 Found 368 hits with Last Name = 'krimm' and Initial = 'i'
Found 368 hits with Last Name = 'krimm' and Initial = 'i' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50162774

(ABT-199 | US11420968, Example ABT-199 | Venetoclax)Show SMILES CC1(C)CCC(CN2CCN(CC2)c2ccc(C(=O)NS(=O)(=O)c3ccc(NCC4CCOCC4)c(c3)N(=O)=O)c(Oc3cnc4[nH]ccc4c3)c2)=C(C1)c1ccc(Cl)cc1 |c:57| Show InChI InChI=1S/C45H50ClN7O7S/c1-45(2)15-11-33(39(26-45)31-3-5-34(46)6-4-31)29-51-17-19-52(20-18-51)35-7-9-38(42(24-35)60-36-23-32-12-16-47-43(32)49-28-36)44(54)50-61(57,58)37-8-10-40(41(25-37)53(55)56)48-27-30-13-21-59-22-14-30/h3-10,12,16,23-25,28,30,48H,11,13-15,17-22,26-27,29H2,1-2H3,(H,47,49)(H,50,54) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCL2 (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50546262
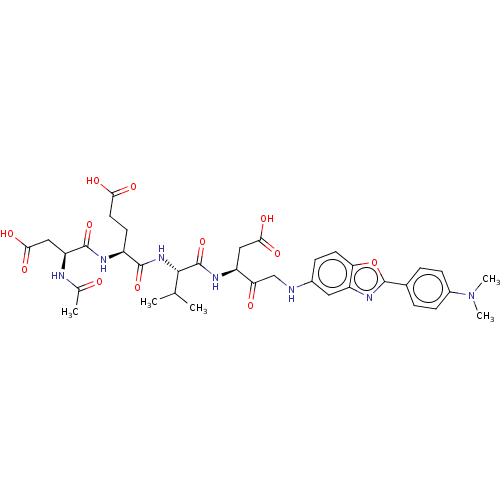
(CHEMBL4751195)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)CNc1ccc2oc(nc2c1)-c1ccc(cc1)N(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Apoptosis regulator Bcl-2
(Homo sapiens (Human)) | BDBM50270877
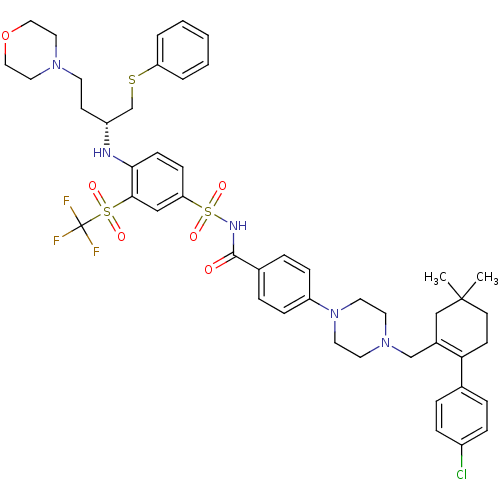
((R)-4-(4-((2-(4-chlorophenyl)-5,5-dimethylcyclohex...)Show SMILES CC1(C)CCC(=C(CN2CCN(CC2)c2ccc(cc2)C(=O)NS(=O)(=O)c2ccc(N[C@H](CCN3CCOCC3)CSc3ccccc3)c(c2)S(=O)(=O)C(F)(F)F)C1)c1ccc(Cl)cc1 |r,t:5| Show InChI InChI=1S/C47H55ClF3N5O6S3/c1-46(2)20-18-42(34-8-12-37(48)13-9-34)36(31-46)32-55-22-24-56(25-23-55)39-14-10-35(11-15-39)45(57)53-65(60,61)41-16-17-43(44(30-41)64(58,59)47(49,50)51)52-38(19-21-54-26-28-62-29-27-54)33-63-40-6-4-3-5-7-40/h3-17,30,38,52H,18-29,31-33H2,1-2H3,(H,53,57)/t38-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| <0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of BCL2 (unknown origin) by TR-FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13954

(3-({5-[(2S)-3-{4-[(2-carboxyphenyl)amidoformic aci...)Show SMILES CC(=O)N[C@@H](Cc1ccc(N(C(=O)C(O)=O)c2ccccc2C(O)=O)c2ccccc12)C(=O)NCCCCCOc1cc2ccccc2cc1C(O)=O |r| Show InChI InChI=1S/C40H37N3O10/c1-24(44)42-32(36(45)41-19-9-2-10-20-53-35-23-26-12-4-3-11-25(26)21-31(35)39(49)50)22-27-17-18-34(29-14-6-5-13-28(27)29)43(37(46)40(51)52)33-16-8-7-15-30(33)38(47)48/h3-8,11-18,21,23,32H,2,9-10,19-20,22H2,1H3,(H,41,45)(H,42,44)(H,47,48)(H,49,50)(H,51,52)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP as substrate measured every 30 secs for 15 mins by Michaelis-Menten plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Caspase-3
(Homo sapiens (Human)) | BDBM50546260
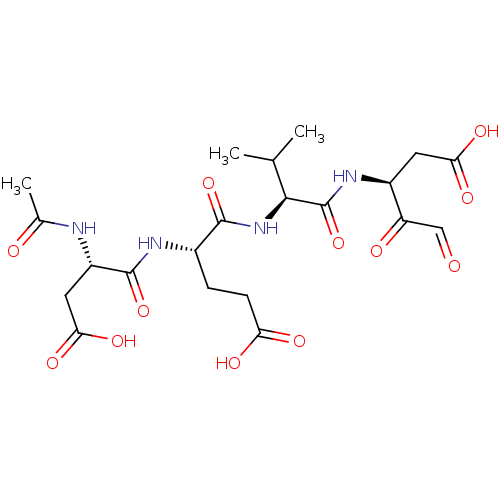
(CHEMBL4782926)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)C=O |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50462575

