 Found 23885 hits with Last Name = 'ren' and Initial = 'j'
Found 23885 hits with Last Name = 'ren' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gastrin-releasing peptide receptor
(RAT) | BDBM50005601
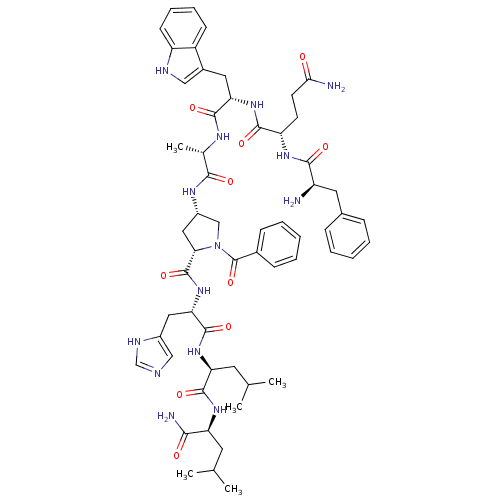
(CHEMBL2369819)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1C[C@@H](CN1C(=O)c1ccccc1)NC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc1ccccc1)C(N)=O Show InChI InChI=1S/C58H76N14O10/c1-32(2)22-44(50(61)74)68-55(79)45(23-33(3)4)69-56(80)47(26-38-29-62-31-64-38)71-57(81)48-27-39(30-72(48)58(82)36-16-10-7-11-17-36)66-51(75)34(5)65-54(78)46(25-37-28-63-42-19-13-12-18-40(37)42)70-53(77)43(20-21-49(60)73)67-52(76)41(59)24-35-14-8-6-9-15-35/h6-19,28-29,31-34,39,41,43-48,63H,20-27,30,59H2,1-5H3,(H2,60,73)(H2,61,74)(H,62,64)(H,65,78)(H,66,75)(H,67,76)(H,68,79)(H,69,80)(H,70,77)(H,71,81)/t34-,39-,41+,43-,44-,45-,46-,47-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Gastrin-releasing peptide receptor
(RAT) | BDBM50408923
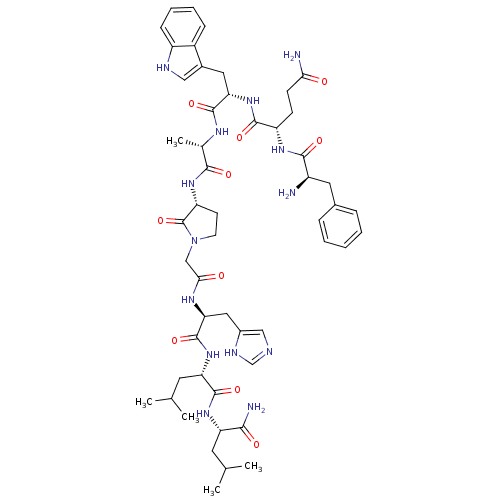
(CHEMBL2114155)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)CN1CC[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](N)Cc2ccccc2)C1=O)C(N)=O Show InChI InChI=1S/C52H72N14O10/c1-28(2)19-39(45(55)69)63-50(74)40(20-29(3)4)64-51(75)42(23-33-25-56-27-58-33)60-44(68)26-66-18-17-38(52(66)76)62-46(70)30(5)59-49(73)41(22-32-24-57-36-14-10-9-13-34(32)36)65-48(72)37(15-16-43(54)67)61-47(71)35(53)21-31-11-7-6-8-12-31/h6-14,24-25,27-30,35,37-42,57H,15-23,26,53H2,1-5H3,(H2,54,67)(H2,55,69)(H,56,58)(H,59,73)(H,60,68)(H,61,71)(H,62,70)(H,63,74)(H,64,75)(H,65,72)/t30-,35+,37-,38+,39-,40-,41-,42-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Universités Montpellier I & II
Curated by ChEMBL
| Assay Description
In vitro binding affinity against bombesin / GRP receptors on rat pancreatic acini. |
J Med Chem 43: 2356-61 (2000)
BindingDB Entry DOI: 10.7270/Q21J990K |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044868
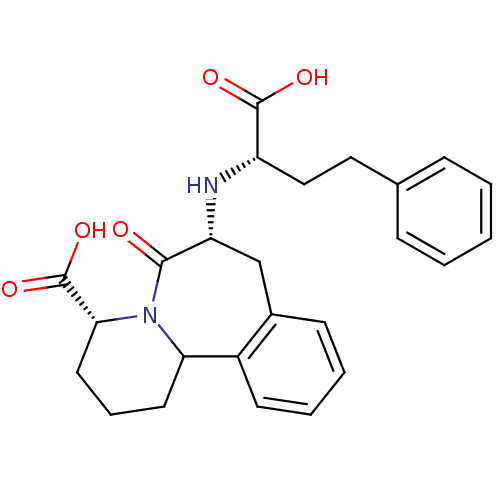
(7-(1-Carboxy-3-phenyl-propylamino)-6-oxo-1,2,3,4,6...)Show SMILES OC(=O)[C@H](CCc1ccccc1)N[C@@H]1Cc2ccccc2C2CCC[C@@H](N2C1=O)C(O)=O Show InChI InChI=1S/C25H28N2O5/c28-23-20(26-19(24(29)30)14-13-16-7-2-1-3-8-16)15-17-9-4-5-10-18(17)21-11-6-12-22(25(31)32)27(21)23/h1-5,7-10,19-22,26H,6,11-15H2,(H,29,30)(H,31,32)/t19-,20+,21?,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500526
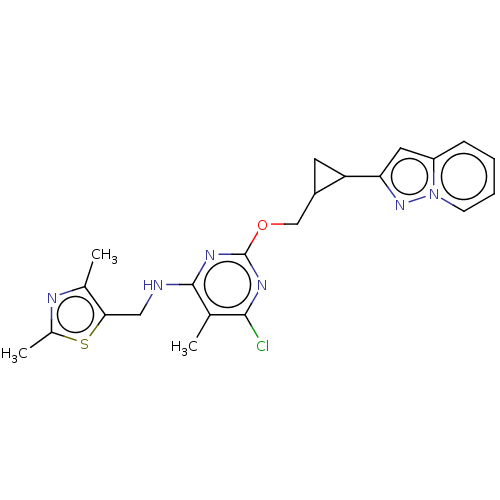
(CHEMBL3747517)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc4ccccn4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-12-20(23)26-22(27-21(12)24-10-19-13(2)25-14(3)31-19)30-11-15-8-17(15)18-9-16-6-4-5-7-29(16)28-18/h4-7,9,15,17H,8,10-11H2,1-3H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458766

