 Found 546 hits with Last Name = 'chai' and Initial = 'j'
Found 546 hits with Last Name = 'chai' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(MOUSE) | BDBM50408679

(CHEMBL5287792)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)o1 Show InChI InChI=1S/C26H38N6O4/c1-30(2)17-18-11-12-21(36-18)25(33)31(3)13-9-7-8-10-14-32(4)26-28-20-16-23(35-6)22(34-5)15-19(20)24(27)29-26/h11-12,15-16H,7-10,13-14,17H2,1-6H3,(H2,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.00260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408509

(CHEMBL5277326)Show InChI InChI=1S/C20H24N2OS/c1-4-18(23)15-10-11-20-17(14-15)22(13-7-12-21(2)3)16-8-5-6-9-19(16)24-20/h5-6,8-11,14H,4,7,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at AT1 receptor in rat aortic rings |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577958
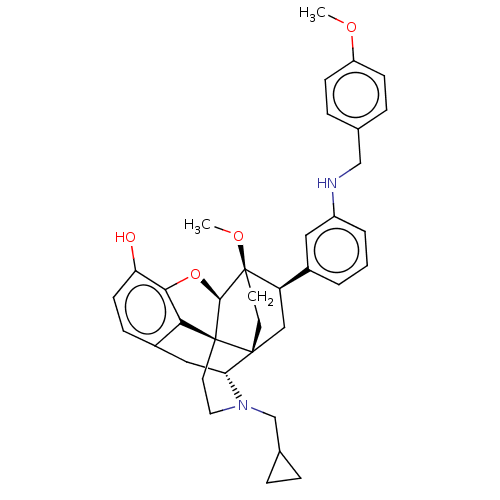
(CHEMBL4859512)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(OC)cc2)c1)ccc3O |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50598866
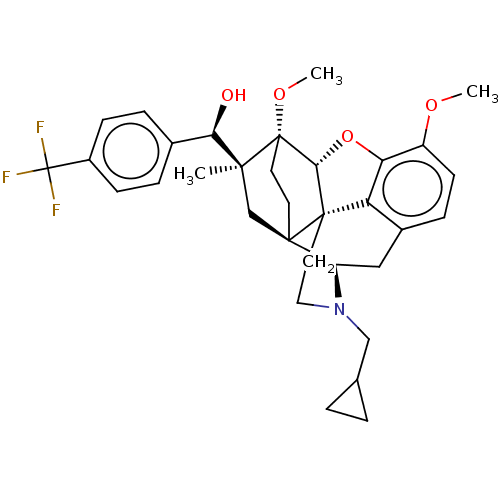
(CHEMBL5205530)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(cc1)C(F)(F)F)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50598866

(CHEMBL5205530)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(cc1)C(F)(F)F)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50577958

(CHEMBL4859512)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(OC)cc2)c1)ccc3O |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DPDPE from rat delta opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting metho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408675
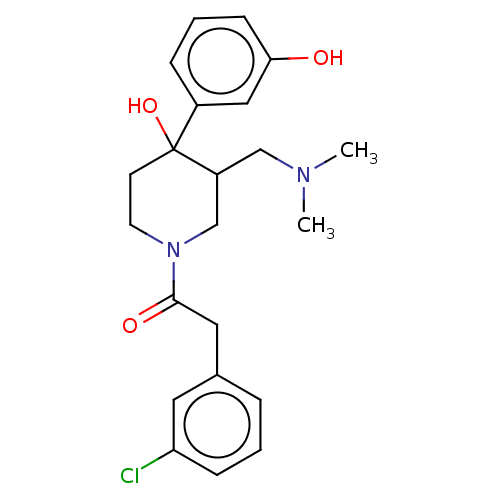
(CHEMBL5270915)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C24H32ClN5O4/c1-29(23(31)19-10-9-16(15-25)34-19)11-7-5-6-8-12-30(2)24-27-18-14-21(33-4)20(32-3)13-17(18)22(26)28-24/h9-10,13-14H,5-8,11-12,15H2,1-4H3,(H2,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50577958

(CHEMBL4859512)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(OC)cc2)c1)ccc3O |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.294 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50506108

(CHEMBL4449252)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1ccc(NC(=O)c2ccccc2)cc1)ccc3OC |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C37H40N2O4/c1-41-29-15-12-26-20-30-35-16-17-37(42-2,34-36(35,31(26)32(29)43-34)18-19-39(30)22-23-8-9-23)28(21-35)24-10-13-27(14-11-24)38-33(40)25-6-4-3-5-7-25/h3-7,10-15,23,28,30,34H,8-9,16-22H2,1-2H3,(H,38,40)/t28-,30-,34-,35-,36+,37-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408677