(CHEMBL4238121)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](COCc2ccccc2)NS(=O)(=O)Cc2ccccc2)cc1 |r| Show InChI InChI=1S/C27H31N5O5S/c28-26(29)23-13-11-20(12-14-23)15-30-25(33)16-31-27(34)24(18-37-17-21-7-3-1-4-8-21)32-38(35,36)19-22-9-5-2-6-10-22/h1-14,24,32H,15-19H2,(H3,28,29)(H,30,33)(H,31,34)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human factor 10a using Boc-Leu-Gly-Arg-AMC as fluorogenic substrate measured after 10 mins by spectrofluorimetry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Caspase-3
(Homo sapiens (Human)) | BDBM220
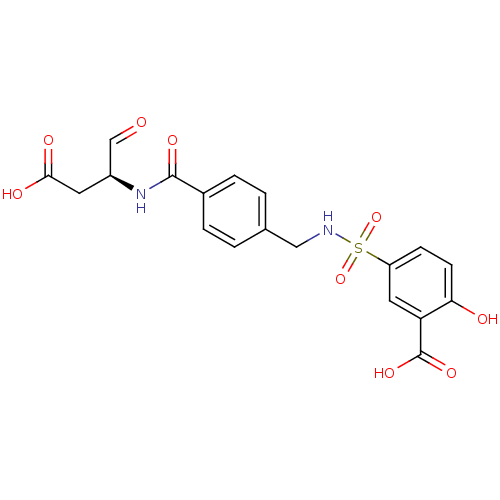
(5-{[(4-{[(2S)-1-carboxy-3-oxopropan-2-yl]carbamoyl...)Show SMILES OC(=O)C[C@H](NC(=O)c1ccc(CNS(=O)(=O)c2ccc(O)c(c2)C(O)=O)cc1)C=O |r| Show InChI InChI=1S/C19H18N2O9S/c22-10-13(7-17(24)25)21-18(26)12-3-1-11(2-4-12)9-20-31(29,30)14-5-6-16(23)15(8-14)19(27)28/h1-6,8,10,13,20,23H,7,9H2,(H,21,26)(H,24,25)(H,27,28)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human caspase 3 using Ac-Asp-Glu-Val-Asp-AFC as substrate preincubated for 30 mins followed by substrate addition and measu... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50530325
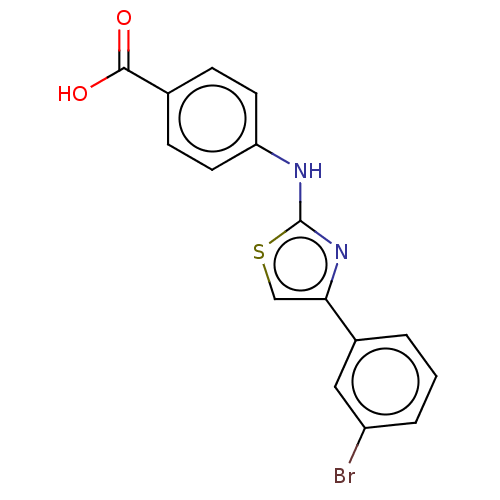
(CHEMBL4458300)Show InChI InChI=1S/C16H11BrN2O2S/c17-12-3-1-2-11(8-12)14-9-22-16(19-14)18-13-6-4-10(5-7-13)15(20)21/h1-9H,(H,18,19)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human GST-tagged CK2alpha in presence of ATP by Lineweaver-Burk plot analysis |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50530325

(CHEMBL4458300)Show InChI InChI=1S/C16H11BrN2O2S/c17-12-3-1-2-11(8-12)14-9-22-16(19-14)18-13-6-4-10(5-7-13)15(20)21/h1-9H,(H,18,19)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human GST-tagged CK2alpha in presence of ATP by Lineweaver-Burk plot analysis |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50530338

(CHEMBL1621959)Show InChI InChI=1S/C16H12N2O2S/c19-15(20)12-6-8-13(9-7-12)17-16-18-14(10-21-16)11-4-2-1-3-5-11/h1-10H,(H,17,18)(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human GST-tagged CK2alpha in presence of ATP by Lineweaver-Burk plot analysis |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50530338

(CHEMBL1621959)Show InChI InChI=1S/C16H12N2O2S/c19-15(20)12-6-8-13(9-7-12)17-16-18-14(10-21-16)11-4-2-1-3-5-11/h1-10H,(H,17,18)(H,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human GST-tagged CK2alpha in presence of ATP by Lineweaver-Burk plot analysis |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bcl-2-like protein 1
(Homo sapiens (Human)) | BDBM50196032
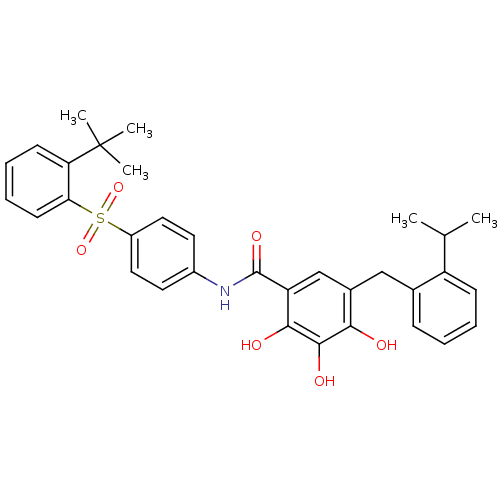
((N-[(2-tert-butylbenzenesulfonyl)phenyl]-2,3,4-tri...)Show SMILES CC(C)c1ccccc1Cc1cc(C(=O)Nc2ccc(cc2)S(=O)(=O)c2ccccc2C(C)(C)C)c(O)c(O)c1O Show InChI InChI=1S/C33H35NO6S/c1-20(2)25-11-7-6-10-21(25)18-22-19-26(30(36)31(37)29(22)35)32(38)34-23-14-16-24(17-15-23)41(39,40)28-13-9-8-12-27(28)33(3,4)5/h6-17,19-20,35-37H,18H2,1-5H3,(H,34,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Lyon
Curated by ChEMBL
| Assay Description
Displacement of FAM-Bid peptide from human recombinant Bcl-xL by fluorescence polarization assay |
J Med Chem 53: 2577-88 (2010)
Article DOI: 10.1021/jm100009z
BindingDB Entry DOI: 10.7270/Q2445MK6 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50530336
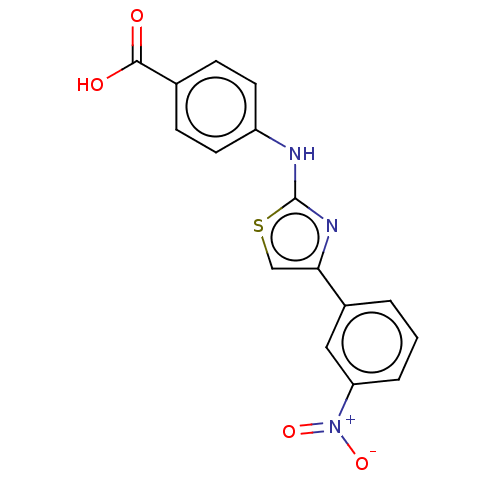
(CHEMBL4462082)Show SMILES OC(=O)c1ccc(Nc2nc(cs2)-c2cccc(c2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C16H11N3O4S/c20-15(21)10-4-6-12(7-5-10)17-16-18-14(9-24-16)11-2-1-3-13(8-11)19(22)23/h1-9H,(H,17,18)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human GST-tagged CK2alpha in presence of ATP by Lineweaver-Burk plot analysis |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM50530336

