

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341332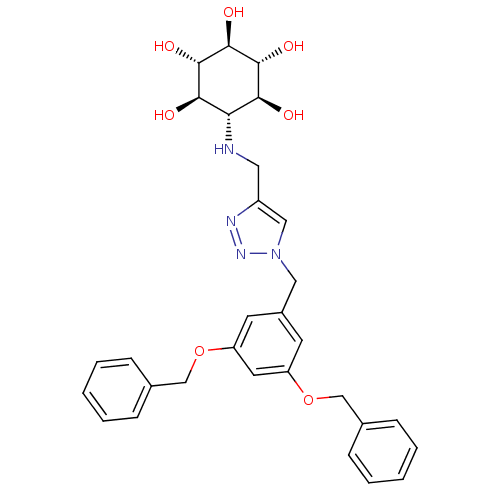 (CHEMBL1766350 | rel-(1R,2S,4R,5S)-6-[(1-(3,5-Bis(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant glucocerebrosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341324 (CHEMBL1766482 | rel-(1R,2S,4R,5S)-6-[[1-Undecyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant glucocerebrosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318564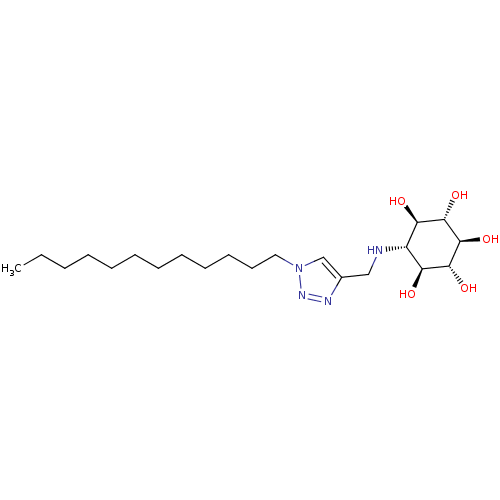 ((1R,2S,3r,4R,5S,6s)-6-((1-dodecyl-1H-1,2,3-triazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318563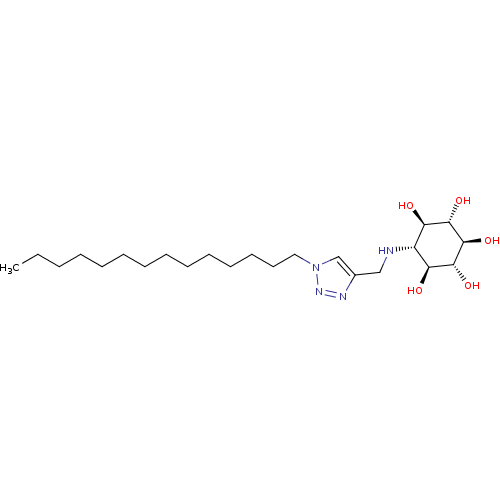 ((1R,2S,3r,4R,5S,6s)-6-[(1-Tetradecyl-1H-1,2,3-tria...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318562 ((1R,2S,3r,4R,5S,6s)-6-[(1-Decyl-1H-1,2,3-triazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341335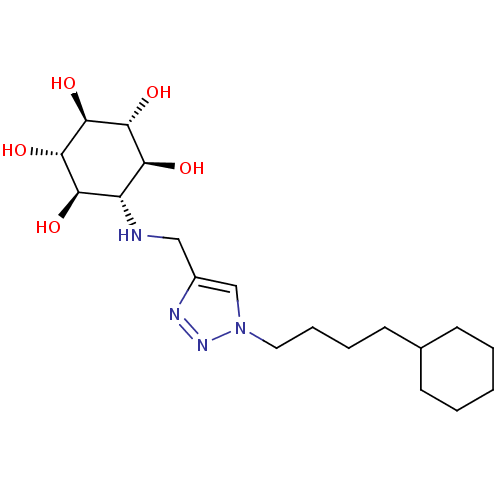 (CHEMBL1766474 | rel-(1R,2S,4R,5S)-6-[[1-(4-Cyclohe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant glucocerebrosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18358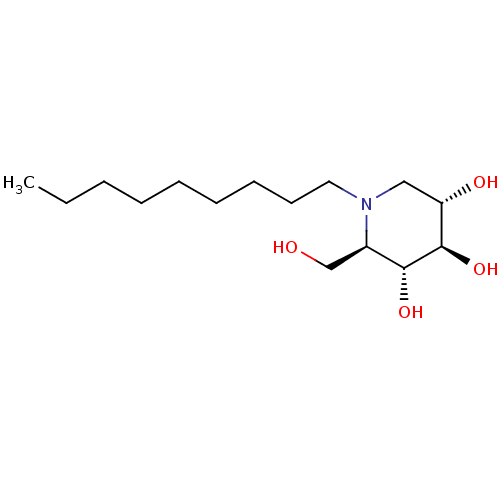 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM18358 ((2R,3R,4R,5S)-2-(hydroxymethyl)-1-nonylpiperidine-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant glucocerebrosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318560 (CHEMBL1083795 | N-decylaminocyclitol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant glucocerebrosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318560 (CHEMBL1083795 | N-decylaminocyclitol) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318561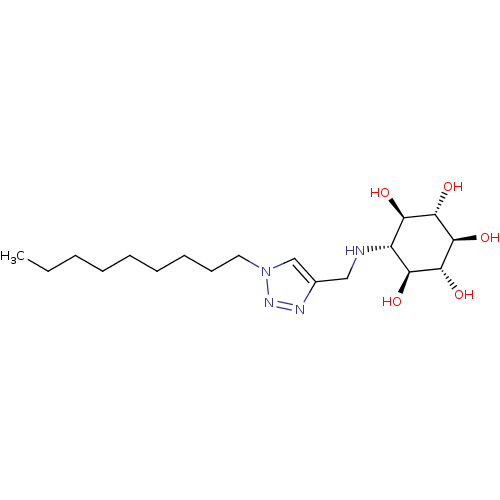 ((1R,2S,3r,4R,5S,6s)-6-[(1-Nonyl-1H-1,2,3-triazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341333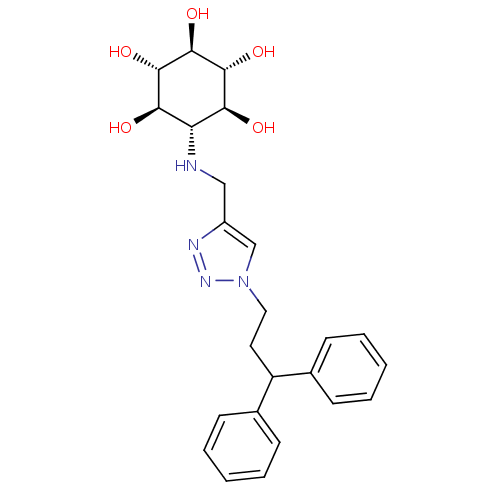 (CHEMBL1766470 | rel-(1R,2S,4R,5S)-6-[[1-(3,3-Diphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant glucocerebrosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral ceramidase (Homo sapiens (Human)) | BDBM50569050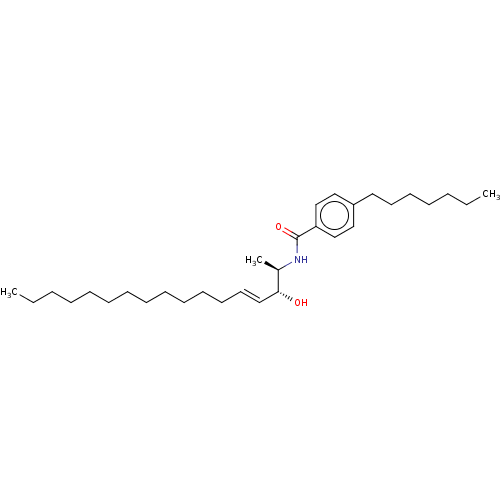 (CHEMBL4848172) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-competitive inhibition of recombinant ASAH2 stably over expressed in human A-375 cell lysate using RBM14C24 as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113296 BindingDB Entry DOI: 10.7270/Q2W66QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341331 (CHEMBL1766489 | rel-(1R,2S,4R,5S)-6-[[1-(10-Hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant glucocerebrosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341334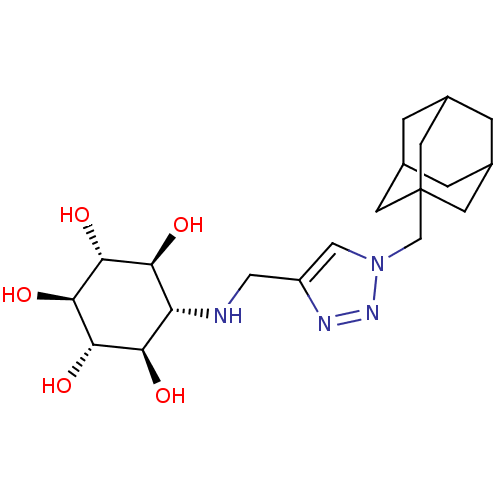 (CHEMBL1766473 | rel-(1R,2S,4R,5S)-6-[(1-Adamantyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Competitive inhibition of recombinant glucocerebrosidase | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318559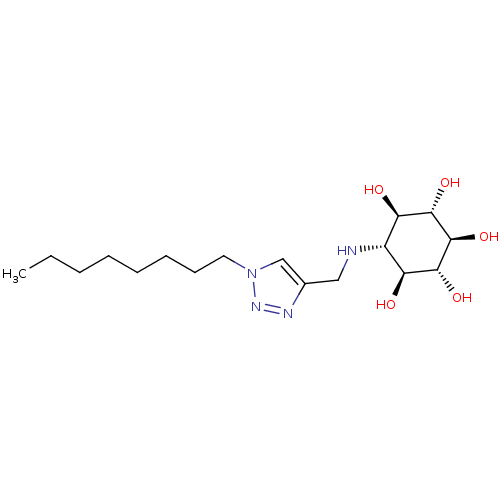 ((1R,2S,3r,4R,5S,6s)-6-[(1-Octyl-1H-1,2,3-triazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral ceramidase (Homo sapiens (Human)) | BDBM50569051 (CHEMBL4865548) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-competitive inhibition of recombinant ASAH2 stably over expressed in human A-375 cell lysate using RBM14C24 as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113296 BindingDB Entry DOI: 10.7270/Q2W66QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318558 ((1R,2S,3r,4R,5S,6s)-6-((1-(6-propoxyhexyl)-1H-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C (Bacillus cereus) | BDBM50332109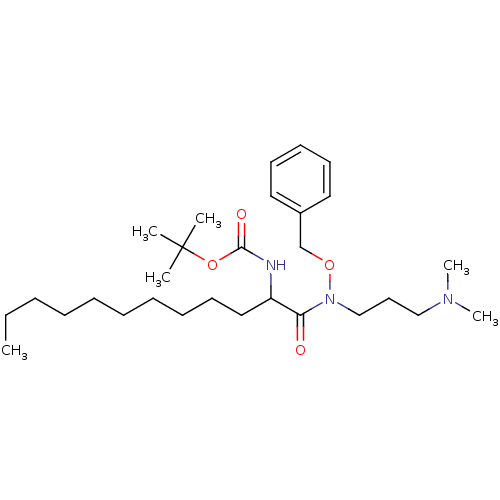 (CHEMBL1287861 | Tert-butyl 1-(benzyloxy[3-(dimethy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advance Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Non-competitive inhibition of Bacillus cereus phosphatidylcholine preferred phospholipase C by Dixon plot analysis | Bioorg Med Chem 18: 8549-55 (2010) Article DOI: 10.1016/j.bmc.2010.10.031 BindingDB Entry DOI: 10.7270/Q2VH5P32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C (Bacillus cereus) | BDBM50332115 (3-(N-(benzyloxy)-2-(tert-butyloxycarbonylamino)dod...