(CHEMBL5274117)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccccc1CNCCCCCCN Show InChI InChI=1S/C32H49N7O3/c1-38(31(40)25-16-10-9-15-24(25)23-35-18-12-6-5-11-17-33)19-13-7-8-14-20-39(2)32-36-27-22-29(42-4)28(41-3)21-26(27)30(34)37-32/h9-10,15-16,21-22,35H,5-8,11-14,17-20,23,33H2,1-4H3,(H2,34,36,37) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21130
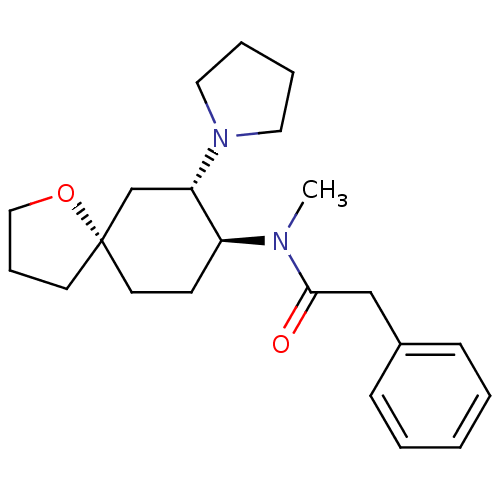
(N-methyl-2-phenyl-N-[(5R,7S,8S)-7-(pyrrolidin-1-yl...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C22H32N2O2/c1-23(21(25)16-18-8-3-2-4-9-18)19-10-12-22(11-7-15-26-22)17-20(19)24-13-5-6-14-24/h2-4,8-9,19-20H,5-7,10-17H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at oxytocin receptor in Wistar rat uterus assessed as inhibition of oxytocin-induced uterotonic activity treated 1 min prior to o... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408676
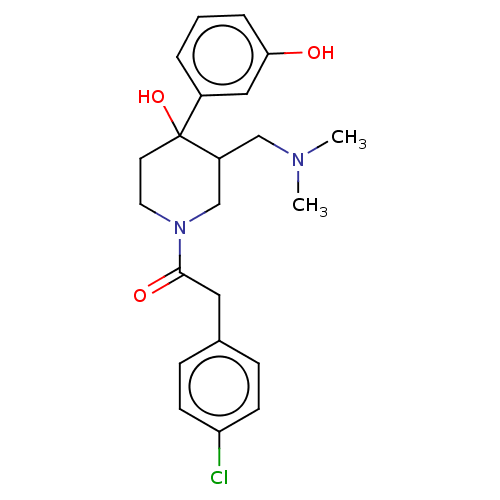
(CHEMBL5272258)Show SMILES CNCCCCCCN(C)Cc1ccc(cc1)C(=O)N(C)CCCCCCN(C)c1nc(N)c2cc(OC)c(OC)cc2n1 Show InChI InChI=1S/C34H53N7O3/c1-36-19-11-7-8-12-20-39(2)25-26-15-17-27(18-16-26)33(42)40(3)21-13-9-10-14-22-41(4)34-37-29-24-31(44-6)30(43-5)23-28(29)32(35)38-34/h15-18,23-24,36H,7-14,19-22,25H2,1-6H3,(H2,35,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408673

(CHEMBL5275873)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCSSCCN(C)C(=O)c1ccc(CCl)o1 Show InChI InChI=1S/C22H28ClN5O4S2/c1-27(21(29)17-6-5-14(13-23)32-17)7-9-33-34-10-8-28(2)22-25-16-12-19(31-4)18(30-3)11-15(16)20(24)26-22/h5-6,11-12H,7-10,13H2,1-4H3,(H2,24,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408816