(CHEMBL4462082)Show SMILES OC(=O)c1ccc(Nc2nc(cs2)-c2cccc(c2)[N+]([O-])=O)cc1 Show InChI InChI=1S/C16H11N3O4S/c20-15(21)10-4-6-12(7-5-10)17-16-18-14(9-24-16)11-2-1-3-13(8-11)19(22)23/h1-9H,(H,17,18)(H,20,21) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Mixed-type inhibition of recombinant human GST-tagged CK2alpha in presence of ATP by Lineweaver-Burk plot analysis |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50546250
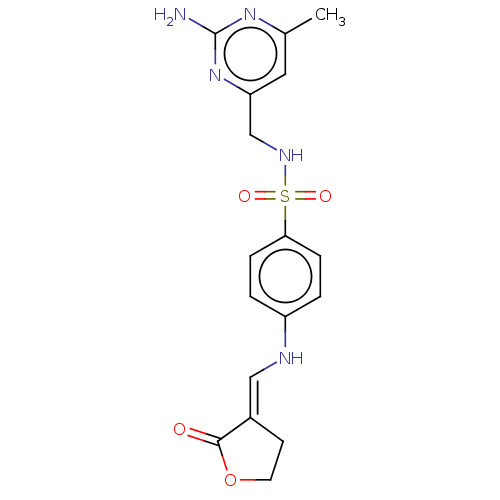
(CHEMBL4754689)Show SMILES Cc1cc(CNS(=O)(=O)c2ccc(N\C=C3/CCOC3=O)cc2)nc(N)n1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of biotin-labelled geldanamycin from human HSP90 (D9 to E236 residues) incubated for 3 hrs by FRET assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-alpha/90-beta
(Homo sapiens (Human)) | BDBM50546251

(CHEMBL4783360) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human HSP90 (D9 to E236 residues) by fluorescence spectroscopy |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13990
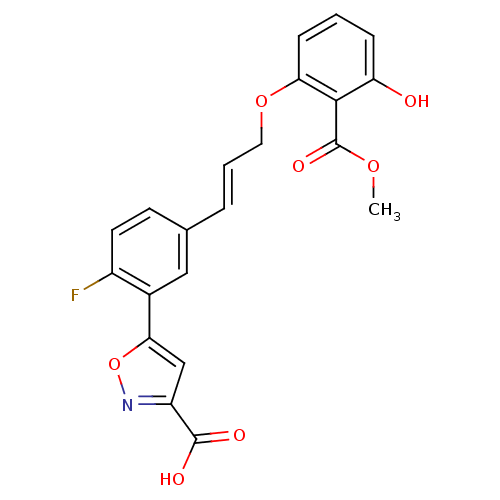
(5-{2-fluoro-5-[(1E)-3-[3-hydroxy-2-(methoxycarbony...)Show SMILES COC(=O)c1c(O)cccc1OC\C=C\c1ccc(F)c(c1)-c1cc(no1)C(O)=O Show InChI InChI=1S/C21H16FNO7/c1-28-21(27)19-16(24)5-2-6-17(19)29-9-3-4-12-7-8-14(22)13(10-12)18-11-15(20(25)26)23-30-18/h2-8,10-11,24H,9H2,1H3,(H,25,26)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PTP1B (unknown origin) using pNPP as substrate measured every 30 secs for 15 mins by Michaelis-Menten plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Creatine kinase M-type
(Oryctolagus cuniculus) | BDBM50370908
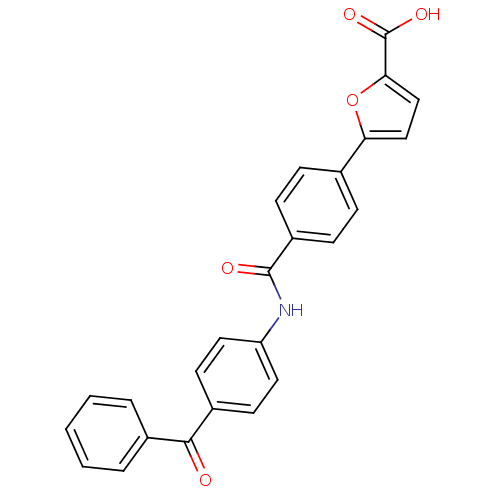
(CHEMBL373782)Show SMILES OC(=O)c1ccc(o1)-c1ccc(cc1)C(=O)Nc1ccc(cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H17NO5/c27-23(17-4-2-1-3-5-17)18-10-12-20(13-11-18)26-24(28)19-8-6-16(7-9-19)21-14-15-22(31-21)25(29)30/h1-15H,(H,26,28)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle creatine kinase by pH-stat assay |
J Med Chem 50: 1865-75 (2007)
Article DOI: 10.1021/jm061460r
BindingDB Entry DOI: 10.7270/Q2HT2Q3B |
More data for this
Ligand-Target Pair | |
Creatine kinase M-type
(Oryctolagus cuniculus) | BDBM50370908