(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.00680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126826
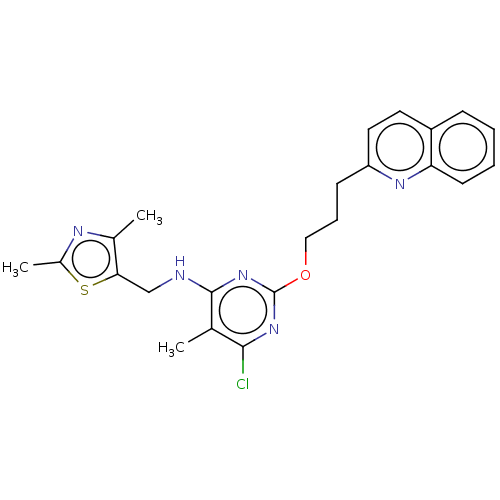
(US8785467, 1-29)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H24ClN5OS/c1-14-21(24)28-23(29-22(14)25-13-20-15(2)26-16(3)31-20)30-12-6-8-18-11-10-17-7-4-5-9-19(17)27-18/h4-5,7,9-11H,6,8,12-13H2,1-3H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500520

(CHEMBL3746993)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C24H24ClN5OS/c1-13-22(25)29-24(30-23(13)26-11-21-14(2)27-15(3)32-21)31-12-17-10-18(17)20-9-8-16-6-4-5-7-19(16)28-20/h4-9,17-18H,10-12H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500521

(CHEMBL3746277)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc(C)ccn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H24ClN5OS/c1-11-5-6-23-17(7-11)16-8-15(16)10-28-21-26-19(22)12(2)20(27-21)24-9-18-13(3)25-14(4)29-18/h5-7,15-16H,8-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500538

(CHEMBL3745790)Show SMILES COc1ccnc(c1)C1CC1COc1nc(Cl)c(C)c(NCc2sc(C)nc2C)n1 Show InChI InChI=1S/C21H24ClN5O2S/c1-11-19(22)26-21(27-20(11)24-9-18-12(2)25-13(3)30-18)29-10-14-7-16(14)17-8-15(28-4)5-6-23-17/h5-6,8,14,16H,7,9-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Mast cell carboxypeptidase A
(Rattus norvegicus) | BDBM50530230

(CHEMBL4445882)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)COCCOCCNC(=O)CCOCCOCCOCCNC(=O)CCc1ccc(cc1)\C(\C=C\C1=[N+](CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S(O)(=O)=O)=C/C=C1/N(CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S([O-])(=O)=O)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r,c:54| Show InChI InChI=1S/C87H109N6O32PS6/c1-58(126(101,102)57-64(85(99)100)23-17-59-13-7-6-8-14-59)90-84(98)72(51-61-18-26-65(94)27-19-61)91-81(97)56-125-48-47-123-42-37-89-80(96)35-40-121-43-45-124-46-44-122-41-36-88-79(95)34-20-60-15-21-62(22-16-60)63(24-32-77-86(2,3)82-70-52-66(129(109,110)111)54-75(131(115,116)117)68(70)28-30-73(82)92(77)38-9-11-49-127(103,104)105)25-33-78-87(4,5)83-71-53-67(130(112,113)114)55-76(132(118,119)120)69(71)29-31-74(83)93(78)39-10-12-50-128(106,107)108/h6-8,13-16,18-19,21-22,24-33,52-55,58,64,72H,9-12,17,20,23,34-51,56-57H2,1-5H3,(H12-,88,89,90,91,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120)/t58-,64-,72+/m1/s1 | MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of rat CPA3 using AAFP as substrate preincubated for 45 mins measured at 30 sec intervals for 15 mins by UV/vis-spectrophotometry |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
Mast cell carboxypeptidase A
(Rattus norvegicus) | BDBM50530230

(CHEMBL4445882)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)COCCOCCNC(=O)CCOCCOCCOCCNC(=O)CCc1ccc(cc1)\C(\C=C\C1=[N+](CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S(O)(=O)=O)=C/C=C1/N(CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S([O-])(=O)=O)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r,c:54| Show InChI InChI=1S/C87H109N6O32PS6/c1-58(126(101,102)57-64(85(99)100)23-17-59-13-7-6-8-14-59)90-84(98)72(51-61-18-26-65(94)27-19-61)91-81(97)56-125-48-47-123-42-37-89-80(96)35-40-121-43-45-124-46-44-122-41-36-88-79(95)34-20-60-15-21-62(22-16-60)63(24-32-77-86(2,3)82-70-52-66(129(109,110)111)54-75(131(115,116)117)68(70)28-30-73(82)92(77)38-9-11-49-127(103,104)105)25-33-78-87(4,5)83-71-53-67(130(112,113)114)55-76(132(118,119)120)69(71)29-31-74(83)93(78)39-10-12-50-128(106,107)108/h6-8,13-16,18-19,21-22,24-33,52-55,58,64,72H,9-12,17,20,23,34-51,56-57H2,1-5H3,(H12-,88,89,90,91,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120)/t58-,64-,72+/m1/s1 | MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of rat CPA3 using AAFP as substrate preincubated for 45 mins measured at 30 sec intervals for 15 mins by UV/vis-spectrophotometry |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500535