(CHEMBL5279890)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NC1(CCCCCCC1)C(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C43H68N12O8/c44-29(15-10-20-49-41(45)46)35(57)51-30(16-11-21-50-42(47)48)36(58)53-43(18-8-2-1-3-9-19-43)40(63)52-31(25-56)37(59)54-24-28-14-5-4-12-26(28)22-33(54)38(60)55-32-17-7-6-13-27(32)23-34(55)39(61)62/h4-5,12,14,27,29-34,56H,1-3,6-11,13,15-25,44H2,(H,51,57)(H,52,63)(H,53,58)(H,61,62)(H4,45,46,49)(H4,47,48,50)/t27-,29+,30-,31-,32-,33+,34-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at histamine H1 receptor in guinea pig ileum assessed as histamine-induced contractions |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408813
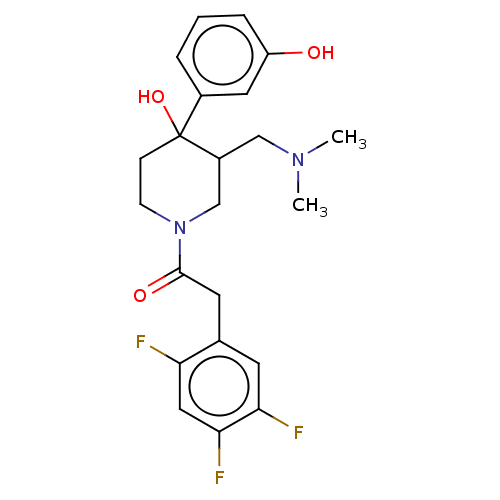
(CHEMBL5273356)Show SMILES N[C@H](CCCNC(N)=N)C(=O)N[C@@H](CCCNC(N)=N)C(=O)NCCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N1Cc2ccccc2C[C@@H]1C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(O)=O Show InChI InChI=1S/C46H76N12O8/c47-33(19-14-24-53-45(48)49)40(61)56-34(20-15-25-54-46(50)51)41(62)52-23-13-7-5-3-1-2-4-6-8-22-39(60)55-35(29-59)42(63)57-28-32-18-10-9-16-30(32)26-37(57)43(64)58-36-21-12-11-17-31(36)27-38(58)44(65)66/h9-10,16,18,31,33-38,59H,1-8,11-15,17,19-29,47H2,(H,52,62)(H,55,60)(H,56,61)(H,65,66)(H4,48,49,53)(H4,50,51,54)/t31-,33+,34-,35-,36-,37+,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at MTL receptor in rabbit duodenum homogenate |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50016848
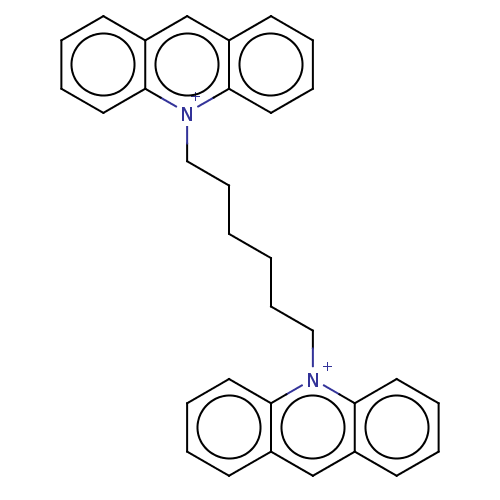
(CHEMBL3276408)Show SMILES [I-].[I-].C(CCC[n+]1c2ccccc2cc2ccccc12)CC[n+]1c2ccccc2cc2ccccc12 Show InChI InChI=1S/C32H30N2.2HI/c1(11-21-33-29-17-7-3-13-25(29)23-26-14-4-8-18-30(26)33)2-12-22-34-31-19-9-5-15-27(31)24-28-16-6-10-20-32(28)34;;/h3-10,13-20,23-24H,1-2,11-12,21-22H2;2*1H/q+2;;/p-2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis |
J Med Chem 20: 1617-23 (1978)
BindingDB Entry DOI: 10.7270/Q29025BQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50016848