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advance Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Mixed-type inhibition of Bacillus cereus phosphatidylcholine preferred phospholipase C by Dixon plot analysis | Bioorg Med Chem 18: 8549-55 (2010) Article DOI: 10.1016/j.bmc.2010.10.031 BindingDB Entry DOI: 10.7270/Q2VH5P32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral ceramidase (Homo sapiens (Human)) | BDBM50569048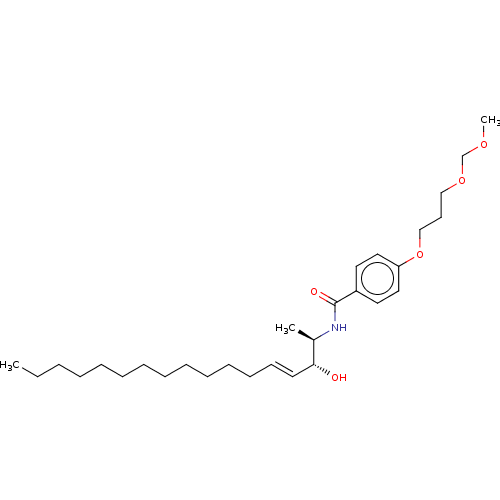 (CHEMBL4861323) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-competitive inhibition of recombinant ASAH2 stably over expressed in human A-375 cell lysate using RBM14C24 as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113296 BindingDB Entry DOI: 10.7270/Q2W66QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Rattus norvegicus) | BDBM50187501 (CHEMBL3827445) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of rat liver microsomal S1PL using varying levels of [3-3H]-D(+) erythro-sphinganine-1-phosphate as substrate incubated for 15... | Citation and Details Article DOI: 10.1016/j.ejmech.2016.08.008 BindingDB Entry DOI: 10.7270/Q29W0K5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Rattus norvegicus) | BDBM50187501 (CHEMBL3827445) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Advanced Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Competitive inhibition of rat S1PL in presence of D(+)-erythro-sphinganine-1-phosphate measured after 15 mins by Dixon plot analysis | Bioorg Med Chem 24: 4381-4389 (2016) Article DOI: 10.1016/j.bmc.2016.07.033 BindingDB Entry DOI: 10.7270/Q2862JDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral ceramidase (Homo sapiens (Human)) | BDBM50569049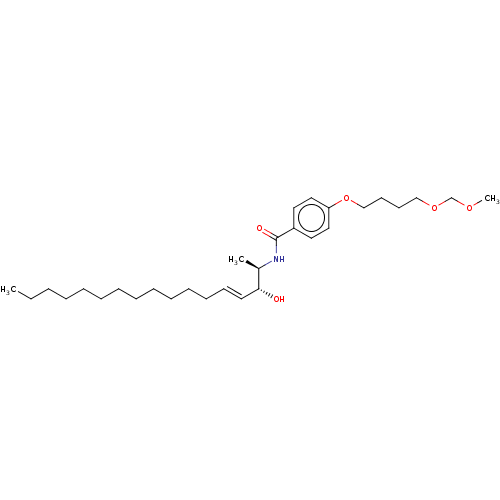 (CHEMBL4878103) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-competitive inhibition of recombinant ASAH2 stably over expressed in human A-375 cell lysate using RBM14C24 as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113296 BindingDB Entry DOI: 10.7270/Q2W66QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Homo sapiens (Human)) | BDBM50558370 (CHEMBL4784427) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human S1PL using varying levels of RBM13 as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.08.008 BindingDB Entry DOI: 10.7270/Q29W0K5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Homo sapiens (Human)) | BDBM50558364 (CHEMBL4754006) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human S1PL using varying levels of RBM13 as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.08.008 BindingDB Entry DOI: 10.7270/Q29W0K5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318557 ((1R,2S,3r,4R,5S,6s)-6-(3-(1-decyl-1H-1,2,3-triazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Homo sapiens (Human)) | BDBM50558371 (CHEMBL4763050) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human S1PL using varying levels of RBM13 as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.08.008 BindingDB Entry DOI: 10.7270/Q29W0K5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C (Bacillus cereus) | BDBM50332105 (1-(hydroxy(2-(trimethylammonio)ethyl)amino)-1-oxod...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advance Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Uncompetitive inhibition of Bacillus cereus phosphatidylcholine preferred phospholipase C by Dixon plot analysis | Bioorg Med Chem 18: 8549-55 (2010) Article DOI: 10.1016/j.bmc.2010.10.031 BindingDB Entry DOI: 10.7270/Q2VH5P32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Homo sapiens (Human)) | BDBM50558367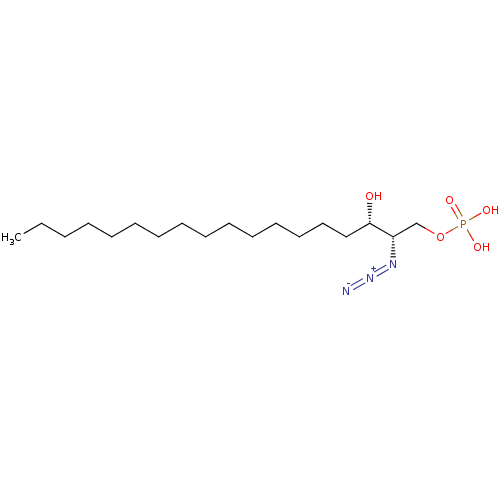 (CHEMBL4754871) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human S1PL using varying levels of RBM13 as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.08.008 BindingDB Entry DOI: 10.7270/Q29W0K5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Homo sapiens (Human)) | BDBM50558368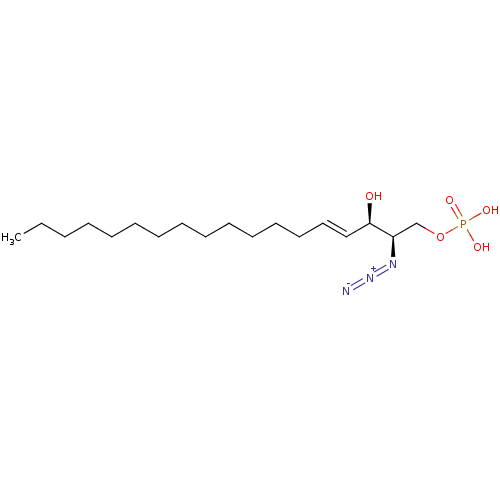 (CHEMBL4748268) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human S1PL using varying levels of RBM13 as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.08.008 BindingDB Entry DOI: 