(CHEMBL3747450)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cn(C)cn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C19H23ClN6OS/c1-10-17(20)24-19(25-18(10)21-6-16-11(2)23-12(3)28-16)27-8-13-5-14(13)15-7-26(4)9-22-15/h7,9,13-14H,5-6,8H2,1-4H3,(H,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126825
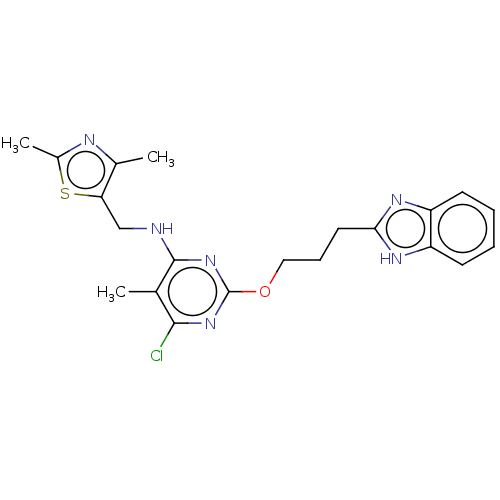
(US8785467, 1-27)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3nc4ccccc4[nH]3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H23ClN6OS/c1-12-19(22)27-21(28-20(12)23-11-17-13(2)24-14(3)30-17)29-10-6-9-18-25-15-7-4-5-8-16(15)26-18/h4-5,7-8H,6,9-11H2,1-3H3,(H,25,26)(H,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500519
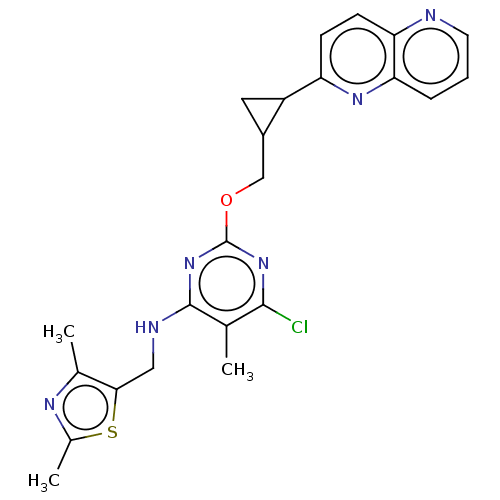
(CHEMBL3746917)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ncccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H23ClN6OS/c1-12-21(24)29-23(30-22(12)26-10-20-13(2)27-14(3)32-20)31-11-15-9-16(15)17-6-7-18-19(28-17)5-4-8-25-18/h4-8,15-16H,9-11H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125967

(CHEMBL3627846)Show SMILES Cc1ccc2c(c1)nc(CCN1C(=O)c3ccccc3C1=O)n(-c1ccc3n(C)ncc3c1)c2=O Show InChI InChI=1S/C27H21N5O3/c1-16-7-9-21-22(13-16)29-24(11-12-31-25(33)19-5-3-4-6-20(19)26(31)34)32(27(21)35)18-8-10-23-17(14-18)15-28-30(23)2/h3-10,13-15H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823
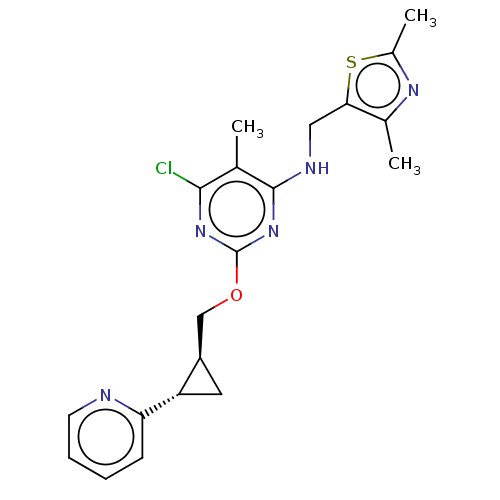
(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823

(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
Tolloid-like protein 1
(Homo sapiens) | BDBM50458766

(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tolloid-like protein 2
(Homo sapiens) | BDBM50458766

(CHEMBL4212386)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1ccc(C(=O)N[C@@H](CC(O)=O)C(O)=O)c(OCC)c1 |r| Show InChI InChI=1S/C30H40N4O11/c1-4-7-8-9-19(22(5-2)34(43)17-35)27(38)31-16-32-29(40)24-13-12-23(45-24)18-10-11-20(25(14-18)44-6-3)28(39)33-21(30(41)42)15-26(36)37/h10-14,17,19,21-22,43H,4-9,15-16H2,1-3H3,(H,31,38)(H,32,40)(H,33,39)(H,36,37)(H,41,42)/t19-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to TLL2 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate incubated for 3.5 hrs followed by subst... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530229
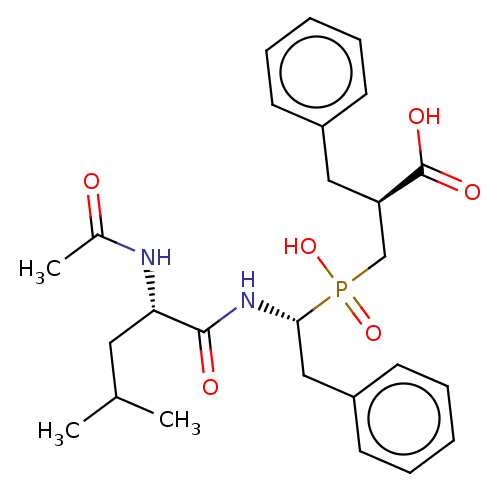
(CHEMBL4460840)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)P(O)(=O)C[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C26H35N2O6P/c1-18(2)14-23(27-19(3)29)25(30)28-24(16-21-12-8-5-9-13-21)35(33,34)17-22(26(31)32)15-20-10-6-4-7-11-20/h4-13,18,22-24H,14-17H2,1-3H3,(H,27,29)(H,28,30)(H,31,32)(H,33,34)/t22-,23+,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50458771

(CHEMBL4214046)Show SMILES CCCCC[C@H]([C@@H](CC)N(O)C=O)C(=O)NCNC(=O)c1ccc(o1)-c1cc(OCC)cc(c1)P(O)(O)=O |r| Show InChI InChI=1S/C25H36N3O9P/c1-4-7-8-9-20(21(5-2)28(32)16-29)24(30)26-15-27-25(31)23-11-10-22(37-23)17-12-18(36-6-3)14-19(13-17)38(33,34)35/h10-14,16,20-21,32H,4-9,15H2,1-3H3,(H,26,30)(H,27,31)(H2,33,34,35)/t20-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to BMP1 (unknown origin) using ((5-FAM)-ELIDQYDVQRDDSSDGSLED-K(5,6 TAMRA)-CONH2 as substrate preincubated for 3 hrs followed by subs... |
ACS Med Chem Lett 9: 736-740 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00173
BindingDB Entry DOI: 10.7270/Q2X35127 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530229

