

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451155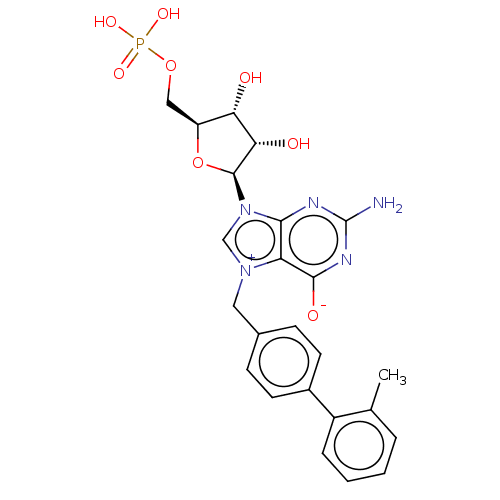 (US10676499, Example 49) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451145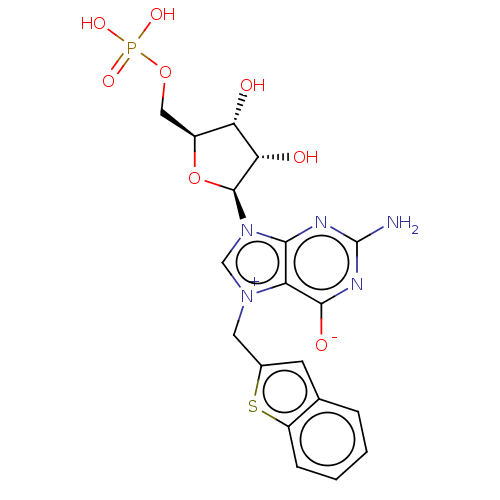 (US10676499, Example 17) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451159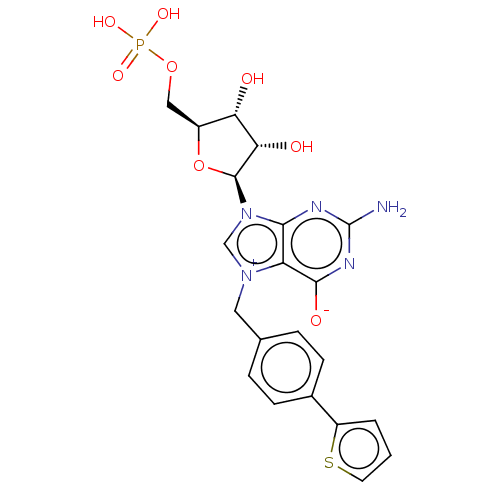 (US10676499, Example 57) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451148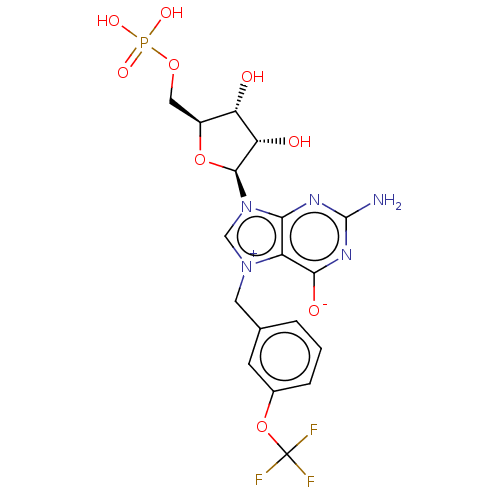 (US10676499, Example 29) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451158 (US10676499, Example 55) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451147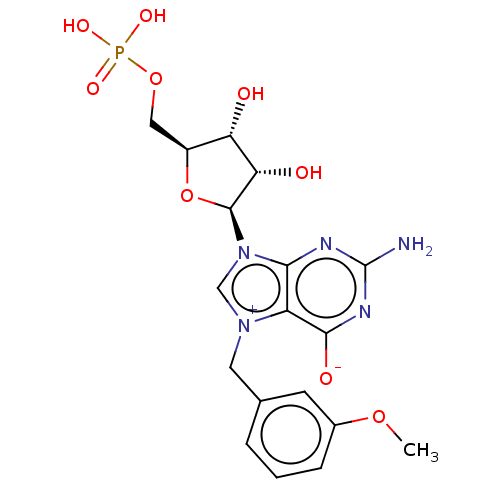 (US10676499, Example 26) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451144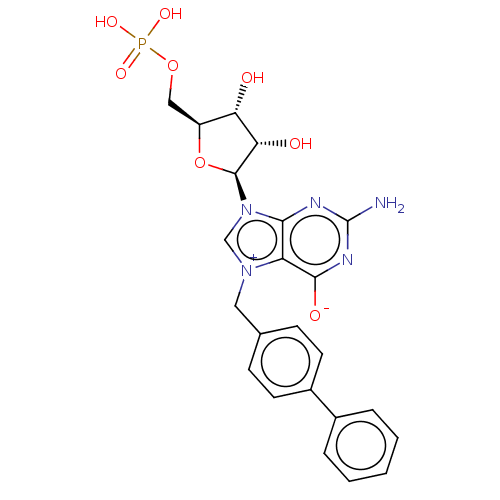 (US10676499, Example 1) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451150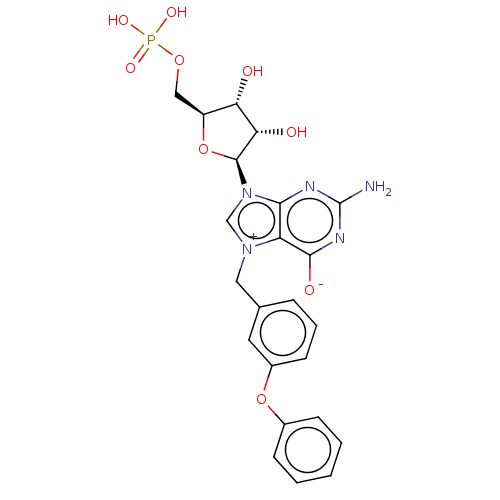 (US10676499, Example 33) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451146 (US10676499, Example 18) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451156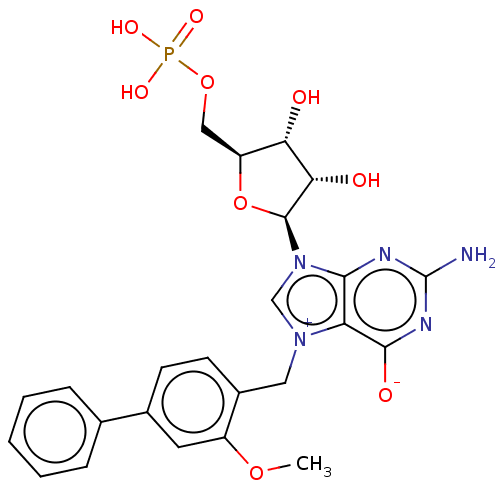 (US10676499, Example 53) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451160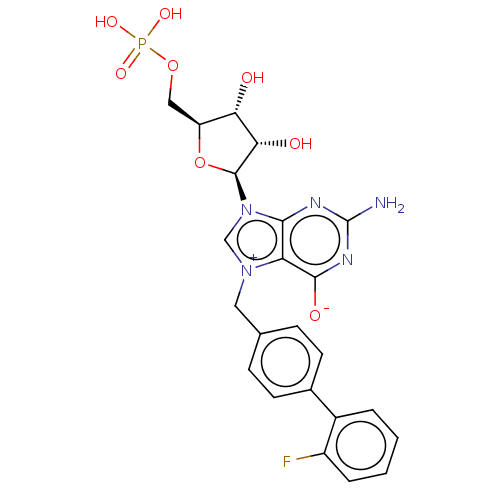 (US10676499, Example 58) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451151 (US10676499, Example 40) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451157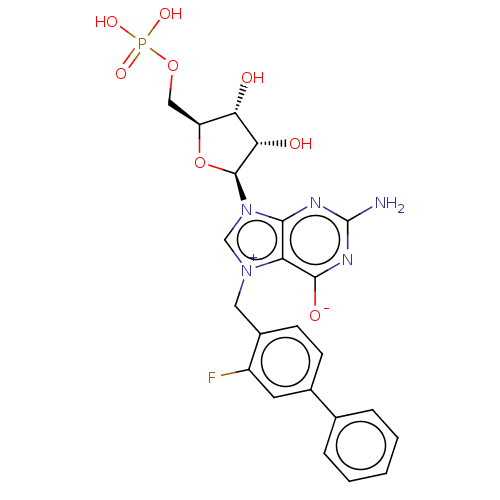 (US10676499, Example 54) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451154 (US10676499, Example 43) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451153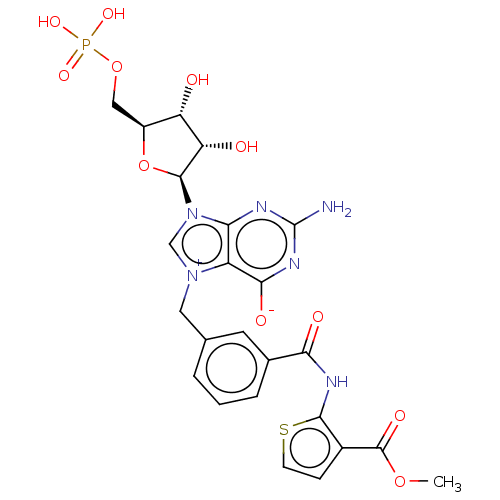 (US10676499, Example 42) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451152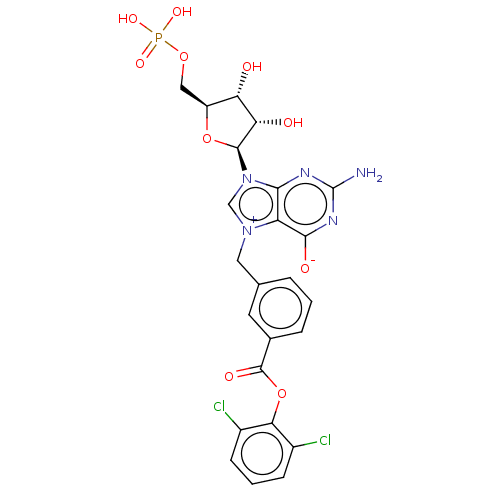 (US10676499, Example 41) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 8.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Eukaryotic translation initiation factor 4E (Homo sapiens (Human)) | BDBM451149 (US10676499, Example 32) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 9.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG US Patent | Assay Description Dual histidine and Avi tagged human EIF4E (His6-3C-avi-eIF4E) was expressed in 8 L of TB Media. Induction by 0.4 mM IPTG occurred at 2.0 OD600, and c... | US Patent