10.7270/Q29W0K5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Homo sapiens (Human)) | BDBM50558365 (CHEMBL4756827) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human S1PL using varying levels of RBM13 as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.08.008 BindingDB Entry DOI: 10.7270/Q29W0K5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318556 ((1R,2S,3r,4R,5S,6s)-6-[(1-Phenethyl-1H-1,2,3-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.91E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318554 ((1R,2S,3r,4R,5S,6s)-6-(3-(1-octyl-1H-1,2,3-triazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318555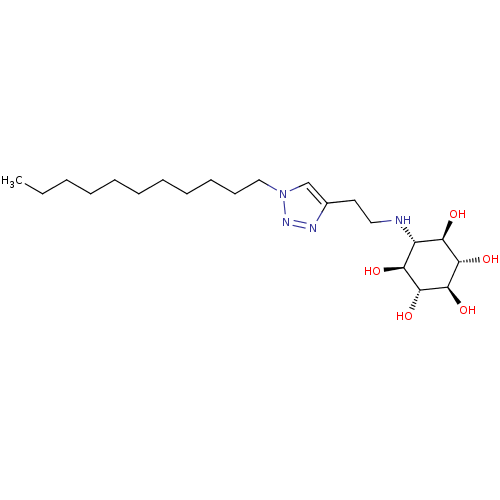 ((1R,2S,3r,4R,5S,6s)-6-[2-(1-Undecyl-1H-1,2,3-triaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of beta-glucocerebrosidase at pH 5.2 by Lineweaver-Burke plot analysis | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Homo sapiens (Human)) | BDBM50558369 (CHEMBL4791053) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human S1PL using varying levels of RBM13 as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.08.008 BindingDB Entry DOI: 10.7270/Q29W0K5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline ceramidase 3 (Mus musculus) | BDBM50569048 (CHEMBL4861323) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-competitive inhibition of ACER3 in ASAH2-null mouse embryonic fibroblast lysate using RBM14C16 as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113296 BindingDB Entry DOI: 10.7270/Q2W66QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase C (Bacillus cereus) | BDBM50332119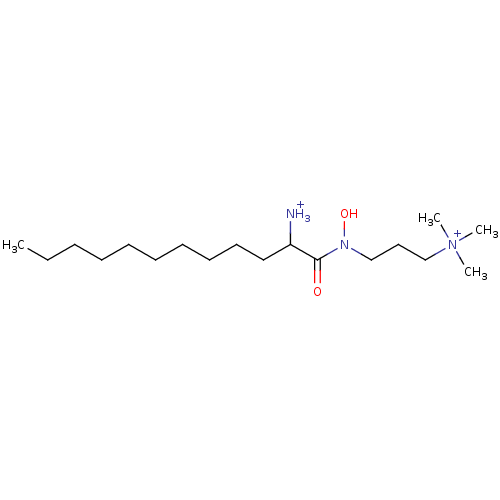 (1-(hydroxy[3-(trimethylammonio)propyl]amino)-1-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Advance Chemistry of Catalonia (IQAC-CSIC) Curated by ChEMBL | Assay Description Mixed-type inhibition of Bacillus cereus phosphatidylcholine preferred phospholipase C by Dixon plot analysis | Bioorg Med Chem 18: 8549-55 (2010) Article DOI: 10.1016/j.bmc.2010.10.031 BindingDB Entry DOI: 