(CHEMBL4460840)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)P(O)(=O)C[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C26H35N2O6P/c1-18(2)14-23(27-19(3)29)25(30)28-24(16-21-12-8-5-9-13-21)35(33,34)17-22(26(31)32)15-20-10-6-4-7-11-20/h4-13,18,22-24H,14-17H2,1-3H3,(H,27,29)(H,28,30)(H,31,32)(H,33,34)/t22-,23+,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126827
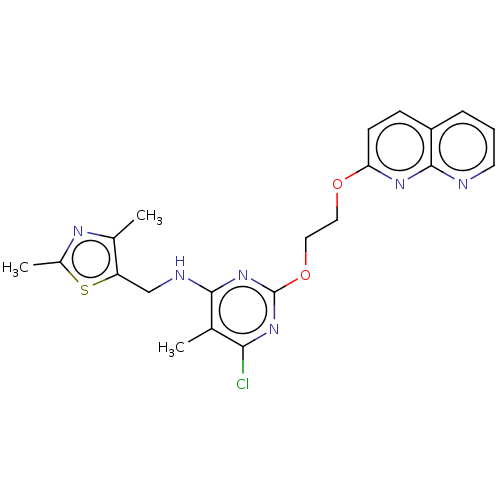
(US8785467, 1-32)Show SMILES Cc1nc(C)c(CNc2nc(OCCOc3ccc4cccnc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H21ClN6O2S/c1-12-18(22)27-21(28-19(12)24-11-16-13(2)25-14(3)31-16)30-10-9-29-17-7-6-15-5-4-8-23-20(15)26-17/h4-8H,9-11H2,1-3H3,(H,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124648
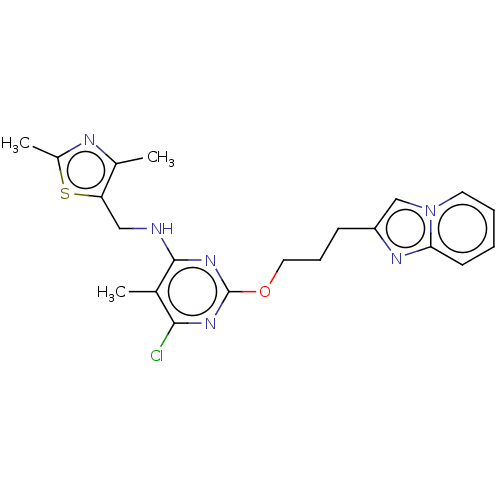
(CHEMBL3622901)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3cn4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H23ClN6OS/c1-13-19(22)26-21(27-20(13)23-11-17-14(2)24-15(3)30-17)29-10-6-7-16-12-28-9-5-4-8-18(28)25-16/h4-5,8-9,12H,6-7,10-11H2,1-3H3,(H,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124649