US10676499 (2020) BindingDB Entry DOI: 10.7270/Q2K64N44 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542738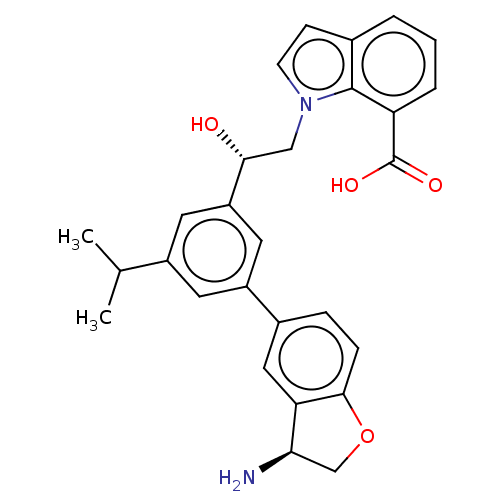 (CHEMBL4637027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542741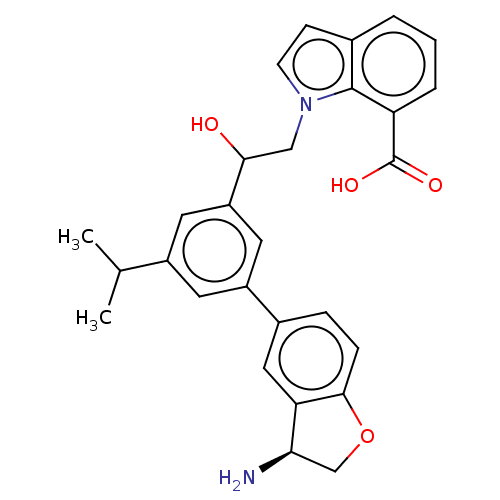 (CHEMBL4647950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542738 (CHEMBL4637027) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542740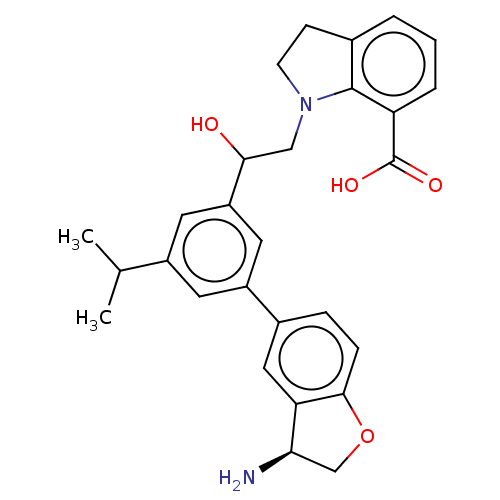 (CHEMBL4646398) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542740 (CHEMBL4646398) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542724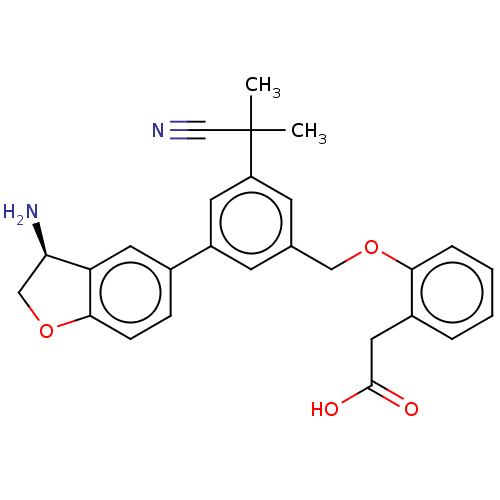 (CHEMBL4636415) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542723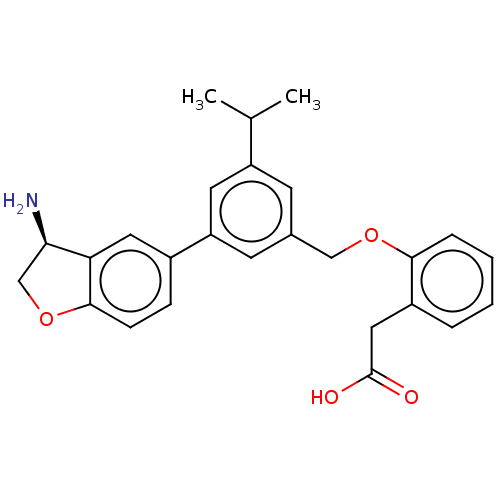 (CHEMBL4643449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542723 (CHEMBL4643449) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542728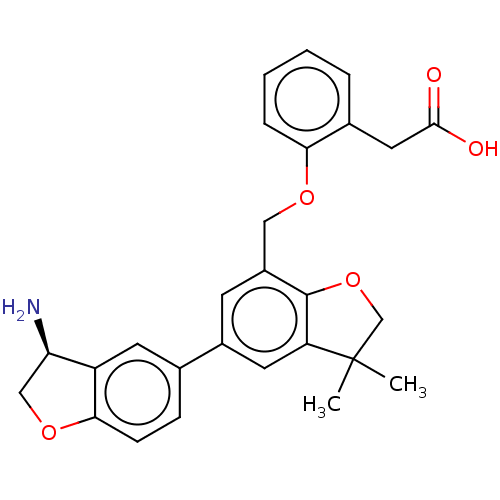 (CHEMBL4635912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542733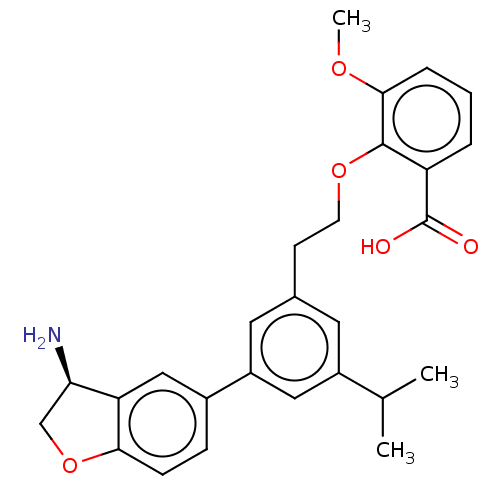 (CHEMBL4646141) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542724 (CHEMBL4636415) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542730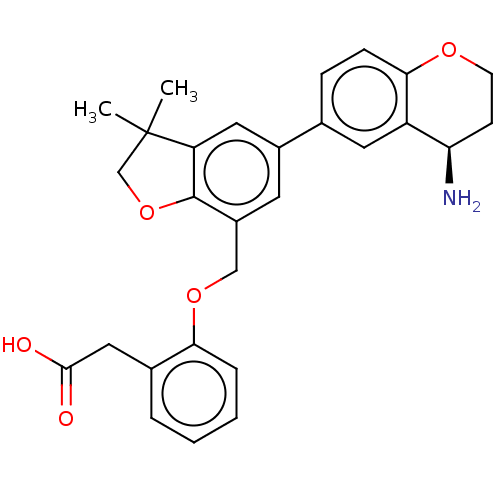 (CHEMBL4647909) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542725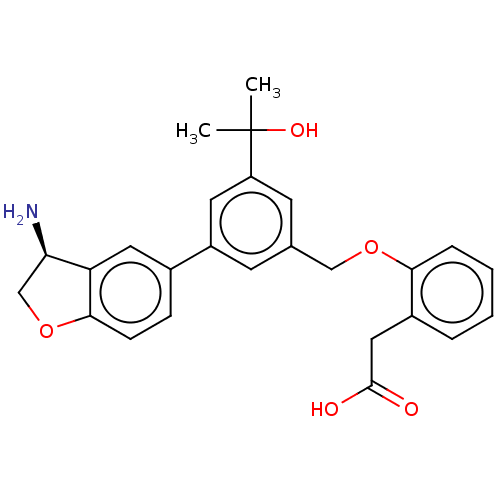 (CHEMBL4637683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542730 (CHEMBL4647909) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542735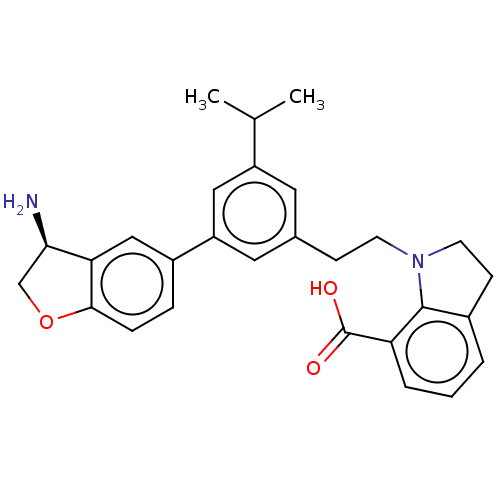 (CHEMBL4635286) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542725 (CHEMBL4637683) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542728 (CHEMBL4635912) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50209006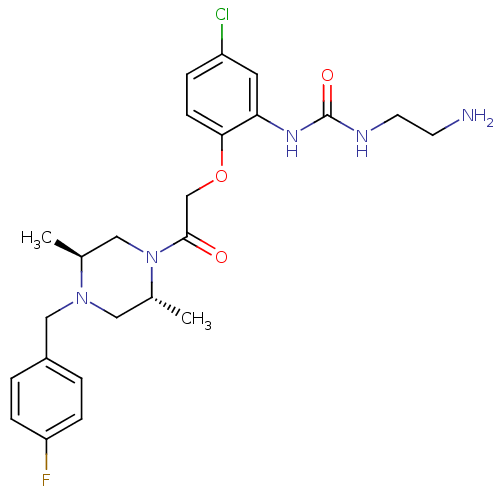 (1-(2-(2-((2R,5S)-4-(4-fluorobenzyl)-2,5-dimethylpi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding Affinity at CCL3 | Bioorg Med Chem Lett 17: 3109-12 (2007) Article DOI: 10.1016/j.bmcl.2007.03.037 BindingDB Entry DOI: 10.7270/Q2CF9PSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Complement factor D (Homo sapiens (Human)) | BDBM50524338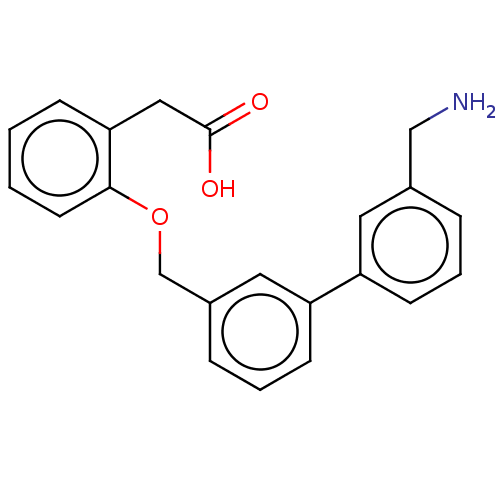 (CHEMBL4468000) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human complement FD by TR-FRET assay | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542726 (CHEMBL4642766) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542733 (CHEMBL4646141) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of N-terminal human plasma F11a catalytic domain expressed in Escherichia coli strain BL21(DE3) using D-Leu-Pro-Arg*Rh110-D-Pro as substra... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542738 (CHEMBL4637027) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of N-terminal human plasma F11a catalytic domain expressed in Escherichia coli strain BL21(DE3) using D-Leu-Pro-Arg*Rh110-D-Pro as substra... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542726 (CHEMBL4642766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542732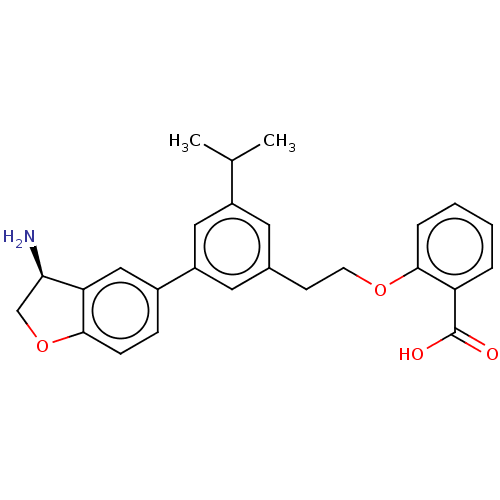 (CHEMBL4647925) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50209001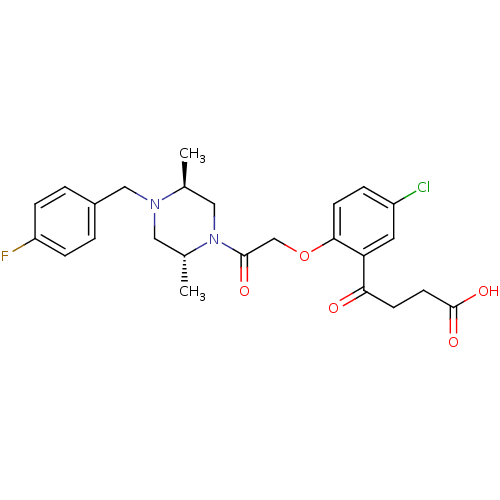 (4-(2-(2-((2R,5S)-4-(4-fluorobenzyl)-2,5-dimethylpi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding Affinity at CCL3 | Bioorg Med Chem Lett 17: 3109-12 (2007) Article DOI: 10.1016/j.bmcl.2007.03.037 BindingDB Entry DOI: 