(CHEMBL373782)Show SMILES OC(=O)c1ccc(o1)-c1ccc(cc1)C(=O)Nc1ccc(cc1)C(=O)c1ccccc1 Show InChI InChI=1S/C25H17NO5/c27-23(17-4-2-1-3-5-17)18-10-12-20(13-11-18)26-24(28)19-8-6-16(7-9-19)21-14-15-22(31-21)25(29)30/h1-15H,(H,26,28)(H,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Claude Bernard Lyon 1
Curated by ChEMBL
| Assay Description
Inhibition of rabbit muscle creatine kinase-MgATP complex by pH-stat assay |
J Med Chem 50: 1865-75 (2007)
Article DOI: 10.1021/jm061460r
BindingDB Entry DOI: 10.7270/Q2HT2Q3B |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50546261
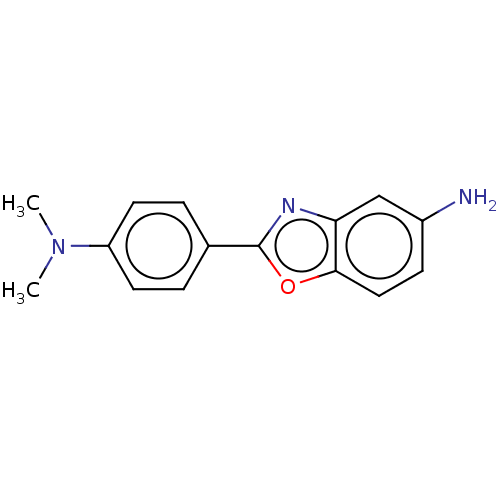
(CHEMBL234378) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of caspase-3 (unknown origin) using Ac-DEVD-AMCA as substrate incubated for 5 mins by Dixon plot analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Cytosolic purine 5'-nucleotidase
(Homo sapiens) | BDBM50500514
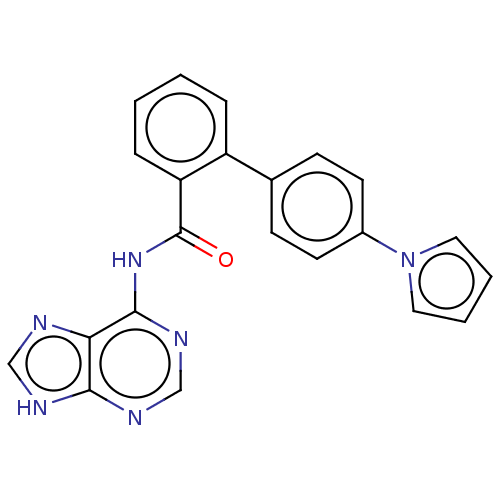
(CHEMBL3746819)Show SMILES O=C(Nc1ncnc2[nH]cnc12)c1ccccc1-c1ccc(cc1)-n1cccc1 Show InChI InChI=1S/C22H16N6O/c29-22(27-21-19-20(24-13-23-19)25-14-26-21)18-6-2-1-5-17(18)15-7-9-16(10-8-15)28-11-3-4-12-28/h1-14H,(H2,23,24,25,26,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 4.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate h... |
J Med Chem 58: 9680-96 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01616
BindingDB Entry DOI: 10.7270/Q2SF3062 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50232690

(4-Aminomethyl-Benzamidine | CHEMBL187301)Show InChI InChI=1S/C8H11N3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4H,5,9H2,(H3,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human factor 10a using Boc-Leu-Gly-Arg-AMC as fluorogenic substrate measured after 10 mins by spectrofluorimetry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Cytosolic purine 5'-nucleotidase
(Homo sapiens) | BDBM50500511
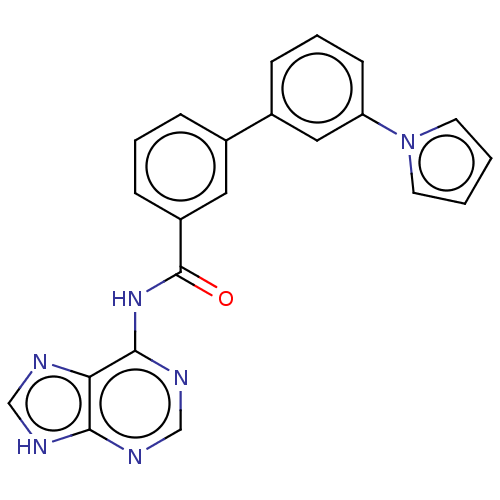
(CHEMBL3746624)Show SMILES O=C(Nc1ncnc2[nH]cnc12)c1cccc(c1)-c1cccc(c1)-n1cccc1 Show InChI InChI=1S/C22H16N6O/c29-22(27-21-19-20(24-13-23-19)25-14-26-21)17-7-3-5-15(11-17)16-6-4-8-18(12-16)28-9-1-2-10-28/h1-14H,(H2,23,24,25,26,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 7.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate h... |
J Med Chem 58: 9680-96 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01616
BindingDB Entry DOI: 10.7270/Q2SF3062 |
More data for this
Ligand-Target Pair | |
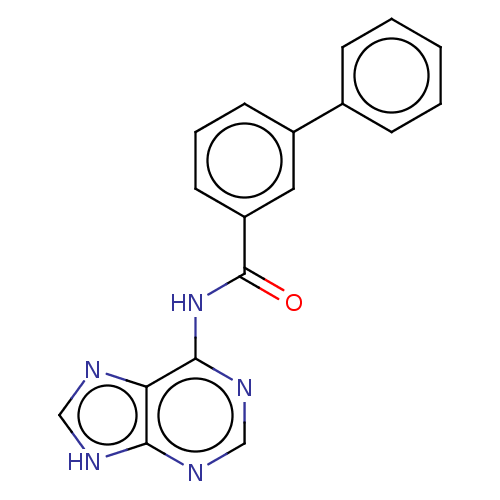
Cytosolic purine 5'-nucleotidase
(Homo sapiens) | BDBM50500515
(CHEMBL3747390)Show InChI InChI=1S/C18H13N5O/c24-18(23-17-15-16(20-10-19-15)21-11-22-17)14-8-4-7-13(9-14)12-5-2-1-3-6-12/h1-11H,(H2,19,20,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 9.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate hydro... |
J Med Chem 58: 9680-96 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01616
BindingDB Entry DOI: 10.7270/Q2SF3062 |
More data for this
Ligand-Target Pair | |
Cytosolic purine 5'-nucleotidase
(Homo sapiens) | BDBM50500513