10.7270/Q2VH5P32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alkaline ceramidase 3 (Mus musculus) | BDBM50569049 (CHEMBL4878103) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-competitive inhibition of ACER3 in ASAH2-null mouse embryonic fibroblast lysate using RBM14C16 as substrate | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113296 BindingDB Entry DOI: 10.7270/Q2W66QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine-1-phosphate lyase 1 (Homo sapiens (Human)) | BDBM50558366 (CHEMBL4763487) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human S1PL using varying levels of RBM13 as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2016.08.008 BindingDB Entry DOI: 10.7270/Q29W0K5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutral ceramidase (Homo sapiens (Human)) | BDBM50569052 (CHEMBL4866400) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human ASAH2 using RBM14C24 fluorogenic substrate measured at pH 7.5 | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113296 BindingDB Entry DOI: 10.7270/Q2W66QJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50586222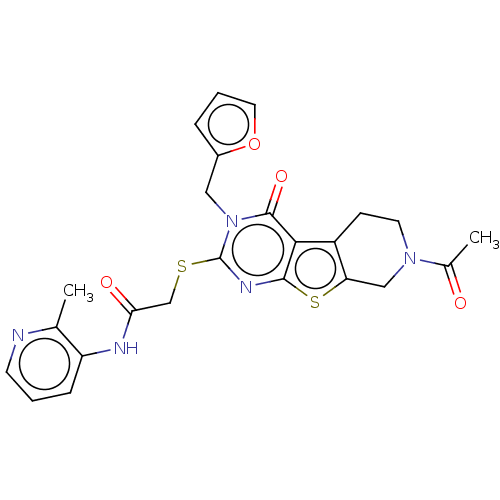 (CHEMBL5082786) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRD3-BD2 (unknown origin) incubated for 6 hrs by HTRF method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02168 BindingDB Entry DOI: 10.7270/Q20Z7752 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318564 ((1R,2S,3r,4R,5S,6s)-6-((1-dodecyl-1H-1,2,3-triazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant beta-glucocerebrosidase at pH 5.2 after 10 mins by fluorimetry | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318564 ((1R,2S,3r,4R,5S,6s)-6-((1-dodecyl-1H-1,2,3-triazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant beta-glucocerebrosidase after 10 mins by fluorimetry | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341332 (CHEMBL1766350 | rel-(1R,2S,4R,5S)-6-[(1-(3,5-Bis(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Inhibition of recombinant glucocerebrosidase in McIlvaine buffer at pH 5.2 after 10 mins by fluorometric