(CHEMBL3622902)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3nc4ccccc4s3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H22ClN5OS2/c1-12-19(22)26-21(27-20(12)23-11-17-13(2)24-14(3)29-17)28-10-6-9-18-25-15-7-4-5-8-16(15)30-18/h4-5,7-8H,6,9-11H2,1-3H3,(H,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50071654
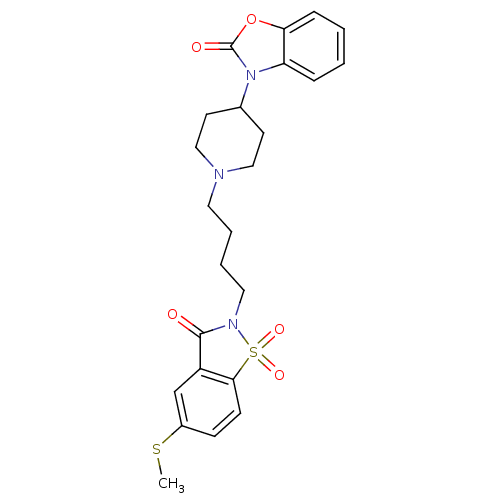
(3-{1-[4-(5-Methylsulfanyl-1,1,3-trioxo-1,3-dihydro...)Show SMILES CSc1ccc2c(c1)C(=O)N(CCCCN1CCC(CC1)n1c3ccccc3oc1=O)S2(=O)=O Show InChI InChI=1S/C24H27N3O5S2/c1-33-18-8-9-22-19(16-18)23(28)26(34(22,30)31)13-5-4-12-25-14-10-17(11-15-25)27-20-6-2-3-7-21(20)32-24(27)29/h2-3,6-9,16-17H,4-5,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... |
Bioorg Med Chem Lett 8: 2467-72 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23K1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50221547
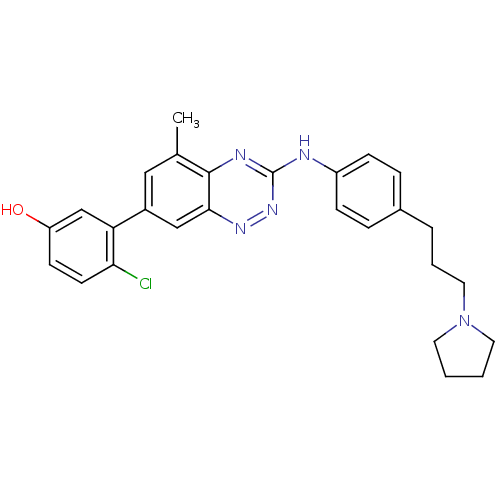
(4-chloro-3-(5-methyl-3-(4-(3-(pyrrolidin-1-yl)prop...)Show SMILES Cc1cc(cc2nnc(Nc3ccc(CCCN4CCCC4)cc3)nc12)-c1cc(O)ccc1Cl Show InChI InChI=1S/C27H28ClN5O/c1-18-15-20(23-17-22(34)10-11-24(23)28)16-25-26(18)30-27(32-31-25)29-21-8-6-19(7-9-21)5-4-14-33-12-2-3-13-33/h6-11,15-17,34H,2-5,12-14H2,1H3,(H,29,30,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TargeGen, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 5812-8 (2007)
Article DOI: 10.1016/j.bmcl.2007.08.043
BindingDB Entry DOI: 10.7270/Q2M32VGM |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50071655
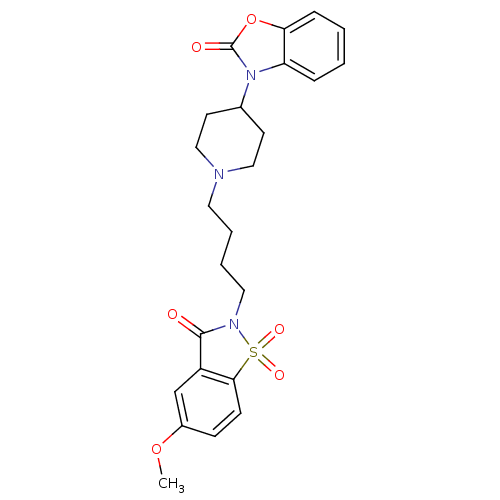
(3-{1-[4-(5-Methoxy-1,1,3-trioxo-1,3-dihydro-1lambd...)Show SMILES COc1ccc2c(c1)C(=O)N(CCCCN1CCC(CC1)n1c3ccccc3oc1=O)S2(=O)=O Show InChI InChI=1S/C24H27N3O6S/c1-32-18-8-9-22-19(16-18)23(28)26(34(22,30)31)13-5-4-12-25-14-10-17(11-15-25)27-20-6-2-3-7-21(20)33-24(27)29/h2-3,6-9,16-17H,4-5,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... |
Bioorg Med Chem Lett 8: 2467-72 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23K1 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50444605
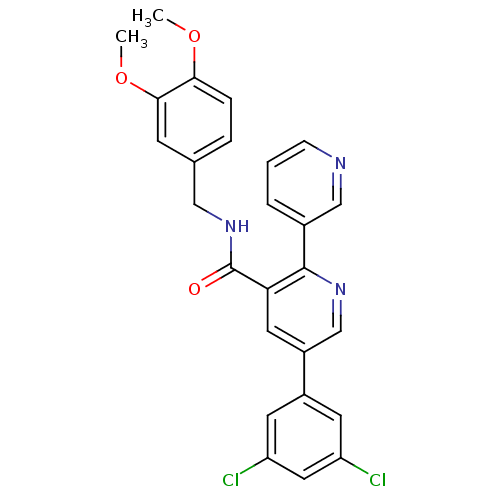
(CHEMBL3099899)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2cccnc2)-c2cc(Cl)cc(Cl)c2)cc1OC Show InChI InChI=1S/C26H21Cl2N3O3/c1-33-23-6-5-16(8-24(23)34-2)13-31-26(32)22-11-19(18-9-20(27)12-21(28)10-18)15-30-25(22)17-4-3-7-29-14-17/h3-12,14-15H,13H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 23: 6620-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.045
BindingDB Entry DOI: 10.7270/Q2WH2RFJ |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530230

(CHEMBL4445882)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)COCCOCCNC(=O)CCOCCOCCOCCNC(=O)CCc1ccc(cc1)\C(\C=C\C1=[N+](CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S(O)(=O)=O)=C/C=C1/N(CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S([O-])(=O)=O)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r,c:54| Show InChI InChI=1S/C87H109N6O32PS6/c1-58(126(101,102)57-64(85(99)100)23-17-59-13-7-6-8-14-59)90-84(98)72(51-61-18-26-65(94)27-19-61)91-81(97)56-125-48-47-123-42-37-89-80(96)35-40-121-43-45-124-46-44-122-41-36-88-79(95)34-20-60-15-21-62(22-16-60)63(24-32-77-86(2,3)82-70-52-66(129(109,110)111)54-75(131(115,116)117)68(70)28-30-73(82)92(77)38-9-11-49-127(103,104)105)25-33-78-87(4,5)83-71-53-67(130(112,113)114)55-76(132(118,119)120)69(71)29-31-74(83)93(78)39-10-12-50-128(106,107)108/h6-8,13-16,18-19,21-22,24-33,52-55,58,64,72H,9-12,17,20,23,34-51,56-57H2,1-5H3,(H12-,88,89,90,91,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120)/t58-,64-,72+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530230

(CHEMBL4445882)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)COCCOCCNC(=O)CCOCCOCCOCCNC(=O)CCc1ccc(cc1)\C(\C=C\C1=[N+](CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S(O)(=O)=O)=C/C=C1/N(CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S([O-])(=O)=O)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r,c:54| Show InChI InChI=1S/C87H109N6O32PS6/c1-58(126(101,102)57-64(85(99)100)23-17-59-13-7-6-8-14-59)90-84(98)72(51-61-18-26-65(94)27-19-61)91-81(97)56-125-48-47-123-42-37-89-80(96)35-40-121-43-45-124-46-44-122-41-36-88-79(95)34-20-60-15-21-62(22-16-60)63(24-32-77-86(2,3)82-70-52-66(129(109,110)111)54-75(131(115,116)117)68(70)28-30-73(82)92(77)38-9-11-49-127(103,104)105)25-33-78-87(4,5)83-71-53-67(130(112,113)114)55-76(132(118,119)120)69(71)29-31-74(83)93(78)39-10-12-50-128(106,107)108/h6-8,13-16,18-19,21-22,24-33,52-55,58,64,72H,9-12,17,20,23,34-51,56-57H2,1-5H3,(H12-,88,89,90,91,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120)/t58-,64-,72+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate measured at pre-steady state by spectrophotometry |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM50109021