10.7270/Q2CF9PSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542737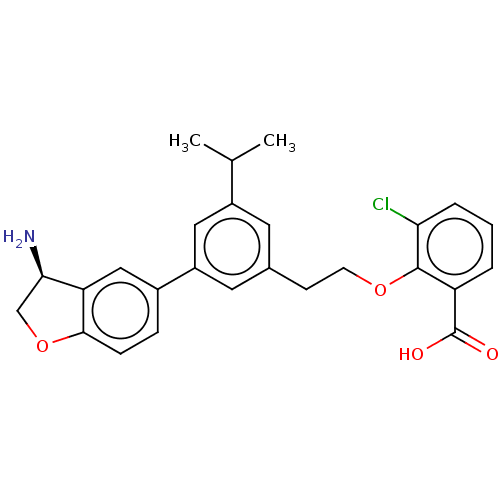 (CHEMBL4645908) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50542728 (CHEMBL4635912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human urokinase using fluorescent peptide as substrate by florescence assay | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50542741 (CHEMBL4647950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKL (unknown origin) using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured ... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50542736 (CHEMBL4633967) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human F11a using D-Leu-Pro-Arg*Rh110-D-Pro as substrate preincubated for 60 mins followed by substrate addition and measured after 60 m... | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50542731 (CHEMBL4642845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human uPA using fluorescent peptide as substrate by florescence assay | J Med Chem 63: 8088-8113 (2020) Article DOI: 10.1021/acs.jmedchem.0c00279 BindingDB Entry DOI: 10.7270/Q2KS6W36 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 228 total ) | Next | Last >> |