(CHEMBL3746419)Show SMILES O=C(Nc1ncnc2[nH]cnc12)c1ccccc1-c1cccc(c1)-n1cccc1 Show InChI InChI=1S/C22H16N6O/c29-22(27-21-19-20(24-13-23-19)25-14-26-21)18-9-2-1-8-17(18)15-6-5-7-16(12-15)28-10-3-4-11-28/h1-14H,(H2,23,24,25,26,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 1.65E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate h... |
J Med Chem 58: 9680-96 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01616
BindingDB Entry DOI: 10.7270/Q2SF3062 |
More data for this
Ligand-Target Pair | |
Cytosolic purine 5'-nucleotidase
(Homo sapiens) | BDBM50500510
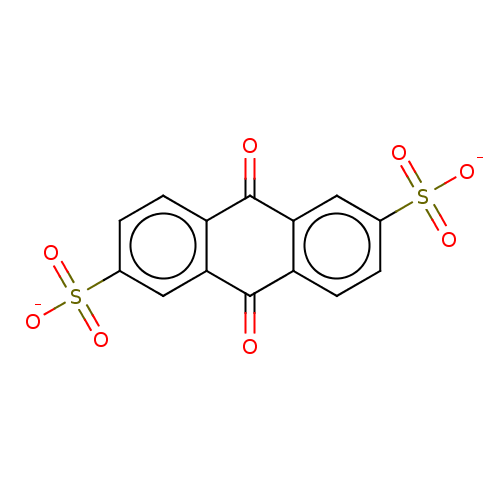
(CHEMBL3746211)Show SMILES [Na;v0+].[Na;v0+].[#8-]S(=O)(=O)c1ccc2-[#6](=O)-c3cc(ccc3-[#6](=O)-c2c1)S([#8-])(=O)=O Show InChI InChI=1S/C14H8O8S2/c15-13-9-3-1-7(23(17,18)19)5-11(9)14(16)10-4-2-8(6-12(10)13)24(20,21)22/h1-6H,(H,17,18,19)(H,20,21,22)/p-2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 2.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate hydrolysis by rap... |
J Med Chem 58: 9680-96 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01616
BindingDB Entry DOI: 10.7270/Q2SF3062 |
More data for this
Ligand-Target Pair | |
Cytosolic purine 5'-nucleotidase
(Homo sapiens) | BDBM50500512
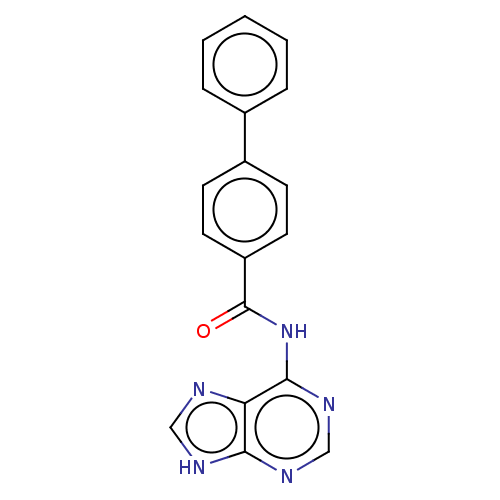
(CHEMBL3746515)Show InChI InChI=1S/C18H13N5O/c24-18(23-17-15-16(20-10-19-15)21-11-22-17)14-8-6-13(7-9-14)12-4-2-1-3-5-12/h1-11H,(H2,19,20,21,22,23,24) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 2.78E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montpellier
Curated by ChEMBL
| Assay Description
Competitive inhibition of recombinant N-terminal truncated human cytosolic 5'-nucleotidase-2 assessed as inhibition of inosine 5'-monophosphate hydro... |
J Med Chem 58: 9680-96 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01616
BindingDB Entry DOI: 10.7270/Q2SF3062 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50514418

(CHEMBL4572188)Show SMILES OC(=O)CNC(=O)[C@@H](COCc1ccccc1)NS(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C19H22N2O6S/c22-18(23)11-20-19(24)17(13-27-12-15-7-3-1-4-8-15)21-28(25,26)14-16-9-5-2-6-10-16/h1-10,17,21H,11-14H2,(H,20,24)(H,22,23)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human factor 10a using Boc-Leu-Gly-Arg-AMC as fluorogenic substrate measured after 10 mins by spectrofluorimetry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM9047
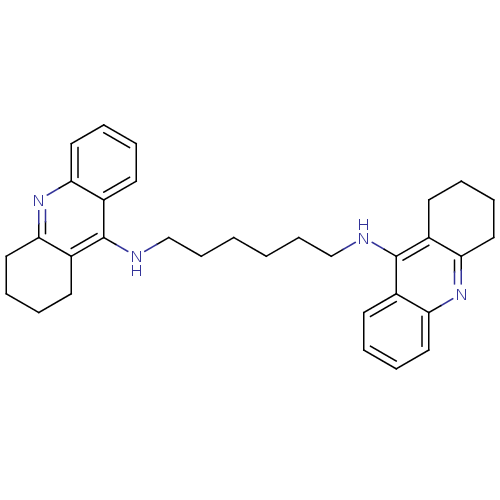
(Bis-THA inhibitor 5 | CHEMBL73800 | Hexylene-Linke...)Show SMILES C(CCCNc1c2CCCCc2nc2ccccc12)CCNc1c2CCCCc2nc2ccccc12 Show InChI InChI=1S/C32H38N4/c1(11-21-33-31-23-13-3-7-17-27(23)35-28-18-8-4-14-24(28)31)2-12-22-34-32-25-15-5-9-19-29(25)36-30-20-10-6-16-26(30)32/h3,5,7,9,13,15,17,19H,1-2,4,6,8,10-12,14,16,18,20-22H2,(H,33,35)(H,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1
(Homo sapiens (Human)) | BDBM50520331
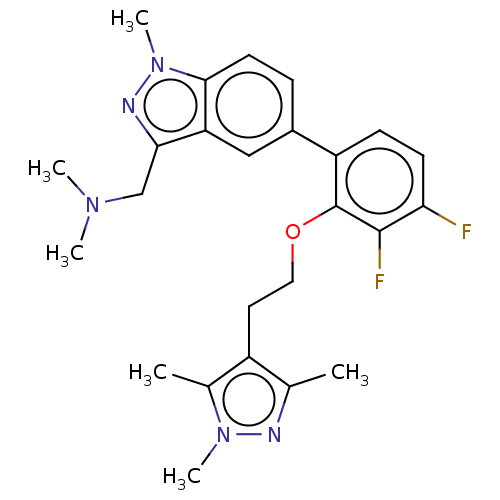
(CHEMBL4445137)Show SMILES CN(C)Cc1nn(C)c2ccc(cc12)-c1ccc(F)c(F)c1OCCc1c(C)nn(C)c1C Show InChI InChI=1S/C25H29F2N5O/c1-15-18(16(2)31(5)28-15)11-12-33-25-19(8-9-21(26)24(25)27)17-7-10-23-20(13-17)22(14-30(3)4)29-32(23)6/h7-10,13H,11-12,14H2,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human NMT1 by fluorescence method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50133435

