

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264192 (CHEMBL4072118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264154 (CHEMBL4100832) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264193 (CHEMBL4084381) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264185 (CHEMBL4070299) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264192 (CHEMBL4072118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264192 (CHEMBL4072118) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264154 (CHEMBL4100832) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264193 (CHEMBL4084381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264184 (CHEMBL4097477) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM41542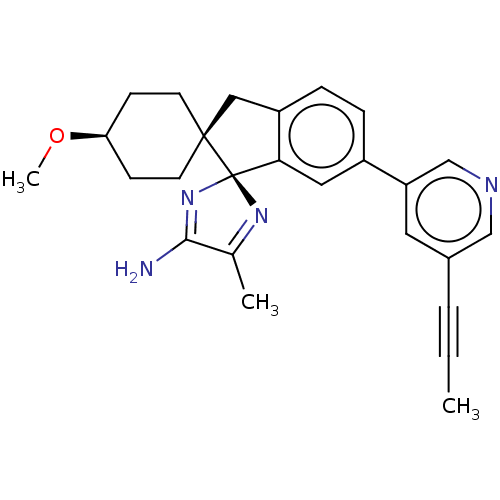 (US8865911, 122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 (1 to 460 residues) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition measu... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264192 (CHEMBL4072118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264186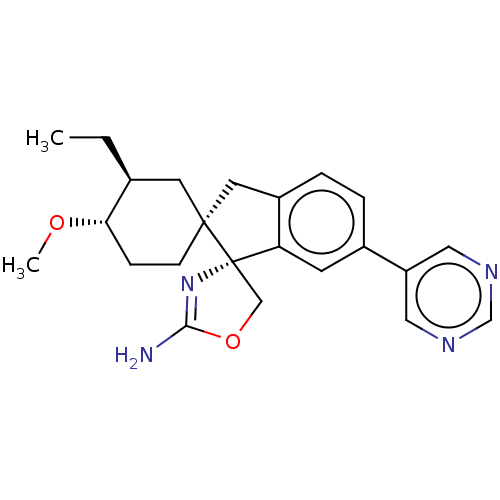 (CHEMBL4067413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264183 (CHEMBL4085364) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264195 (CHEMBL4103296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264181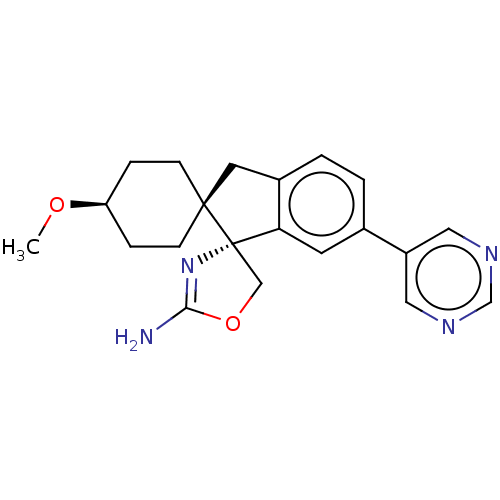 (CHEMBL4065448) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM41542 (US8865911, 122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 372 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition measured after 6.... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264180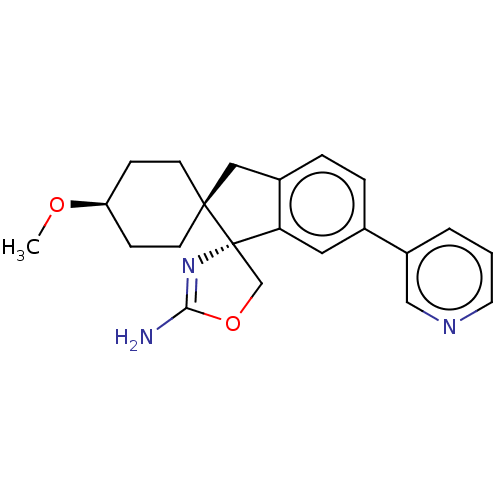 (CHEMBL4074071) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264184 (CHEMBL4097477) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 443 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264194 (CHEMBL4093123) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264185 (CHEMBL4070299) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 