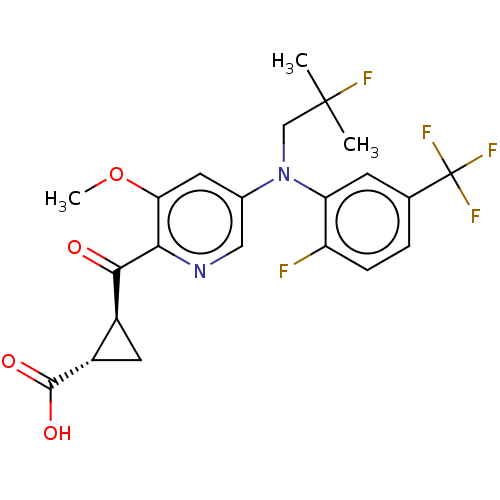 (CHEMBL4584584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290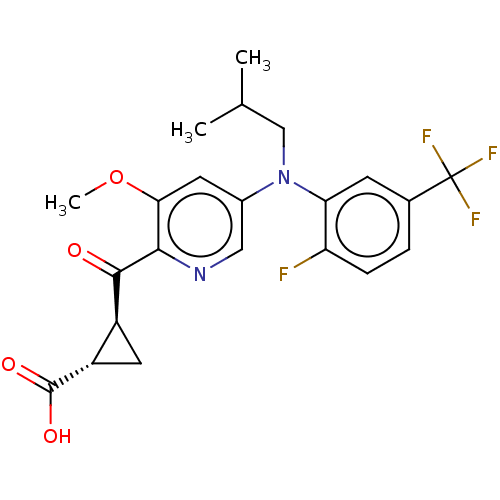 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM140009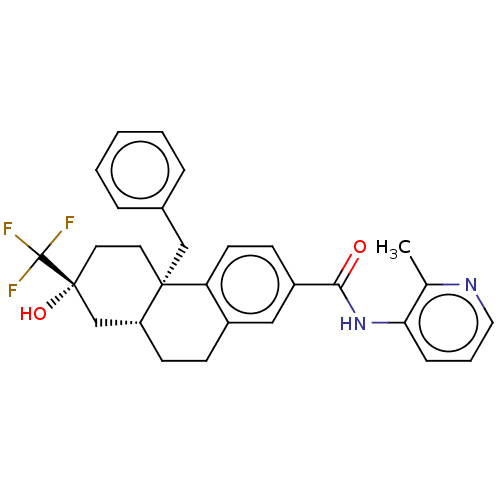 (US8901310, Example 1 ) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519580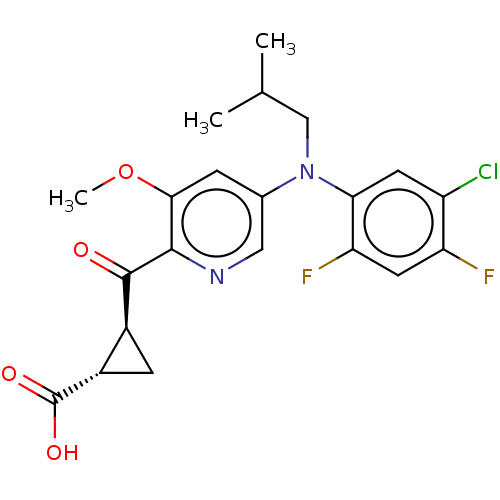 (CHEMBL4574439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519565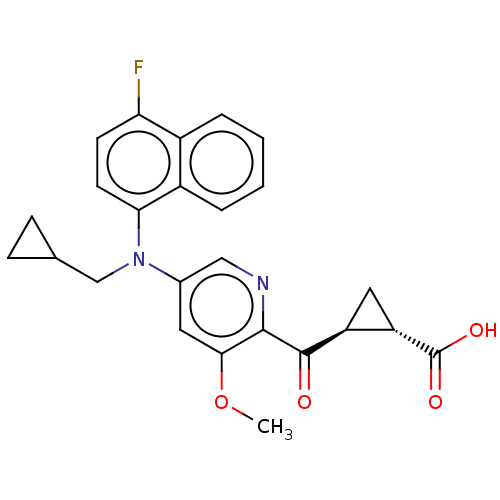 (CHEMBL4441362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.758 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
National Health Research Institutes Curated by ChEMBL | Assay Description Agonist activity at human KOR expressed in HEK293 cells assessed as inhibition of forskolin-induced adenylyl cyclase-mediated cAMP accumulation after... | Eur J Med Chem 126: 202-217 (2017) Article DOI: 