(4-(2-Chloro-phenyl)-1-ethyl-6-methyl-1,4-dihydro-p...)Show SMILES CCN1C(C)=C(C(C(C(O)=O)=C1C(O)=O)c1ccccc1Cl)C(=O)OC(C)C |c:4,10| Show InChI InChI=1S/C20H22ClNO6/c1-5-22-11(4)14(20(27)28-10(2)3)15(12-8-6-7-9-13(12)21)16(18(23)24)17(22)19(25)26/h6-10,15H,5H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit muscular GPb by NMR binding assay |
J Med Chem 55: 1287-95 (2012)
Article DOI: 10.1021/jm201439b
BindingDB Entry DOI: 10.7270/Q21J9BS9 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CLK2 in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity protein kinase CLK2
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CLK2 in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM616483

(US20230278983, Code AB1455)Show SMILES COc1cccc(CO)c1-c1ccc(cc1C)S(=O)(=O)NCCN1CCC(Cn2cc(nn2)-c2c([nH]c3ccc(F)cc23)C(C)=O)CC1 |(-8.41,5.78,;-7.01,6.41,;-6.85,7.94,;-8.09,8.85,;-7.93,10.38,;-6.52,11,;-5.28,10.1,;-3.87,10.72,;-2.62,9.82,;-5.44,8.57,;-4.19,7.66,;-2.79,8.29,;-1.54,7.38,;-1.7,5.85,;-3.11,5.22,;-4.35,6.13,;-5.76,5.5,;-.46,4.95,;.45,6.19,;-1.36,3.7,;.79,4.04,;.63,2.51,;1.88,1.6,;1.71,.07,;.31,-.55,;.15,-2.09,;1.39,-2.99,;1.23,-4.52,;2.48,-5.43,;2.48,-6.97,;3.94,-7.44,;4.85,-6.2,;3.94,-4.95,;4.42,-8.91,;3.51,-10.15,;4.42,-11.4,;5.88,-10.92,;7.22,-11.69,;8.55,-10.92,;8.55,-9.38,;9.88,-8.61,;7.22,-8.61,;5.88,-9.38,;1.97,-10.15,;1.2,-8.82,;1.2,-11.49,;2.8,-2.36,;2.96,-.83,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WH2V4R |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DYRK1B in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1B
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DYRK1B in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DYRK1A in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity tyrosine-phosphorylation-regulated kinase 1A
(Homo sapiens (Human)) | BDBM50335638

(5-(3-Chlorophenylamino)benzo[c][2,6]naphthyridine-...)Show SMILES OC(=O)c1ccc2c(c1)nc(Nc1cccc(Cl)c1)c1ccncc21 Show InChI InChI=1S/C19H12ClN3O2/c20-12-2-1-3-13(9-12)22-18-15-6-7-21-10-16(15)14-5-4-11(19(24)25)8-17(14)23-18/h1-10H,(H,22,23)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human DYRK1A in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM616482
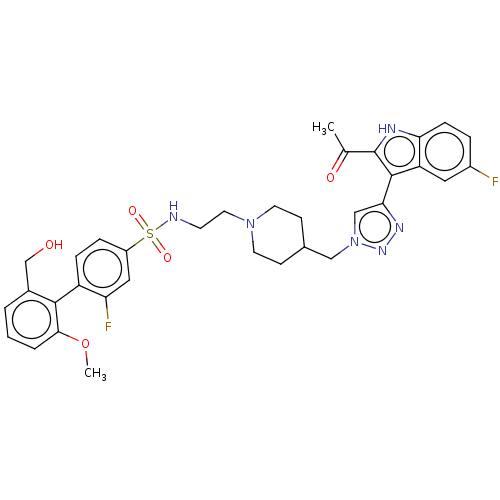
(US20230278983, Code AB1454)Show SMILES COc1cccc(CO)c1-c1ccc(cc1F)S(=O)(=O)NCCN1CCC(Cn2cc(nn2)-c2c([nH]c3ccc(F)cc23)C(C)=O)CC1 |(-8.41,5.78,;-7.01,6.41,;-6.85,7.94,;-8.09,8.85,;-7.93,10.38,;-6.52,11,;-5.28,10.1,;-3.87,10.72,;-2.62,9.82,;-5.44,8.57,;-4.19,7.66,;-2.79,8.29,;-1.54,7.38,;-1.7,5.85,;-3.11,5.22,;-4.35,6.13,;-5.76,5.5,;-.46,4.95,;.45,6.19,;-1.36,3.7,;.79,4.04,;.63,2.51,;1.88,1.6,;1.71,.07,;.31,-.55,;.15,-2.09,;1.39,-2.99,;1.23,-4.52,;2.48,-5.43,;2.48,-6.97,;3.94,-7.44,;4.85,-6.2,;3.94,-4.95,;4.42,-8.91,;3.51,-10.15,;4.42,-11.4,;5.88,-10.92,;7.22,-11.69,;8.55,-10.92,;8.55,-9.38,;9.88,-8.61,;7.22,-8.61,;5.88,-9.38,;1.97,-10.15,;1.2,-8.82,;1.2,-11.49,;2.8,-2.36,;2.96,-.83,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WH2V4R |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM165207
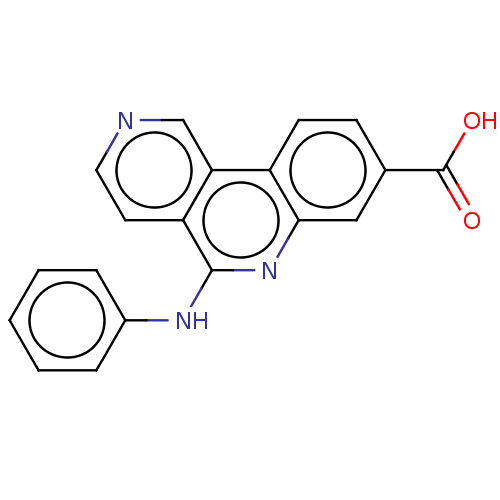
(US8168651, Compound TABLE 16.1 | US9062043, Table ...)Show InChI InChI=1S/C19H13N3O2/c23-19(24)12-6-7-14-16-11-20-9-8-15(16)18(22-17(14)10-12)21-13-4-2-1-3-5-13/h1-11H,(H,21,22)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged CK2alpha in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM165207

