

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM9019 (CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50138279 (CHEMBL3752467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-inhibitor complex by Lineweaver-Burk d... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50138279 (CHEMBL3752467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of recombinant human AChE using acetylthiocholine iodide as substrate assessed as enzyme-substrate-inhibitor complex by Linewea... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279 (CHEMBL3752467) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Noncompetitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometr... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM50138279 (CHEMBL3752467) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Competitive inhibition of Torpedo californica AChE using ATCh as substrate preincubated for 90 mins followed by substrate addition by potentiometric ... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50317074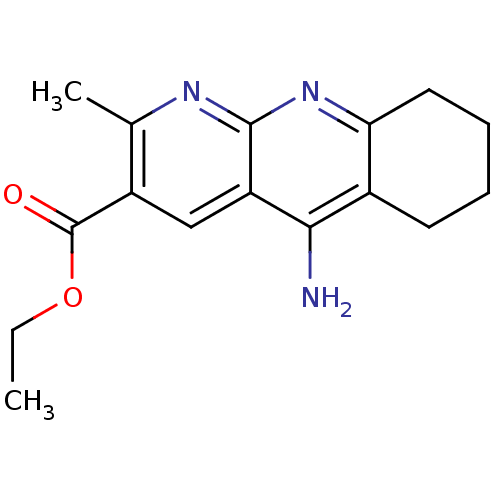 (CHEMBL1087194 | ethyl 5-amino-2-methyl-6,7,8,9-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE (unknown orign) by Lineweaver-Burke plot | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50519742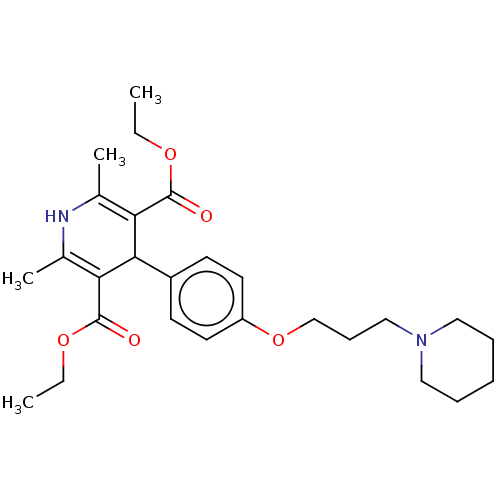 (CHEMBL4469017) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50519739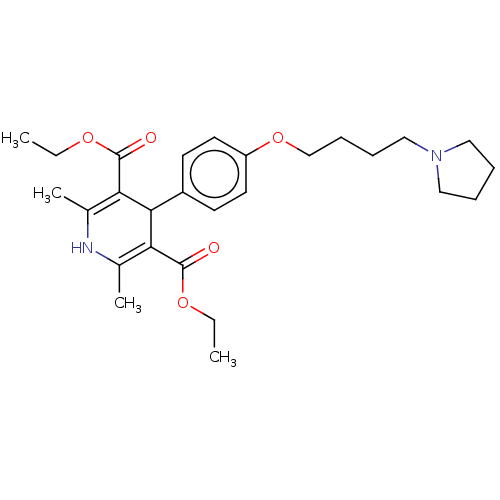 (CHEMBL4443523) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50519746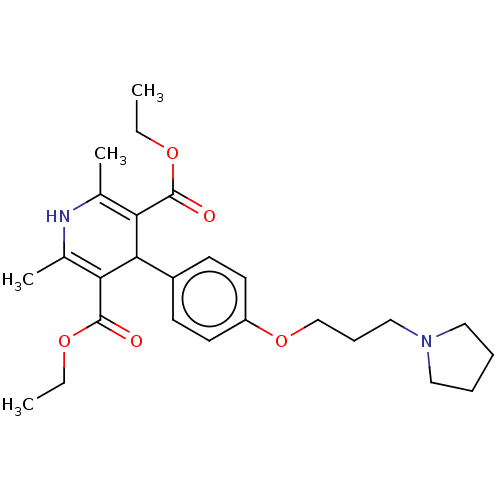 (CHEMBL4455698) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50519740 (CHEMBL4528980) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50519735 (CHEMBL4573309) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50357943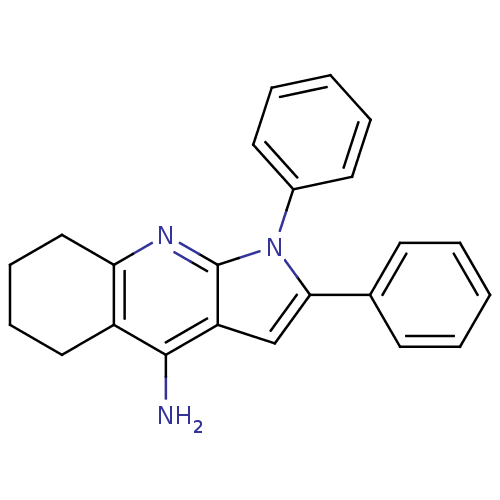 (CHEMBL1916768) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 621 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Mixed-type inhibition of Electrophorus electricus AChE assessed as hydrolysis of acetylthiocholine by Lineweaver-Burk plot analysis | Eur J Med Chem 46: 6119-30 (2011) Article DOI: 10.1016/j.ejmech.2011.09.038 BindingDB Entry DOI: 10.7270/Q27M08BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50519745 (CHEMBL4518949) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Bourgogne Franche-Comt£ Curated by ChEMBL | Assay Description Displacement of [3H]-N-alpha-methylhistamine from human H3 receptor