(CHEMBL3276408)Show SMILES [I-].[I-].C(CCC[n+]1c2ccccc2cc2ccccc12)CC[n+]1c2ccccc2cc2ccccc12 Show InChI InChI=1S/C32H30N2.2HI/c1(11-21-33-29-17-7-3-13-25(29)23-26-14-4-8-18-30(26)33)2-12-22-34-31-19-9-5-15-27(31)24-28-16-6-10-20-32(28)34;;/h3-10,13-20,23-24H,1-2,11-12,21-22H2;2*1H/q+2;;/p-2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... |
J Med Chem 20: 1617-23 (1978)
BindingDB Entry DOI: 10.7270/Q29025BQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408514
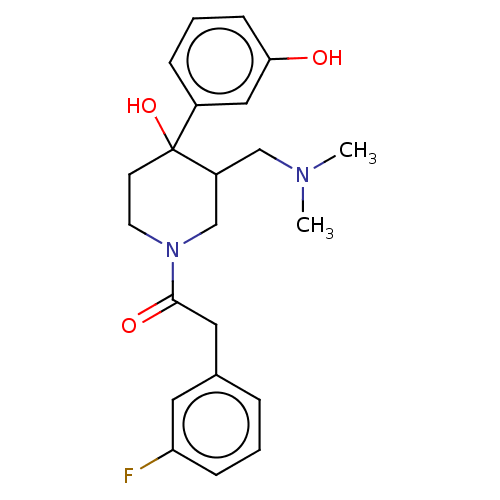
(CHEMBL5281561)Show InChI InChI=1S/C18H22N2OS/c1-19(2)10-5-11-20-15-6-3-4-7-17(15)22-18-9-8-14(13-21)12-16(18)20/h3-4,6-9,12,21H,5,10-11,13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21008
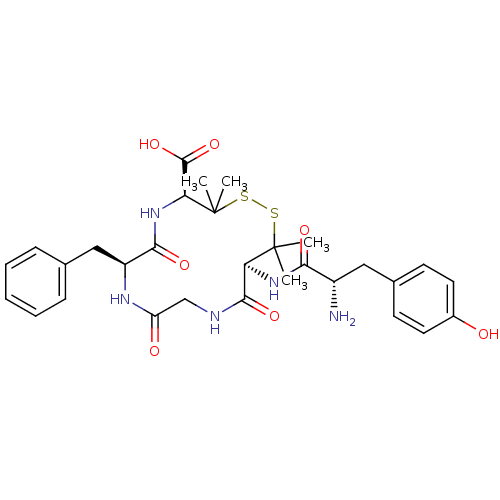
((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant EP1 receptor expressed in CHO cells assessed as luciferase activity by Schild assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408669
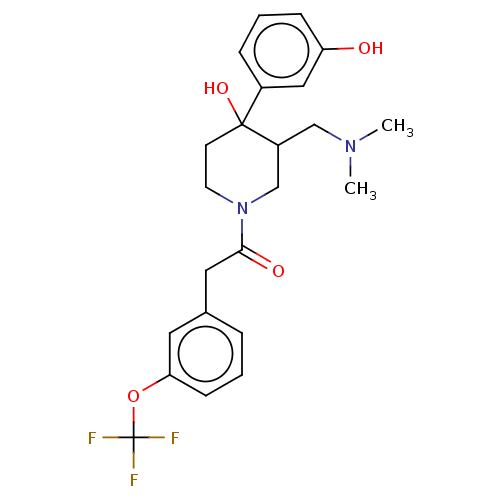
(CHEMBL5272171)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccccc1CN(C)C Show InChI InChI=1S/C28H40N6O3/c1-32(2)19-20-13-9-10-14-21(20)27(35)33(3)15-11-7-8-12-16-34(4)28-30-23-18-25(37-6)24(36-5)17-22(23)26(29)31-28/h9-10,13-14,17-18H,7-8,11-12,15-16,19H2,1-6H3,(H2,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50598860
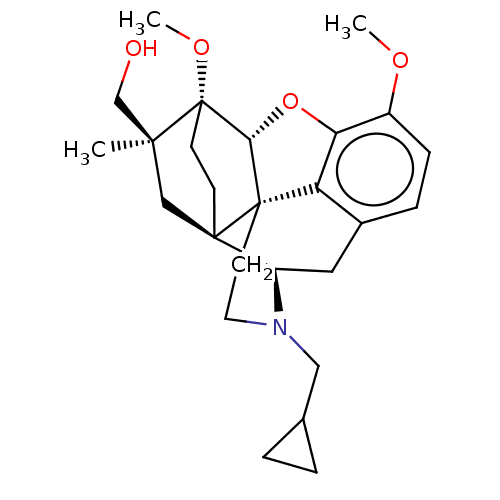
(CHEMBL5177908)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(CO)C1)ccc3OC |r,THB:10:9:17:4.6.5| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50598862
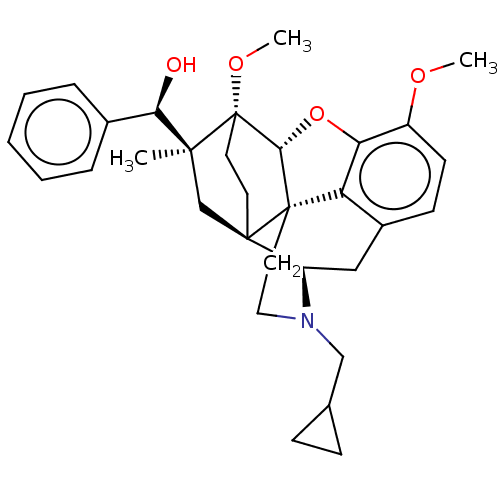
(CHEMBL5178795)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccccc1)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408678
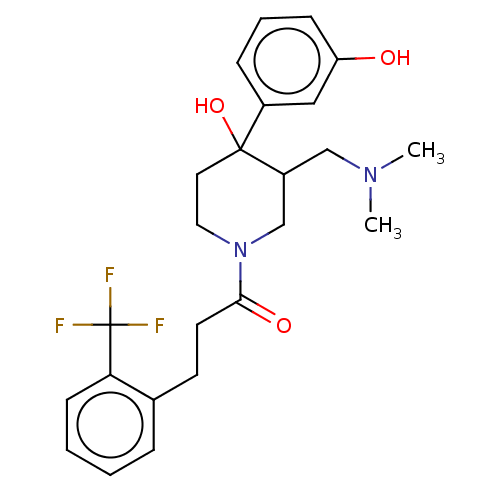
(CHEMBL5281054)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)cc1 Show InChI InChI=1S/C28H40N6O3/c1-32(2)19-20-11-13-21(14-12-20)27(35)33(3)15-9-7-8-10-16-34(4)28-30-23-18-25(37-6)24(36-5)17-22(23)26(29)31-28/h11-14,17-18H,7-10,15-16,19H2,1-6H3,(H2,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M2 receptor-Gqi5 chimeric protein expressed in CHO cells assessed as acetylcholine-induced change in cytosol... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50598864
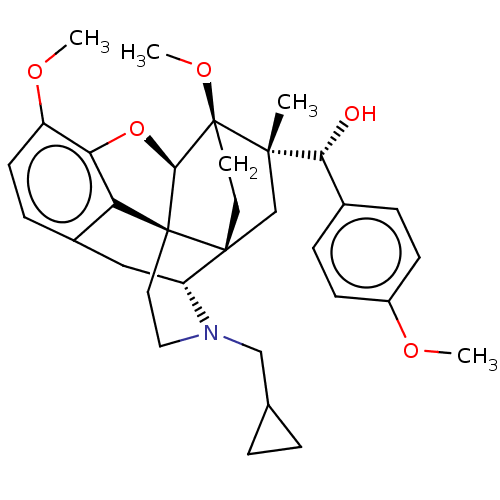
(CHEMBL5171735)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408513
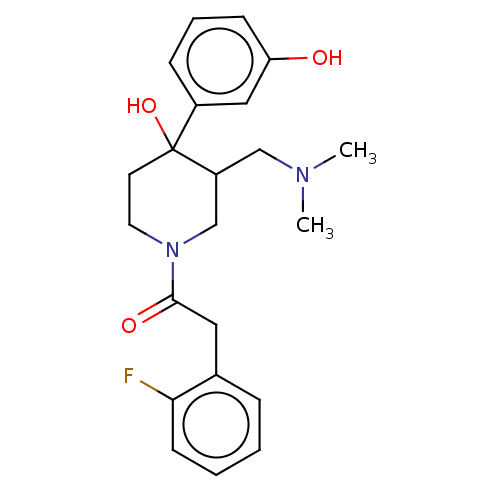
(CHEMBL5272744)Show InChI InChI=1S/C14H9BrClNOS/c15-8-14(18)17-10-3-1-2-4-12(10)19-13-6-5-9(16)7-11(13)17/h1-7H,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M3 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50598860