(US8168651, Compound TABLE 16.1 | US9062043, Table ...)Show InChI InChI=1S/C19H13N3O2/c23-19(24)12-6-7-14-16-11-20-9-8-15(16)18(22-17(14)10-12)21-13-4-2-1-3-5-13/h1-11H,(H,21,22)(H,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged CK2alpha in presence of ATP by radiometric filter-binding assay |
J Med Chem 62: 1803-1816 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01766
BindingDB Entry DOI: 10.7270/Q2NS0ZCZ |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50149219
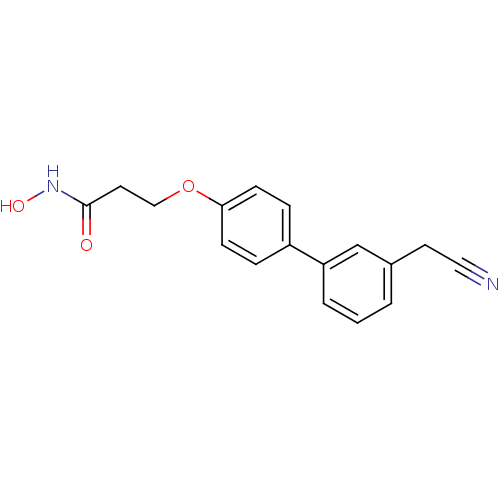
(3-(3'-Cyanomethyl-biphenyl-4-yloxy)-N-hydroxy-prop...)Show InChI InChI=1S/C17H16N2O3/c18-10-8-13-2-1-3-15(12-13)14-4-6-16(7-5-14)22-11-9-17(20)19-21/h1-7,12,21H,8-9,11H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of MMP3 (unknown origin) using Ac-Pro-Leu-Gly-[2-mercapto-4-methyl-pentanoyl]-Leu-Gly-OEt as substrate incubated for 30 mins |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM616454
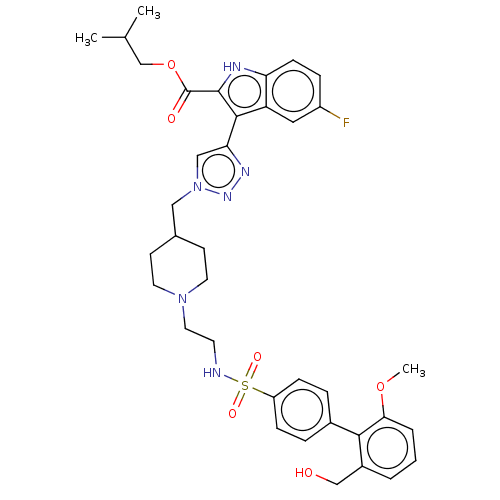
(US20230278983, Code AB1206)Show SMILES COc1cccc(CO)c1-c1ccc(cc1)S(=O)(=O)NCCN1CCC(Cn2cc(nn2)-c2c([nH]c3ccc(F)cc23)C(=O)OCC(C)C)CC1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WH2V4R |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM616473
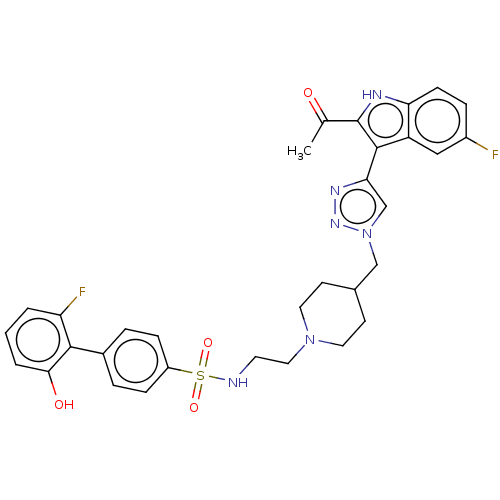
(US20230278983, Code AB1381)Show SMILES CC(=O)c1[nH]c2ccc(F)cc2c1-c1cn(CC2CCN(CCNS(=O)(=O)c3ccc(cc3)-c3c(O)cccc3F)CC2)nn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WH2V4R |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM616446
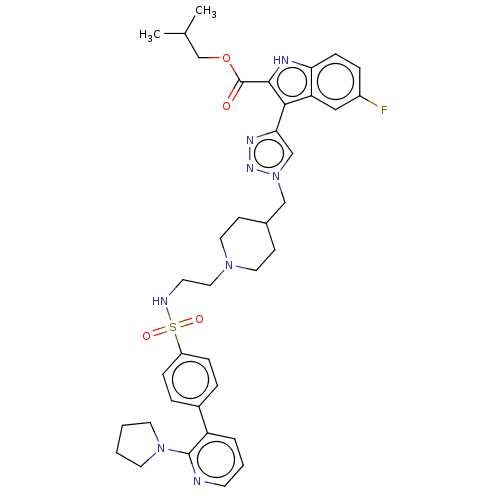
(US20230278983, Code AB1073)Show SMILES CC(C)COC(=O)c1[nH]c2ccc(F)cc2c1-c1cn(CC2CCN(CCNS(=O)(=O)c3ccc(cc3)-c3cccnc3N3CCCC3)CC2)nn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WH2V4R |
More data for this
Ligand-Target Pair | |
Chitinase B
(Serratia marcescens) | BDBM50462584
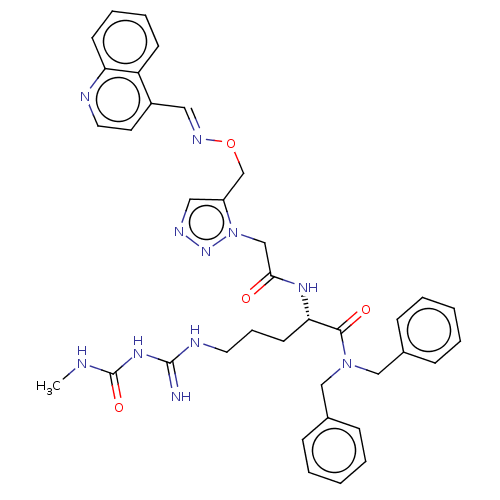