((R)-8-(Benzylamino-methyl)-3,6,7,8-tetrahydro-1H-c...)Show SMILES O=c1[nH]c2ccc3CC[C@H](CNCc4ccccc4)Oc3c2[nH]1 Show InChI InChI=1S/C18H19N3O2/c22-18-20-15-9-7-13-6-8-14(23-17(13)16(15)21-18)11-19-10-12-4-2-1-3-5-12/h1-5,7,9,14,19H,6,8,10-11H2,(H2,20,21,22)/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity of compound for D2 receptor in rat striatal membrane determined for agonist state (high affinity state, D2 High) [3H]quinpirole as radioliga... |
Bioorg Med Chem Lett 12: 271-4 (2002)
BindingDB Entry DOI: 10.7270/Q22J6CDZ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600
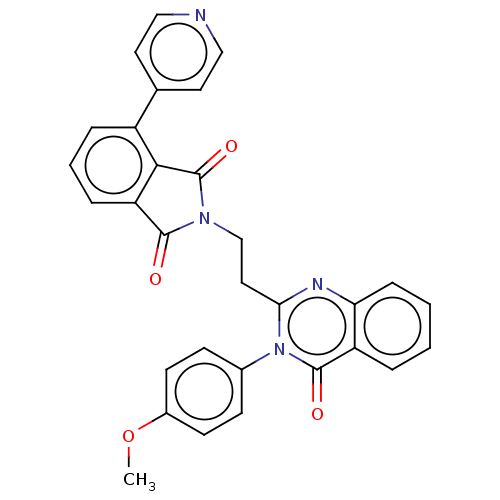
(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50071647
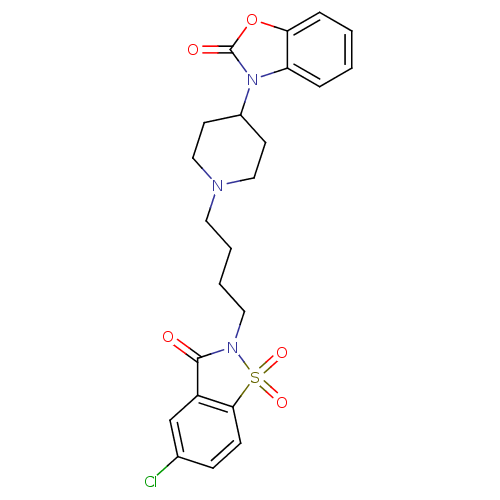
(3-{1-[4-(5-Chloro-1,1,3-trioxo-1,3-dihydro-1lambda...)Show SMILES Clc1ccc2c(c1)C(=O)N(CCCCN1CCC(CC1)n1c3ccccc3oc1=O)S2(=O)=O Show InChI InChI=1S/C23H24ClN3O5S/c24-16-7-8-21-18(15-16)22(28)26(33(21,30)31)12-4-3-11-25-13-9-17(10-14-25)27-19-5-1-2-6-20(19)32-23(27)29/h1-2,5-8,15,17H,3-4,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its affinity for Alpha-1a adrenergic receptor in human aorta preparations |
Bioorg Med Chem Lett 8: 2467-72 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23K1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50044866
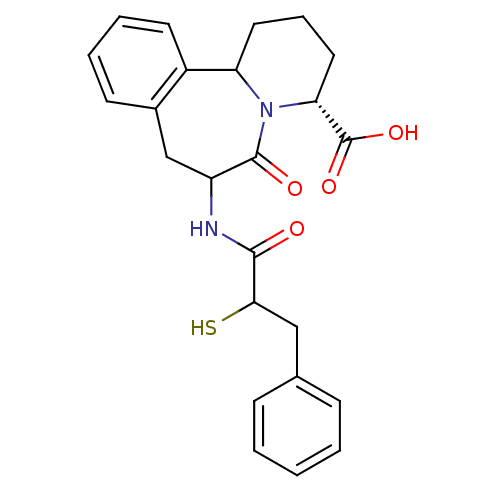
(7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)C(Cc1ccccc21)NC(=O)C(S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Rattus norvegicus (Rat)) | BDBM50044866

(7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)C(Cc1ccccc21)NC(=O)C(S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18?,19?,20-,21?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rat kidney neutral endopeptidase (NEP) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530235

(CHEMBL4436298)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C27H37N2O6P/c1-19(2)16-24(28-20(3)30)26(31)29-25(17-22-12-8-5-9-13-22)36(34,35)18-23(27(32)33)15-14-21-10-6-4-7-11-21/h4-13,19,23-25H,14-18H2,1-3H3,(H,28,30)(H,29,31)(H,32,33)(H,34,35)/t23-,24+,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530235

(CHEMBL4436298)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C27H37N2O6P/c1-19(2)16-24(28-20(3)30)26(31)29-25(17-22-12-8-5-9-13-22)36(34,35)18-23(27(32)33)15-14-21-10-6-4-7-11-21/h4-13,19,23-25H,14-18H2,1-3H3,(H,28,30)(H,29,31)(H,32,33)(H,34,35)/t23-,24+,25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50071652
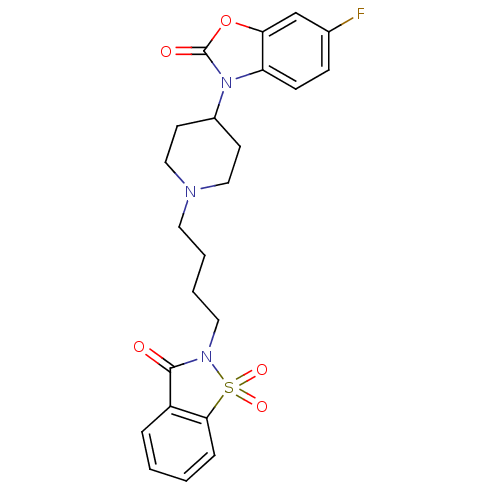
(6-Fluoro-3-{1-[4-(1,1,3-trioxo-1,3-dihydro-1lambda...)Show SMILES Fc1ccc2n(C3CCN(CCCCN4C(=O)c5ccccc5S4(=O)=O)CC3)c(=O)oc2c1 Show InChI InChI=1S/C23H24FN3O5S/c24-16-7-8-19-20(15-16)32-23(29)27(19)17-9-13-25(14-10-17)11-3-4-12-26-22(28)18-5-1-2-6-21(18)33(26,30)31/h1-2,5-8,15,17H,3-4,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylamino methyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in... |
Bioorg Med Chem Lett 8: 2467-72 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23K1 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530231
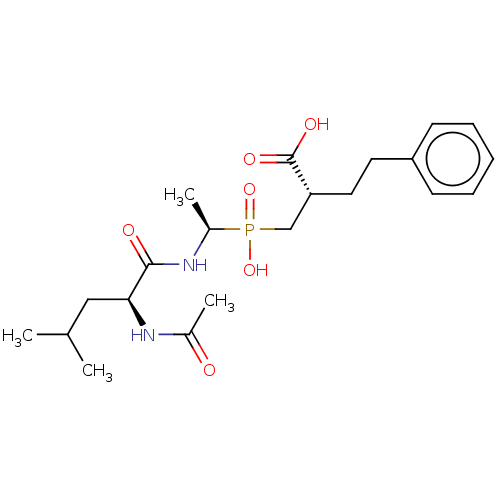
(CHEMBL4528407)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](C)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C21H33N2O6P/c1-14(2)12-19(22-15(3)24)20(25)23-16(4)30(28,29)13-18(21(26)27)11-10-17-8-6-5-7-9-17/h5-9,14,16,18-19H,10-13H2,1-4H3,(H,22,24)(H,23,25)(H,26,27)(H,28,29)/t16-,18-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530231