(CHEMBL5177908)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(CO)C1)ccc3OC |r,THB:10:9:17:4.6.5| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577936
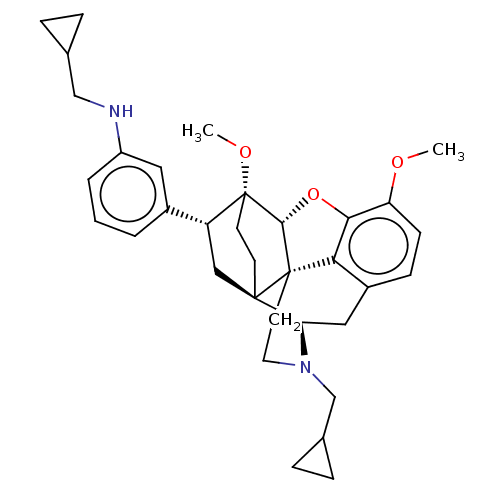
(CHEMBL4864911)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCC2CC2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408511
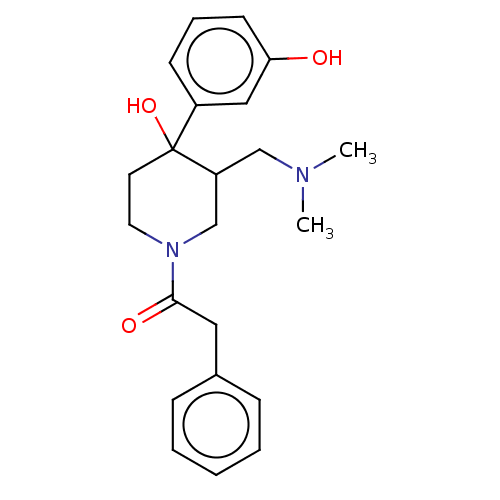
(CHEMBL5274208)Show InChI InChI=1S/C23H30N2OS/c1-4-5-6-11-21(26)18-13-14-23-20(17-18)25(16-9-15-24(2)3)19-10-7-8-12-22(19)27-23/h7-8,10,12-14,17H,4-6,9,11,15-16H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant CXCR3 receptor assessed as human IP10-induced calcium mobilization by FLIPR assay |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50598864

(CHEMBL5171735)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(OC)cc1)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000780
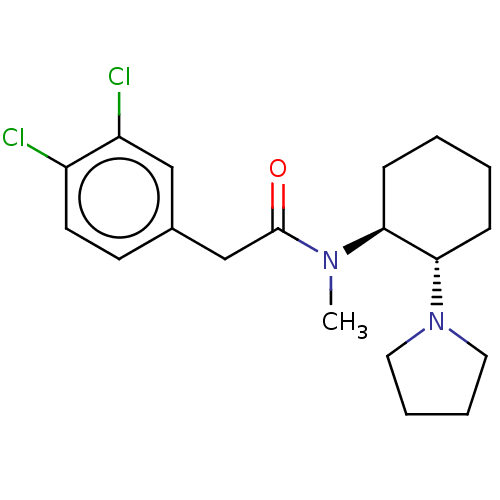
(2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...)Show SMILES CN([C@H]1CCCC[C@@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50016849
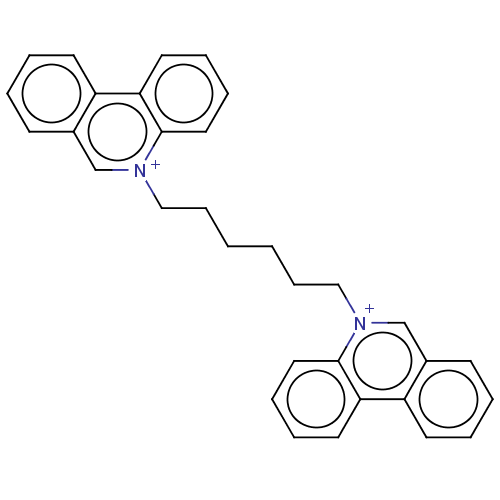
(CHEMBL3276410)Show SMILES [Br-].[Br-].C(CCC[n+]1cc2ccccc2c2ccccc12)CC[n+]1cc2ccccc2c2ccccc12 Show InChI InChI=1S/C32H30N2.2BrH/c1(11-21-33-23-25-13-3-5-15-27(25)29-17-7-9-19-31(29)33)2-12-22-34-24-26-14-4-6-16-28(26)30-18-8-10-20-32(30)34;;/h3-10,13-20,23-24H,1-2,11-12,21-22H2;2*1H/q+2;;/p-2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... |
J Med Chem 20: 1617-23 (1978)
BindingDB Entry DOI: 10.7270/Q29025BQ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50016849