817 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264183 (CHEMBL4085364) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264194 (CHEMBL4093123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264195 (CHEMBL4103296) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264180 (CHEMBL4074071) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264186 (CHEMBL4067413) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264181 (CHEMBL4065448) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50264190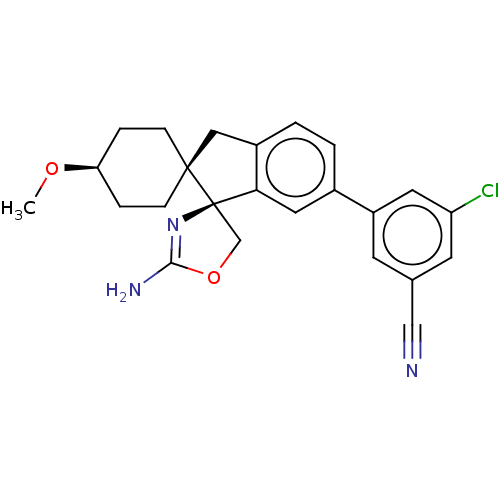 (CHEMBL4092875) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264190 (CHEMBL4092875) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE1 (1 to 460 residues) fused with Fc domain of IgG1 expressed in HEK293 cells preincubated for 10 mins followed by substrate a... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50398264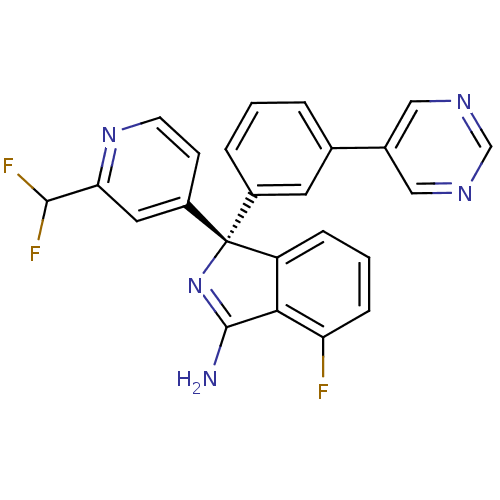 (CHEMBL2177913) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human BACE1 (1 to 460 residues) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition measu... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264195 (CHEMBL4103296) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50398264 (CHEMBL2177913) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human BACE2 (1 to 473 residues) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition measured after 6.... | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264184 (CHEMBL4097477) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264185 (CHEMBL4070299) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264183 (CHEMBL4085364) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264186 (CHEMBL4067413) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257167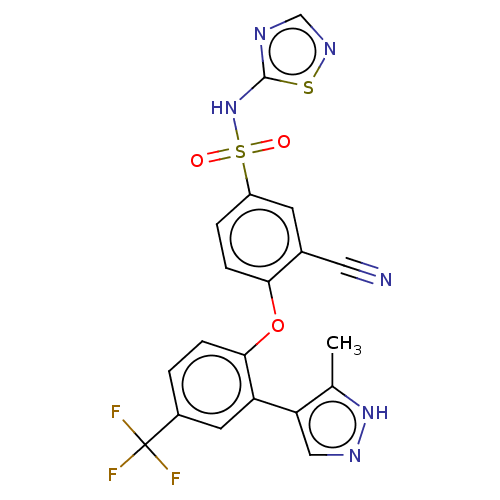 (CHEMBL2325619) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264193 (CHEMBL4084381) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264154 (CHEMBL4100832) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257179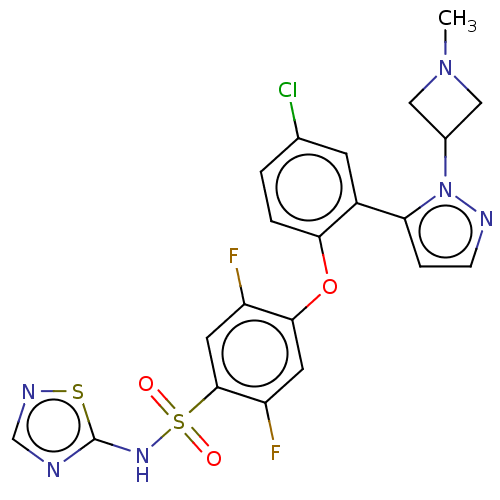 (CHEMBL2325622) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264192 (CHEMBL4072118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257166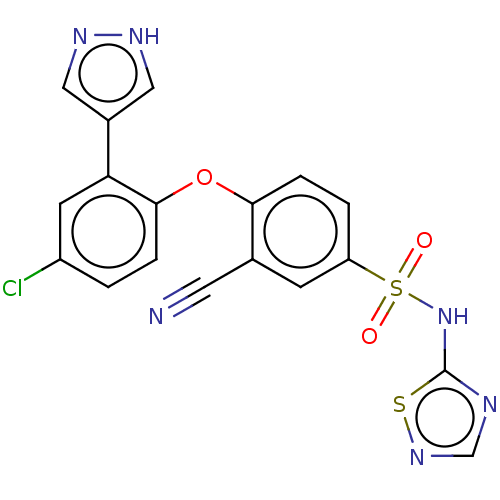 (CHEMBL2325330) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264192 (CHEMBL4072118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257216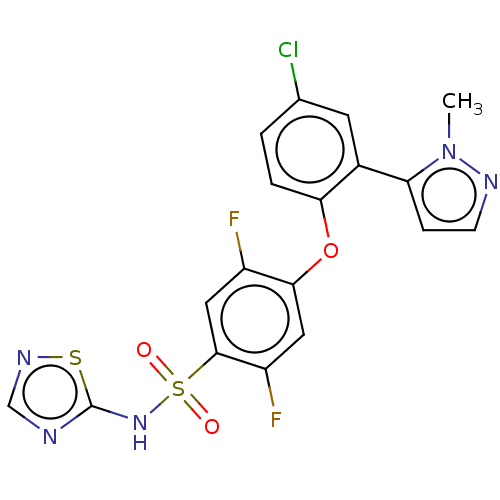 (CHEMBL2325350) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264194 (CHEMBL4093123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264181 (CHEMBL4065448) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50264180 (CHEMBL4074071) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of BACE1 in human SH-SY5Y cells assessed as decrease in sAPP-beta release after 16 to 17 hrs | J Med Chem 61: 3491-3502 (2018) Article DOI: 10.1021/acs.jmedchem.7b01716 BindingDB Entry DOI: 10.7270/Q2TX3HVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257180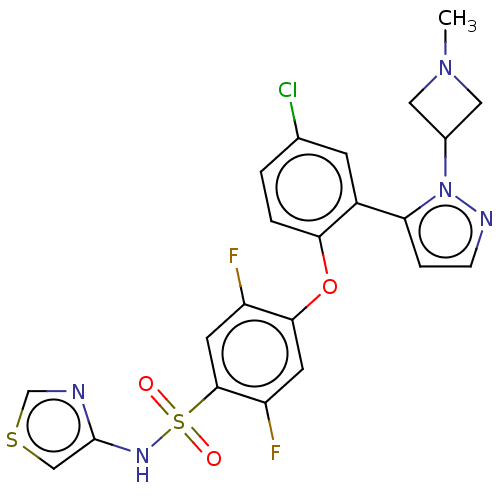 (CHEMBL2325317) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257178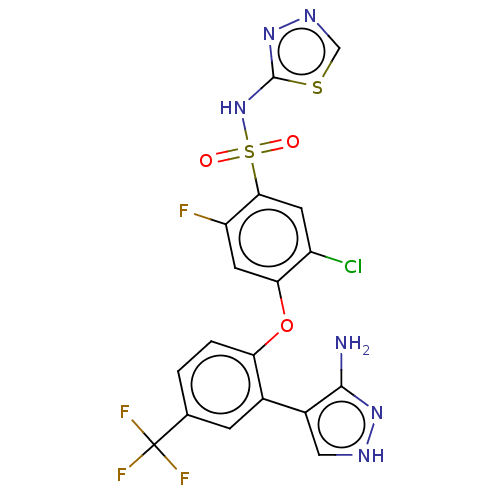 (CHEMBL2325627) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257214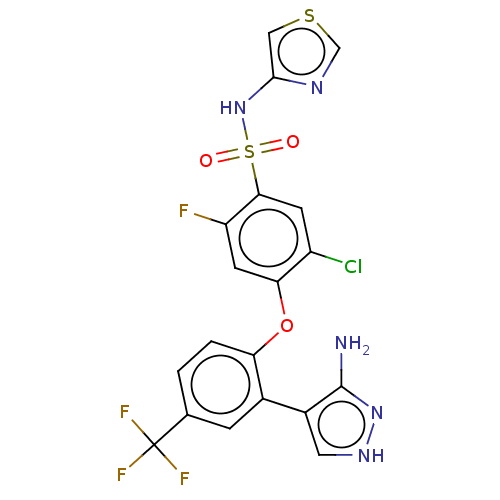 (CHEMBL2325601) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 9 subunit alpha (Homo sapiens (Human)) | BDBM50257168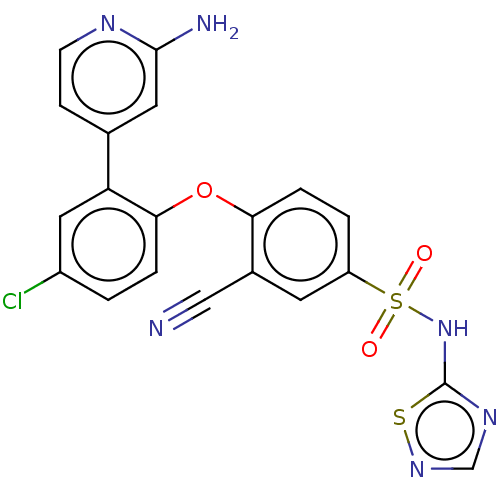 (CHEMBL2325013) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Icagen Inc. , 4222 Emperor Blvd no. 350, Durham, North Carolina 27703, United States. Curated by ChEMBL | Assay Description Inhibition of human NaV1.7 expressed in HEK cells assessed as half inactivation potential at -120 mV holding potential by automated patch clamp metho... | J Med Chem 60: 7029-7042 (2017) Article DOI: 10.1021/acs.jmedchem.7b00598 BindingDB Entry DOI: 10.7270/Q21G0PQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 178 total ) | Next | Last >> |