(CHEMBL4528407)Show SMILES CC(C)C[C@H](NC(C)=O)C(=O)N[C@@H](C)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C21H33N2O6P/c1-14(2)12-19(22-15(3)24)20(25)23-16(4)30(28,29)13-18(21(26)27)11-10-17-8-6-5-7-9-17/h5-9,14,16,18-19H,10-13H2,1-4H3,(H,22,24)(H,23,25)(H,26,27)(H,28,29)/t16-,18-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530230

(CHEMBL4445882)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)COCCOCCNC(=O)CCOCCOCCOCCNC(=O)CCc1ccc(cc1)\C(\C=C\C1=[N+](CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S(O)(=O)=O)=C/C=C1/N(CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S([O-])(=O)=O)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r,c:54| Show InChI InChI=1S/C87H109N6O32PS6/c1-58(126(101,102)57-64(85(99)100)23-17-59-13-7-6-8-14-59)90-84(98)72(51-61-18-26-65(94)27-19-61)91-81(97)56-125-48-47-123-42-37-89-80(96)35-40-121-43-45-124-46-44-122-41-36-88-79(95)34-20-60-15-21-62(22-16-60)63(24-32-77-86(2,3)82-70-52-66(129(109,110)111)54-75(131(115,116)117)68(70)28-30-73(82)92(77)38-9-11-49-127(103,104)105)25-33-78-87(4,5)83-71-53-67(130(112,113)114)55-76(132(118,119)120)69(71)29-31-74(83)93(78)39-10-12-50-128(106,107)108/h6-8,13-16,18-19,21-22,24-33,52-55,58,64,72H,9-12,17,20,23,34-51,56-57H2,1-5H3,(H12-,88,89,90,91,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120)/t58-,64-,72+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530230

(CHEMBL4445882)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)COCCOCCNC(=O)CCOCCOCCOCCNC(=O)CCc1ccc(cc1)\C(\C=C\C1=[N+](CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S(O)(=O)=O)=C/C=C1/N(CCCCS(O)(=O)=O)c2ccc3c(cc(cc3c2C1(C)C)S(O)(=O)=O)S([O-])(=O)=O)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r,c:54| Show InChI InChI=1S/C87H109N6O32PS6/c1-58(126(101,102)57-64(85(99)100)23-17-59-13-7-6-8-14-59)90-84(98)72(51-61-18-26-65(94)27-19-61)91-81(97)56-125-48-47-123-42-37-89-80(96)35-40-121-43-45-124-46-44-122-41-36-88-79(95)34-20-60-15-21-62(22-16-60)63(24-32-77-86(2,3)82-70-52-66(129(109,110)111)54-75(131(115,116)117)68(70)28-30-73(82)92(77)38-9-11-49-127(103,104)105)25-33-78-87(4,5)83-71-53-67(130(112,113)114)55-76(132(118,119)120)69(71)29-31-74(83)93(78)39-10-12-50-128(106,107)108/h6-8,13-16,18-19,21-22,24-33,52-55,58,64,72H,9-12,17,20,23,34-51,56-57H2,1-5H3,(H12-,88,89,90,91,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120)/t58-,64-,72+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50374443
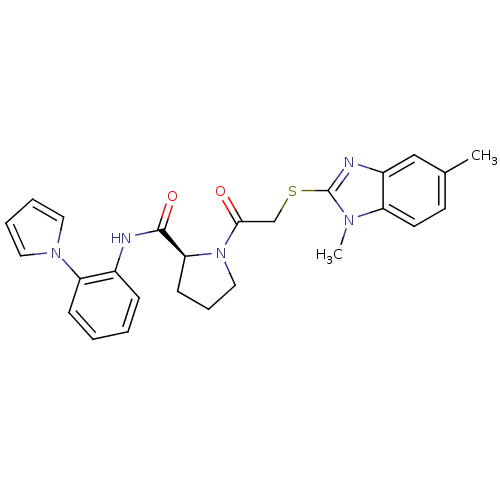
(CHEMBL272715)Show SMILES Cc1ccc2n(C)c(SCC(=O)N3CCC[C@H]3C(=O)Nc3ccccc3-n3cccc3)nc2c1 Show InChI InChI=1S/C26H27N5O2S/c1-18-11-12-21-20(16-18)28-26(29(21)2)34-17-24(32)31-15-7-10-23(31)25(33)27-19-8-3-4-9-22(19)30-13-5-6-14-30/h3-6,8-9,11-14,16,23H,7,10,15,17H2,1-2H3,(H,27,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
Bioorg Med Chem Lett 18: 1425-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.001
BindingDB Entry DOI: 10.7270/Q2N87BM4 |
More data for this
Ligand-Target Pair | |
Alpha-1A adrenergic receptor
(Homo sapiens (Human)) | BDBM50071651
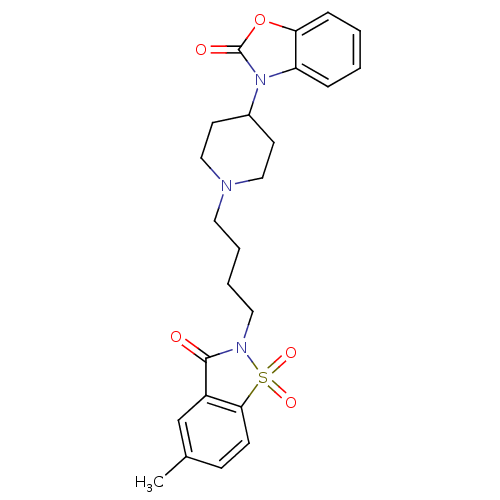
(3-{1-[4-(5-Methyl-1,1,3-trioxo-1,3-dihydro-1lambda...)Show SMILES Cc1ccc2c(c1)C(=O)N(CCCCN1CCC(CC1)n1c3ccccc3oc1=O)S2(=O)=O Show InChI InChI=1S/C24H27N3O5S/c1-17-8-9-22-19(16-17)23(28)26(33(22,30)31)13-5-4-12-25-14-10-18(11-15-25)27-20-6-2-3-7-21(20)32-24(27)29/h2-3,6-9,16,18H,4-5,10-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Ability to displace beta ([125I]-iodo-4-hydroxyphenyl)-ethylaminomethyl tetralone from human cloned Alpha-1a adrenergic receptor stably expressed in ... |
Bioorg Med Chem Lett 8: 2467-72 (1999)
BindingDB Entry DOI: 10.7270/Q2NP23K1 |
More data for this
Ligand-Target Pair | |
Neuraminidase
(Influenza A virus) | BDBM4994