(CHEMBL3276410)Show SMILES [Br-].[Br-].C(CCC[n+]1cc2ccccc2c2ccccc12)CC[n+]1cc2ccccc2c2ccccc12 Show InChI InChI=1S/C32H30N2.2BrH/c1(11-21-33-23-25-13-3-5-15-27(25)29-17-7-9-19-31(29)33)2-12-22-34-24-26-14-4-6-16-28(26)30-18-8-10-20-32(30)34;;/h3-10,13-20,23-24H,1-2,11-12,21-22H2;2*1H/q+2;;/p-2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate by Lineweaver-Burk plot analysis |
J Med Chem 20: 1617-23 (1978)
BindingDB Entry DOI: 10.7270/Q29025BQ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577953
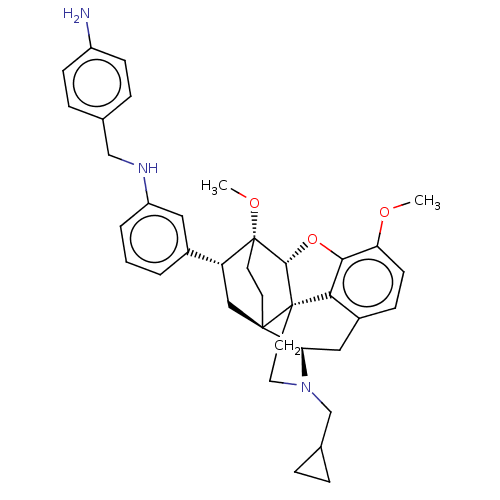
(CHEMBL4876542)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(N)cc2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]DAMGO from rat mu opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting method |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577940
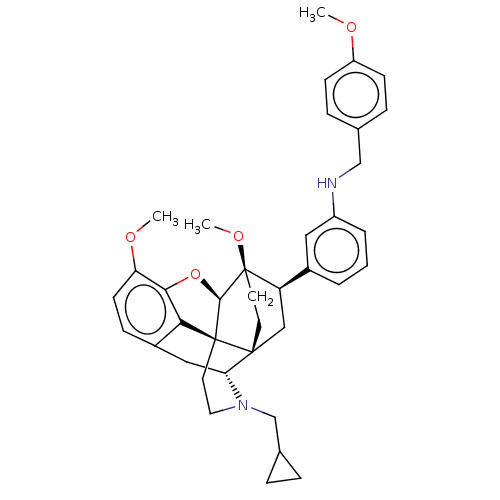
(CHEMBL4848808)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(OC)cc2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577937
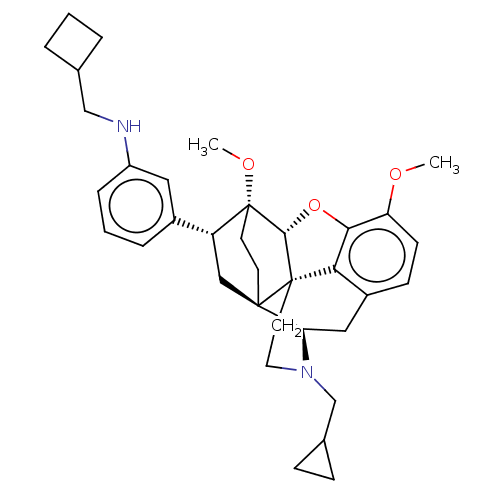
(CHEMBL4860416)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCC2CCC2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50016848

(CHEMBL3276408)Show SMILES [I-].[I-].C(CCC[n+]1c2ccccc2cc2ccccc12)CC[n+]1c2ccccc2cc2ccccc12 Show InChI InChI=1S/C32H30N2.2HI/c1(11-21-33-29-17-7-3-13-25(29)23-26-14-4-8-18-30(26)33)2-12-22-34-31-19-9-5-15-27(31)24-28-16-6-10-20-32(28)34;;/h3-10,13-20,23-24H,1-2,11-12,21-22H2;2*1H/q+2;;/p-2 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible mixed-type inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... |
J Med Chem 20: 1617-23 (1978)
BindingDB Entry DOI: 10.7270/Q29025BQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50408679