10.1016/j.ejmech.2016.09.003 BindingDB Entry DOI: 10.7270/Q2RB76TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519567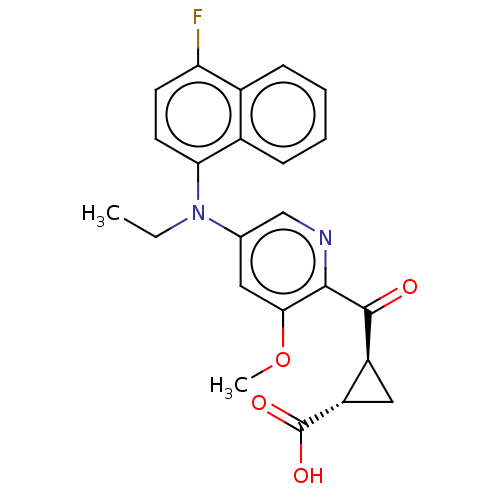 (CHEMBL4476024) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457428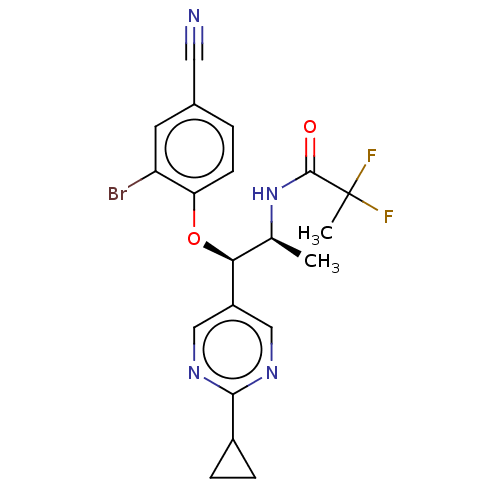 (CHEMBL4213734) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519571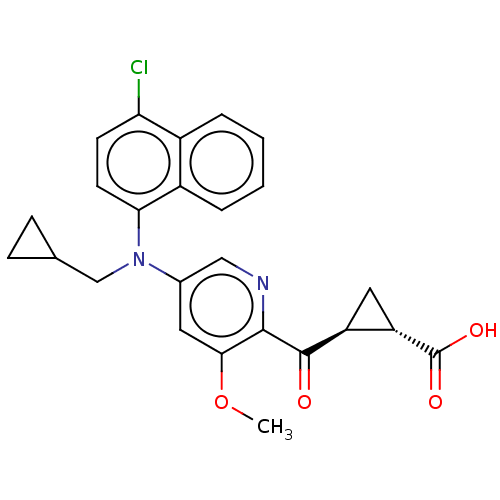 (CHEMBL4454609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457407 (CHEMBL4210441) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457421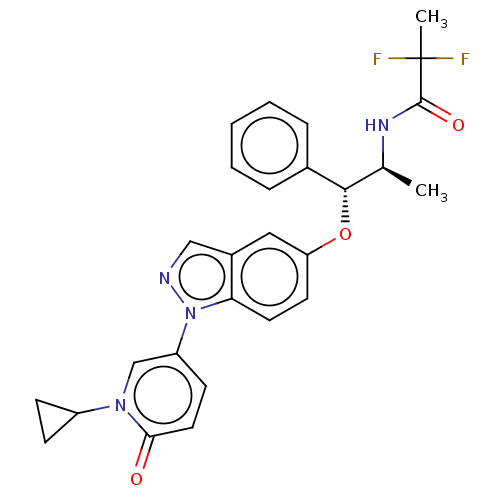 (CHEMBL4214847) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone RED from human full length glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457404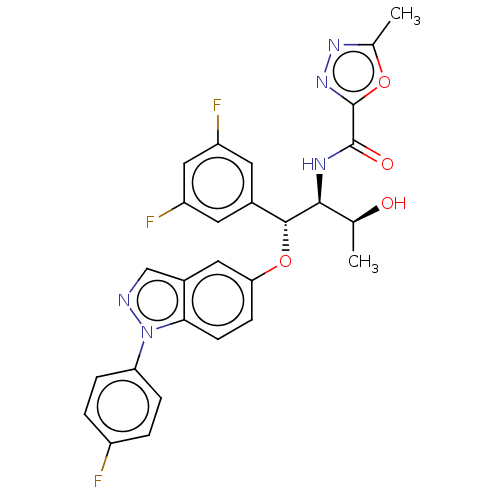 (CHEMBL4217597) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332677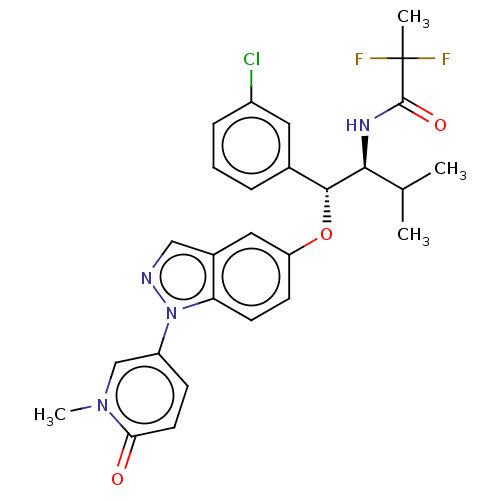 (US10196374, Example 5) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457408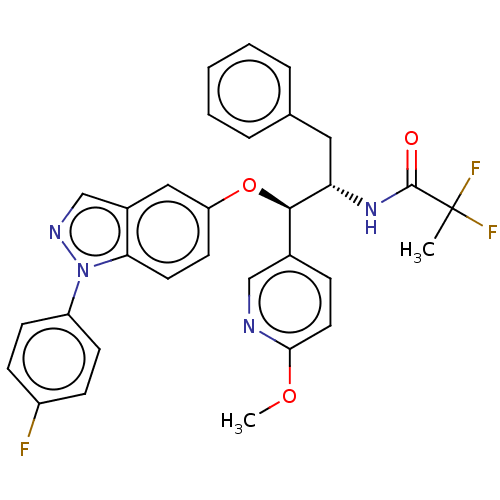 (CHEMBL4211936) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519595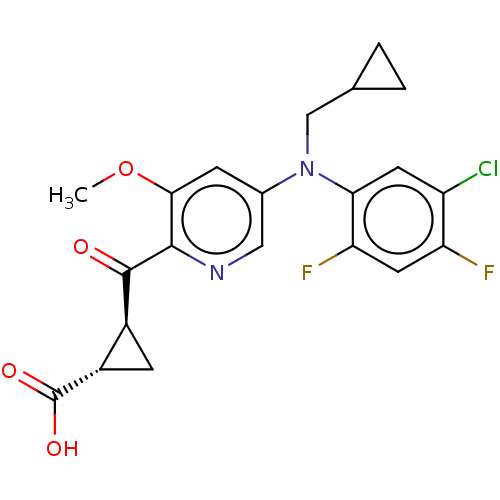 (CHEMBL4545014) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519569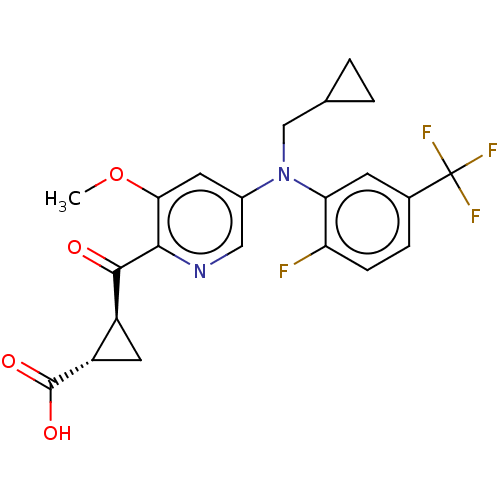 (CHEMBL4545950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332669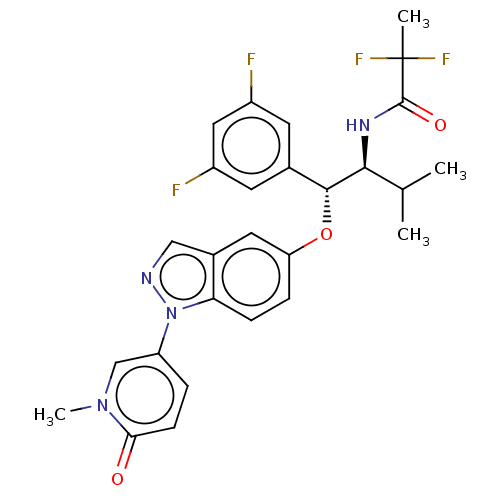 (US10196374, Example 3) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519585 (CHEMBL4464773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457428 (CHEMBL4213734) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone RED from human full length glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457419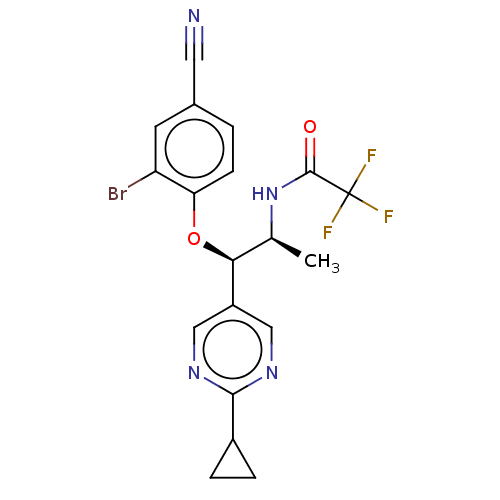 (CHEMBL4204466) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone RED from human full length glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519582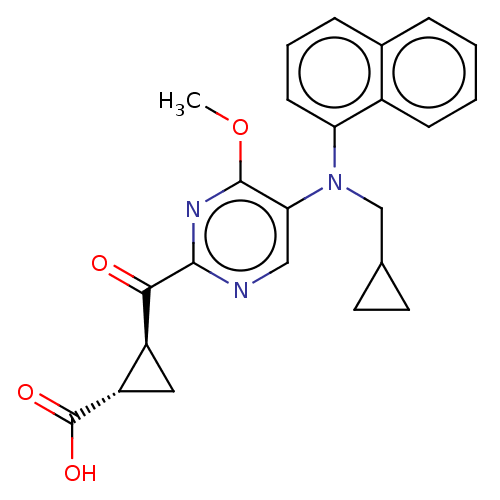 (CHEMBL4525972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457419 (CHEMBL4204466) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene e... | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50457414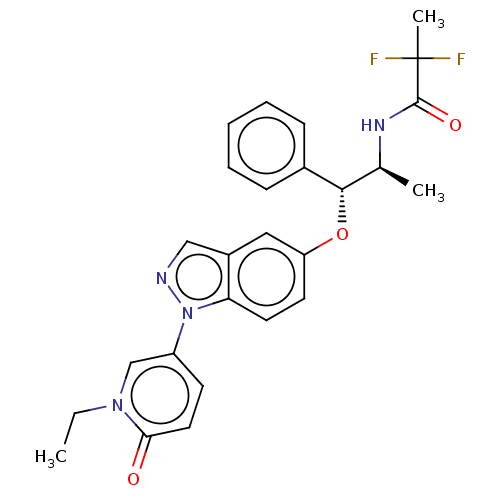 (CHEMBL4215390) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone RED from human full length glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM332677 (US10196374, Example 5) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of fluormone RED from human full length glucocorticoid receptor after 4 hrs by fluorescence polarization assay | J Med Chem 61: 1785-1799 (2018) Article DOI: 10.1021/acs.jmedchem.7b01690 BindingDB Entry DOI: 10.7270/Q2XK8J5R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 342 total ) | Next | Last >> |