((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...)Show SMILES CCC(CC)O[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:7| Show InChI InChI=1S/C14H24N2O4/c1-4-10(5-2)20-12-7-9(14(18)19)6-11(15)13(12)16-8(3)17/h7,10-13H,4-6,15H2,1-3H3,(H,16,17)(H,18,19)/t11-,12+,13+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of Influenza A virus (A/Thailand/1(KAN-1)/2004(H5N1)) neuraminidase by by Michaelis Menten equation analysis |
Antimicrob Agents Chemother 53: 3088-96 (2009)
Article DOI: 10.1128/AAC.01667-08
BindingDB Entry DOI: 10.7270/Q2VM4CHC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50374442
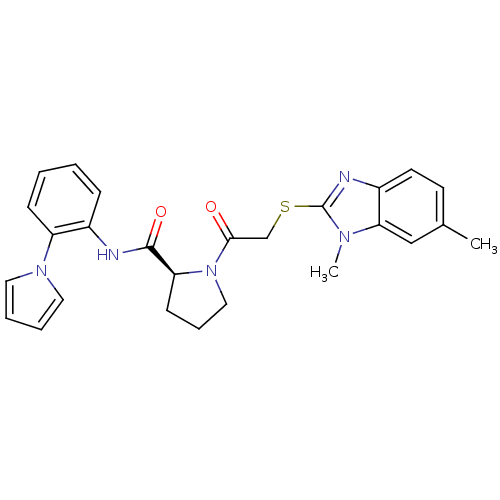
(CHEMBL255763)Show SMILES Cc1ccc2nc(SCC(=O)N3CCC[C@H]3C(=O)Nc3ccccc3-n3cccc3)n(C)c2c1 Show InChI InChI=1S/C26H27N5O2S/c1-18-11-12-20-23(16-18)29(2)26(28-20)34-17-24(32)31-15-7-10-22(31)25(33)27-19-8-3-4-9-21(19)30-13-5-6-14-30/h3-6,8-9,11-14,16,22H,7,10,15,17H2,1-2H3,(H,27,33)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
Bioorg Med Chem Lett 18: 1425-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.001
BindingDB Entry DOI: 10.7270/Q2N87BM4 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530228

(CHEMBL4483485)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H31N2O7P/c1-16(27)25-22(14-19-9-12-21(28)13-10-19)23(29)26-17(2)34(32,33)15-20(24(30)31)11-8-18-6-4-3-5-7-18/h3-7,9-10,12-13,17,20,22,28H,8,11,14-15H2,1-2H3,(H,25,27)(H,26,29)(H,30,31)(H,32,33)/t17-,20-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50530228

(CHEMBL4483485)Show SMILES C[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)P(O)(=O)C[C@@H](CCc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C24H31N2O7P/c1-16(27)25-22(14-19-9-12-21(28)13-10-19)23(29)26-17(2)34(32,33)15-20(24(30)31)11-8-18-6-4-3-5-7-18/h3-7,9-10,12-13,17,20,22,28H,8,11,14-15H2,1-2H3,(H,25,27)(H,26,29)(H,30,31)(H,32,33)/t17-,20-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat Aut£noma de Barcelona
Curated by ChEMBL
| Assay Description
Inhibition of human CPA1 using AAFP as substrate preincubated for 45 mins to 2 hrs measured at 30 sec intervals for 15 mins by UV/vis-spectrophotomet... |
J Med Chem 62: 1917-1931 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01465
BindingDB Entry DOI: 10.7270/Q2K077R9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50044866

(7-(2-Mercapto-3-phenyl-propionylamino)-6-oxo-1,2,3...)Show SMILES OC(=O)[C@H]1CCCC2N1C(=O)C(Cc1ccccc21)NC(=O)C(S)Cc1ccccc1 Show InChI InChI=1S/C24H26N2O4S/c27-22(21(31)13-15-7-2-1-3-8-15)25-18-14-16-9-4-5-10-17(16)19-11-6-12-20(24(29)30)26(19)23(18)28/h1-5,7-10,18-21,31H,6,11-14H2,(H,25,27)(H,29,30)/t18?,19?,20-,21?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Marion Merrell Dow Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity of compound against rabbit lung angiotensin I-converting enzyme (ACE) was determined |
J Med Chem 36: 2420-3 (1993)
BindingDB Entry DOI: 10.7270/Q22N51C1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data