(CHEMBL5287792)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CN(C)C)o1 Show InChI InChI=1S/C26H38N6O4/c1-30(2)17-18-11-12-21(36-18)25(33)31(3)13-9-7-8-10-14-32(4)26-28-20-16-23(35-6)22(34-5)15-19(20)24(27)29-26/h11-12,15-16H,7-10,13-14,17H2,1-6H3,(H2,27,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human recombinant PAC1 receptor expressed in CHO cells assessed as reduction in PACAP38-induced calcium mobilization by FLIPR ... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50408509

(CHEMBL5277326)Show InChI InChI=1S/C20H24N2OS/c1-4-18(23)15-10-11-20-17(14-15)22(13-7-12-21(2)3)16-8-5-6-9-19(16)24-20/h5-6,8-11,14H,4,7,12-13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at human histamine H4 receptor expressed in human SK-N-MC cells assessed as inhibition of 2-[3-(imidazol-4-yl)-propyl]-1-(4-iodo-... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577941
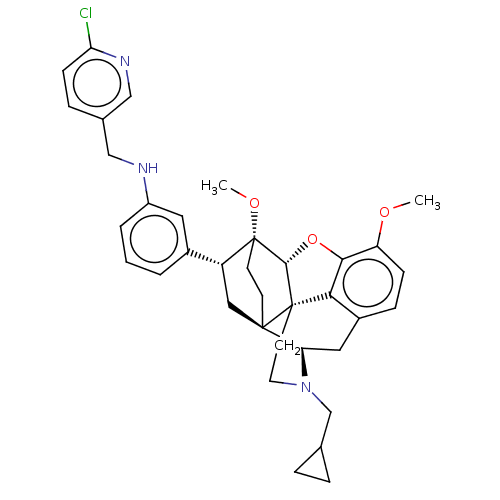
(CHEMBL4873458)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(Cl)nc2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408674
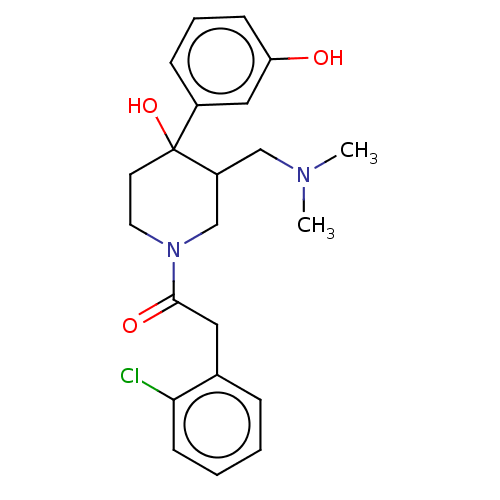
(CHEMBL5282901)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N(C)CCCCCCN(C)C(=O)c1ccc(CCl)cc1 Show InChI InChI=1S/C26H34ClN5O3/c1-31(25(33)19-11-9-18(17-27)10-12-19)13-7-5-6-8-14-32(2)26-29-21-16-23(35-4)22(34-3)15-20(21)24(28)30-26/h9-12,15-16H,5-8,13-14,17H2,1-4H3,(H2,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577947
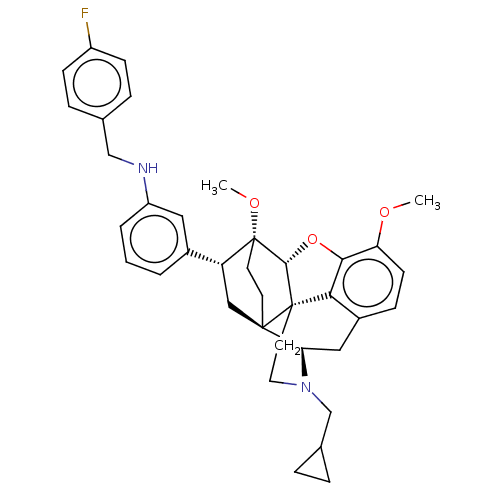
(CHEMBL4875933)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2ccc(F)cc2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(MOUSE) | BDBM50408672
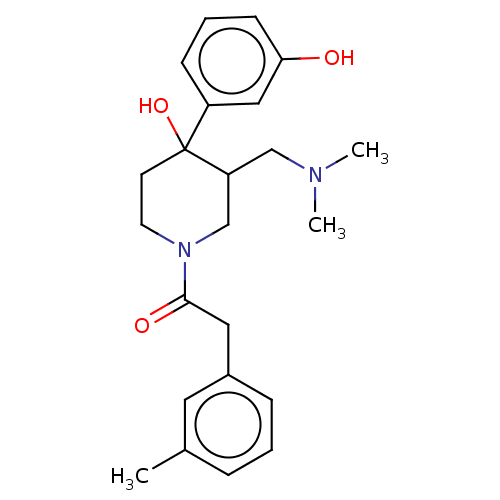
(CHEMBL5274664)Show SMILES CNCCCCCCN(C)Cc1cccc(c1)C(=O)N(C)CCCCCCN(C)c1nc(N)c2cc(OC)c(OC)cc2n1 Show InChI InChI=1S/C34H53N7O3/c1-36-18-11-7-8-12-19-39(2)25-26-16-15-17-27(22-26)33(42)40(3)20-13-9-10-14-21-41(4)34-37-29-24-31(44-6)30(43-5)23-28(29)32(35)38-34/h15-17,22-24,36H,7-14,18-21,25H2,1-6H3,(H2,35,37,38) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at cloned muscarinic M1 receptor expressed in CHO cells assessed as acetylcholine-induced change in cytosolic calcium concentrati... |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50016850