analysis | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341324 (CHEMBL1766482 | rel-(1R,2S,4R,5S)-6-[[1-Undecyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 5.2 | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Inhibition of recombinant glucocerebrosidase in McIlvaine buffer at pH 5.2 after 10 mins by fluorometric analysis | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50341332 (CHEMBL1766350 | rel-(1R,2S,4R,5S)-6-[(1-(3,5-Bis(b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Spanish National Research Council (Consejo Superior de Investigaciones Cienti£?ficas) Curated by ChEMBL | Assay Description Inhibition of recombinant glucocerebrosidase in McIlvaine buffer at pH 7.4 after 10 mins by fluorometric analysis | J Med Chem 54: 2069-79 (2011) Article DOI: 10.1021/jm101204u BindingDB Entry DOI: 10.7270/Q24F1R18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal acid glucosylceramidase (Homo sapiens (Human)) | BDBM50318564 ((1R,2S,3r,4R,5S,6s)-6-((1-dodecyl-1H-1,2,3-triazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.4 | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of recombinant beta-glucocerebrosidase at pH 7.4 after 10 mins by fluorimetry | J Med Chem 53: 5248-55 (2010) Article DOI: 10.1021/jm100198t BindingDB Entry DOI: 10.7270/Q2VH5PS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 2 (Homo sapiens (Human)) | BDBM50586222 (CHEMBL5082786) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRD2-BD2 (unknown origin) incubated for 6 hrs by HTRF method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02168 BindingDB Entry DOI: 10.7270/Q20Z7752 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 3 (Homo sapiens (Human)) | BDBM50586230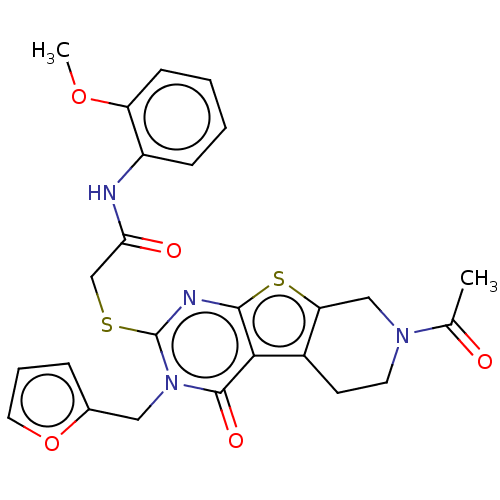 (CHEMBL5081350) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BRD3-BD2 (unknown origin) incubated for 6 hrs by HTRF method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c02168 BindingDB Entry DOI: 10.7270/Q20Z7752 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 300 total ) | Next | Last >> |