expressed in HEK293 cell membranes incubated for 60 mins by scintillation countin... | J Med Chem 62: 11416-11422 (2019) Article DOI: 10.1021/acs.jmedchem.9b00937 BindingDB Entry DOI: 10.7270/Q2736V9G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Mus musculus) | BDBM50605051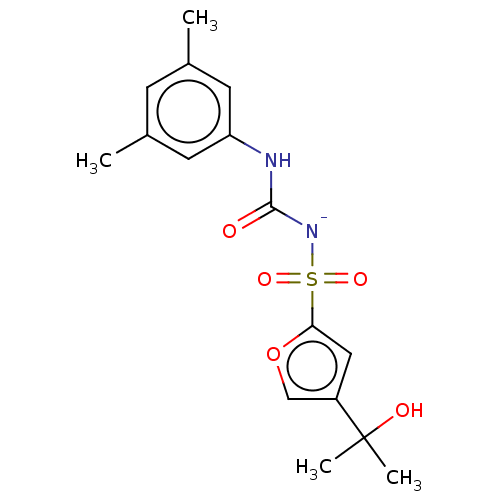 (CHEMBL5184952) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00149 BindingDB Entry DOI: 10.7270/Q2MP57D8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| NACHT, LRR and PYD domains-containing protein 3 (Mus musculus) | BDBM50155926 (CHEMBL3183703) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00149 BindingDB Entry DOI: 10.7270/Q2MP57D8 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138463 (CHEMBL3753957) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138438 (CHEMBL3752969) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50138279 (CHEMBL3752467) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138440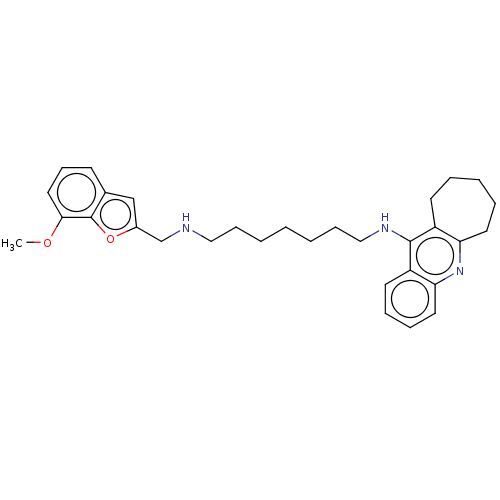 (CHEMBL3753405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550232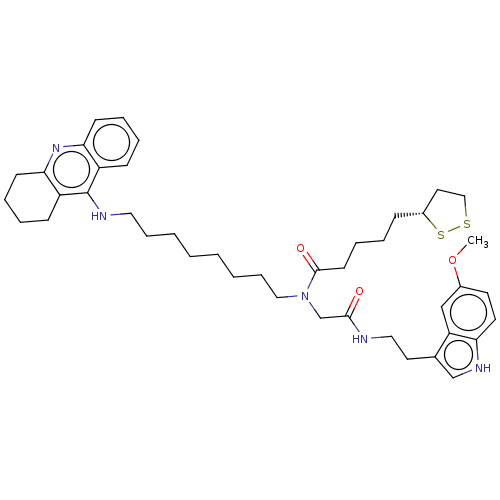 (CHEMBL4762083) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138437 (CHEMBL3752356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50336640 ((nifedipine) 2,6-Dimethyl-4-(2-nitro-phenyl)-1,4-d...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Complutense Curated by ChEMBL | Assay Description Inhibition of rat Cav1.2 channel in rat mesenteric artery assessed as relaxation of 70 mM K+ induced contraction | J Med Chem 57: 4313-23 (2014) Article DOI: 10.1021/jm500263v BindingDB Entry DOI: 10.7270/Q22R3T6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138279 (CHEMBL3752467) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550236 (CHEMBL4759893) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138462 (CHEMBL3754694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138464 (CHEMBL3754778) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138431 (CHEMBL3751941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138439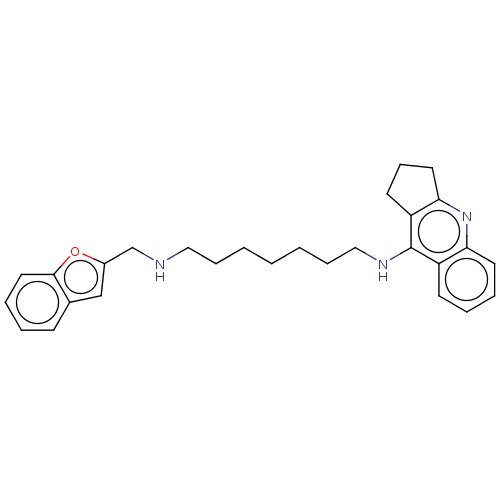 (CHEMBL3752543) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50138440 (CHEMBL3753405) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's ... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550234 (CHEMBL4784757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550235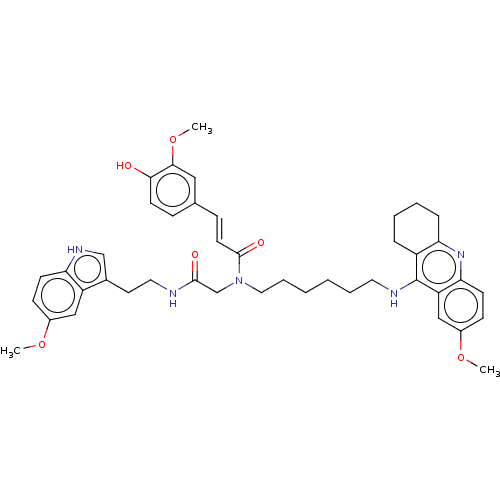 (CHEMBL4790426) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550232 (CHEMBL4762083) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550231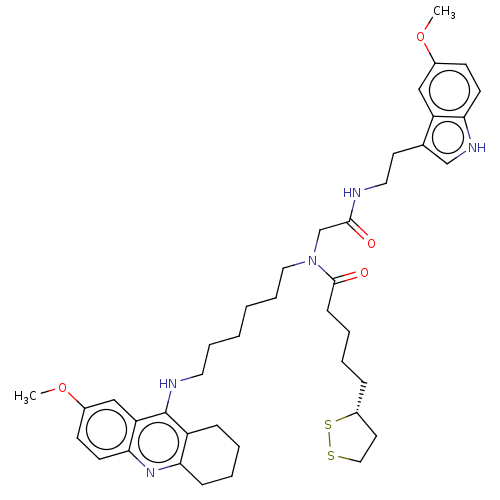 (CHEMBL4752488) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138286 (CHEMBL3752451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138284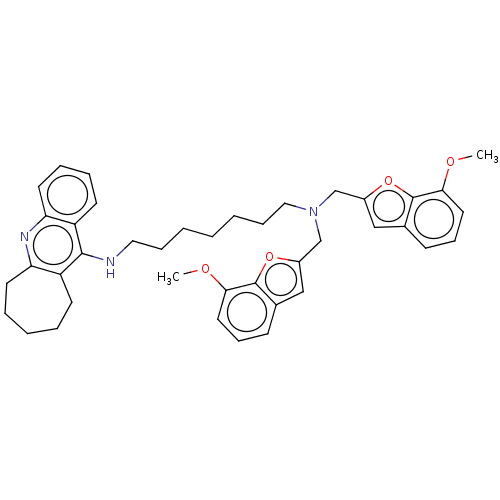 (CHEMBL3754005) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Lisbon Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butylthiocholine iodide as substrate preincubated for 10 mins before substrate addition by Ellman's method | Eur J Med Chem 46: 6119-30 (2011) Article DOI: 10.1016/j.ejmech.2011.09.038 BindingDB Entry DOI: 10.7270/Q27M08BS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550230 (CHEMBL4761802) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8963 (CHEMBL32823 | Homodimeric Tacrine Analog 3b | N-[7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138426 (CHEMBL3751866) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138282 (CHEMBL3753360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138429 (CHEMBL3752926) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550230 (CHEMBL4761802) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50550229 (CHEMBL4755679) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of horse serum BuChE pre-incubated for 10 mins before addition of butyrylthiocholine iodide substrate and further incubated for 15 mins by... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Autonoma de Madrid Curated by ChEMBL | Assay Description Inhibition of AChE in human erythrocytes by Ellman's method | J Med Chem 53: 5129-43 (2010) Article DOI: 10.1021/jm901902w BindingDB Entry DOI: 10.7270/Q25T3MFV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50550234 (CHEMBL4784757) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus AChE pre-incubated for 10 mins before addition of acetylthiocholine iodide substrate and further incubated for... | Citation and Details BindingDB Entry DOI: 10.7270/Q2WH2TMM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138450 (CHEMBL3754341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50138326 (CHEMBL3753850) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellman's metho... | J Med Chem 59: 114-31 (2016) Article DOI: 10.1021/acs.jmedchem.5b01119 BindingDB Entry DOI: 10.7270/Q2S75J5X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 312 total ) | Next | Last >> |