(CHEMBL4245260)Show SMILES CNC(=O)NC(=N)NCCC[C@H](NC(=O)Cn1nncc1CO\N=C\c1ccnc2ccccc12)C(=O)N(Cc1ccccc1)Cc1ccccc1 |r| Show InChI InChI=1S/C37H41N11O4/c1-39-37(51)45-36(38)41-19-10-17-33(35(50)47(23-27-11-4-2-5-12-27)24-28-13-6-3-7-14-28)44-34(49)25-48-30(22-42-46-48)26-52-43-21-29-18-20-40-32-16-9-8-15-31(29)32/h2-9,11-16,18,20-22,33H,10,17,19,23-26H2,1H3,(H,44,49)(H4,38,39,41,45,51)/b43-21+/t33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of Serratia marcescens chitinase B |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM616474
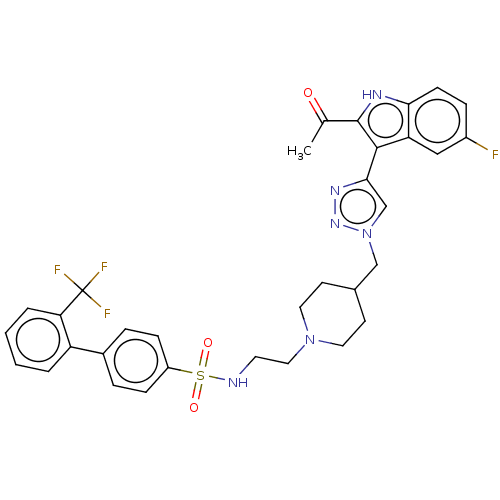
(US20230278983, Code AB1394)Show SMILES CC(=O)c1[nH]c2ccc(F)cc2c1-c1cn(CC2CCN(CCNS(=O)(=O)c3ccc(cc3)-c3ccccc3C(F)(F)F)CC2)nn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WH2V4R |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Oryctolagus cuniculus (rabbit)) | BDBM50382964
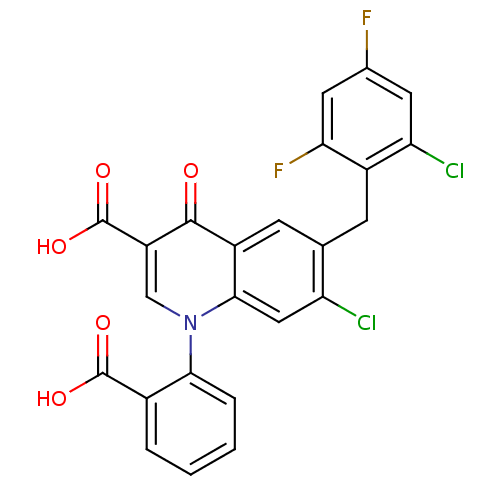
(CHEMBL2030481)Show SMILES OC(=O)c1ccccc1-n1cc(C(O)=O)c(=O)c2cc(Cc3c(F)cc(F)cc3Cl)c(Cl)cc12 Show InChI InChI=1S/C24H13Cl2F2NO5/c25-17-9-21-15(6-11(17)5-14-18(26)7-12(27)8-19(14)28)22(30)16(24(33)34)10-29(21)20-4-2-1-3-13(20)23(31)32/h1-4,6-10H,5H2,(H,31,32)(H,33,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lyon
Curated by ChEMBL
| Assay Description
Binding affinity to rabbit muscular GPb by NMR binding assay |
J Med Chem 55: 1287-95 (2012)
Article DOI: 10.1021/jm201439b
BindingDB Entry DOI: 10.7270/Q21J9BS9 |
More data for this
Ligand-Target Pair | |
Casein kinase II subunit alpha
(Homo sapiens (Human)) | BDBM616451
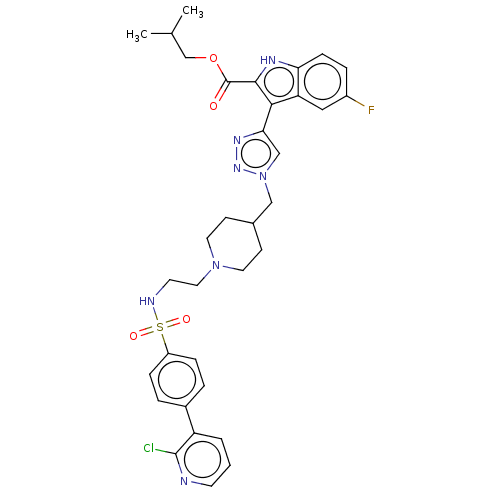
(US20230278983, Code AB1133)Show SMILES CC(C)COC(=O)c1[nH]c2ccc(F)cc2c1-c1cn(CC2CCN(CCNS(=O)(=O)c3ccc(cc3)-c3cccnc3Cl)CC2)nn1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2WH2V4R |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50504620

(GW280670X)Show SMILES NS(=O)(=O)c1ccc(N\N=C2/C(=O)Nc3ccc(Cl)cc23)cc1 Show InChI InChI=1S/C14H11ClN4O3S/c15-8-1-6-12-11(7-8)13(14(20)17-12)19-18-9-2-4-10(5-3-9)23(16,21)22/h1-7,18H,(H2,16,21,22)(H,17,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CDK2/Cyclin A (unknown origin) using histone H1 as substrate by scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00242
BindingDB Entry DOI: 10.7270/Q2KD22HB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data