(CHEMBL3276411)Show SMILES [Br-].[Br-].C(CCC[n+]1cccc2c1ccc1ccccc21)CC[n+]1cccc2c1ccc1ccccc21 Show InChI InChI=1S/C32H30N2.2BrH/c1(7-21-33-23-9-15-29-27-13-5-3-11-25(27)17-19-31(29)33)2-8-22-34-24-10-16-30-28-14-6-4-12-26(28)18-20-32(30)34;;/h3-6,9-20,23-24H,1-2,7-8,21-22H2;2*1H/q+2;;/p-2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible mixed-type inhibition of electric eel acetylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor comp... |
J Med Chem 20: 1617-23 (1978)
BindingDB Entry DOI: 10.7270/Q29025BQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50598863
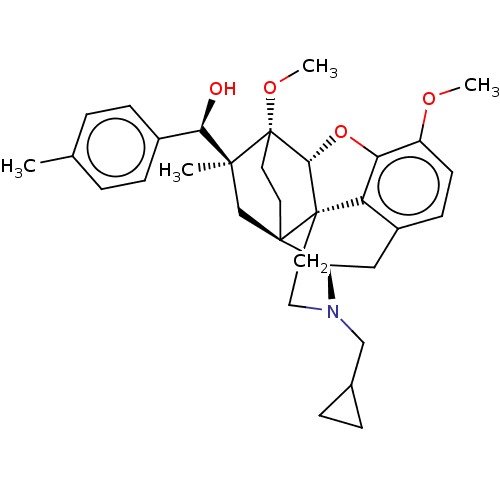
(CHEMBL5188701)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(C)cc1)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50016871
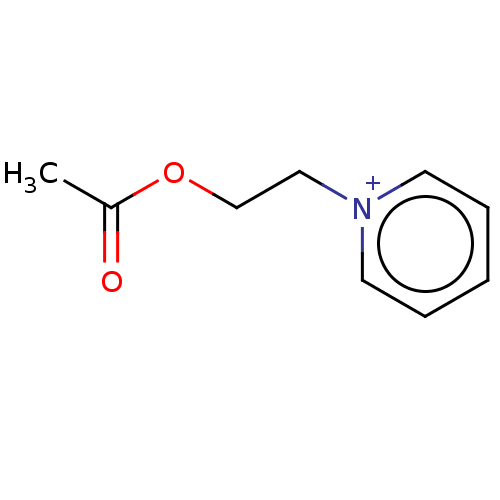
(CHEMBL3276432)Show InChI InChI=1S/C9H12NO2.BrH/c1-9(11)12-8-7-10-5-3-2-4-6-10;/h2-6H,7-8H2,1H3;1H/q+1;/p-1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible competitive inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... |
J Med Chem 20: 1617-23 (1978)
BindingDB Entry DOI: 10.7270/Q29025BQ |
More data for this
Ligand-Target Pair | |
Carboxylic ester hydrolase
(Equus caballus (Horse)) | BDBM50016875

(CHEMBL3276431)Show InChI InChI=1S/C17H16NO2.BrH/c1-13(19)20-11-10-18-16-8-4-2-6-14(16)12-15-7-3-5-9-17(15)18;/h2-9,12H,10-11H2,1H3;1H/q+1;/p-1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Reversible competitive inhibition of horse serum butyrylcholinesterase using acetylcholine bromide as substrate assessed as free enzyme-inhibitor com... |
J Med Chem 20: 1617-23 (1978)
BindingDB Entry DOI: 10.7270/Q29025BQ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50577949
(CHEMBL4860580)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@H](C1)c1cccc(NCc2cccc(OC)c2)c1)ccc3OC |r,THB:10:9:5.4.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membrane measured after 30 mins by liquid scintillation counting m... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01082
BindingDB Entry DOI: 10.7270/Q2NZ8CG1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50598863

(CHEMBL5188701)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@@](C)(C1)[C@H](O)c1ccc(C)cc1)ccc3OC |r,THB:10:9:4.5.6:17| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00014
BindingDB Entry DOI: 10.7270/Q2QJ7N96 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data