

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543404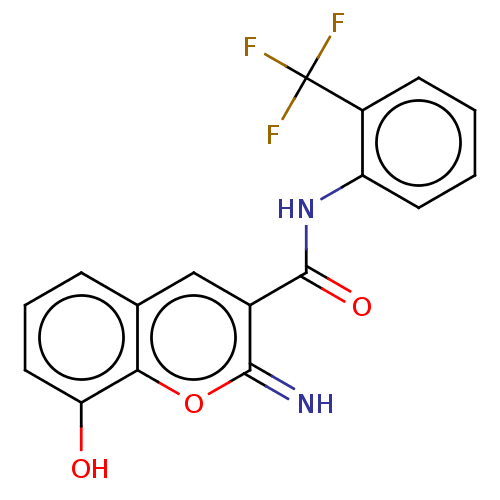 (CHEMBL4640154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543398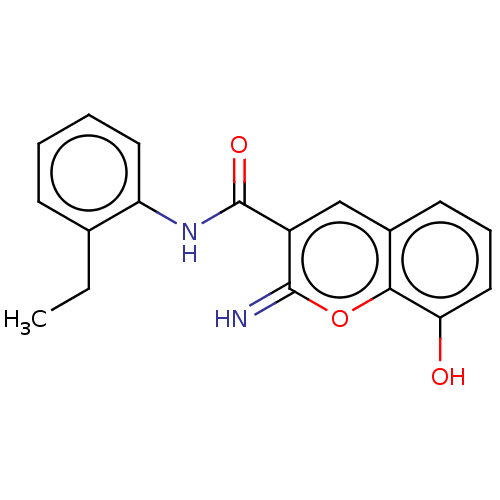 (CHEMBL4642187) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543409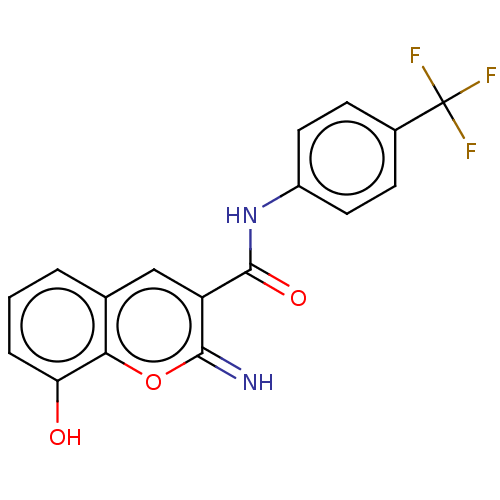 (CHEMBL4642852) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543401 (CHEMBL4644092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543399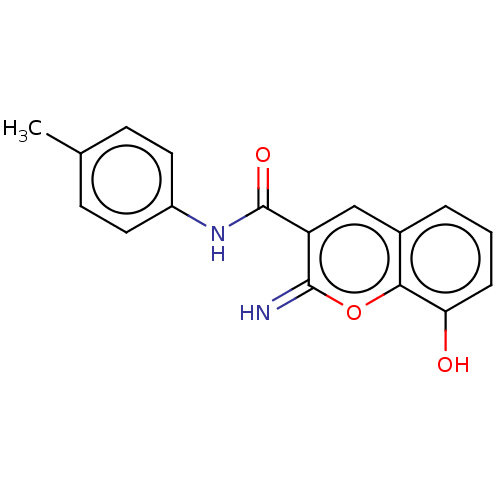 (CHEMBL4648793) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543402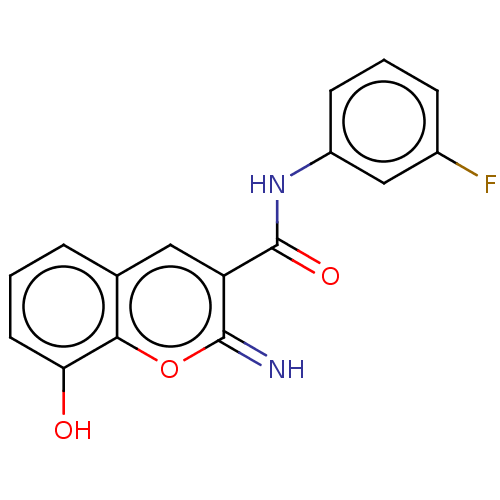 (CHEMBL4634960) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543407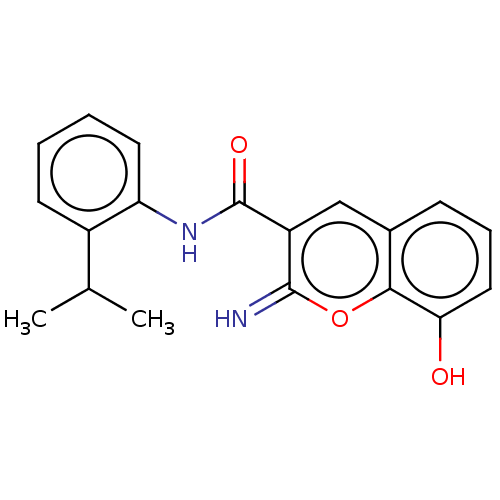 (CHEMBL4641899) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543397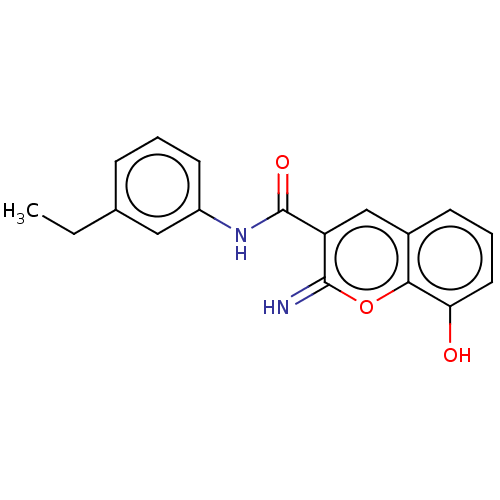 (CHEMBL4646593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50543400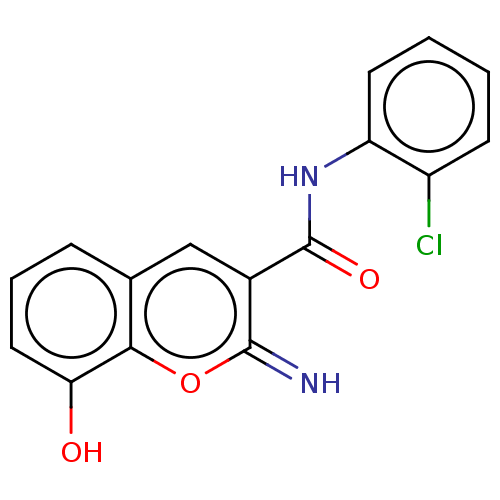 (CHEMBL4640248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of CBR1 (unknown origin) | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543416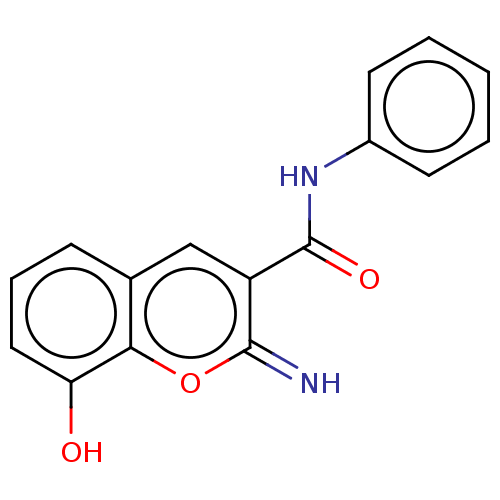 (CHEMBL4639909) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543396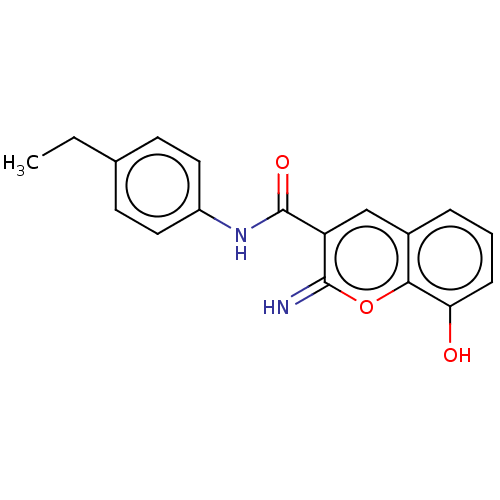 (CHEMBL4641012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543405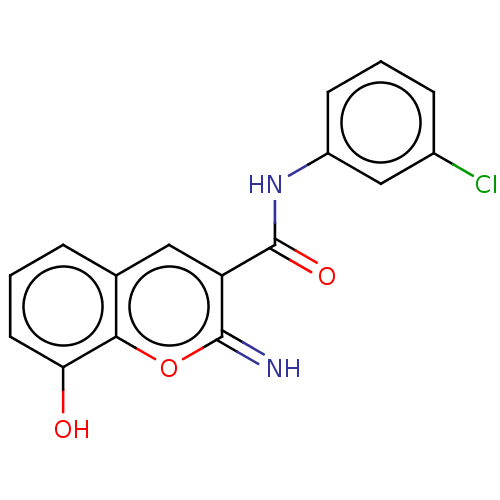 (CHEMBL4639417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543406 (CHEMBL4637597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543411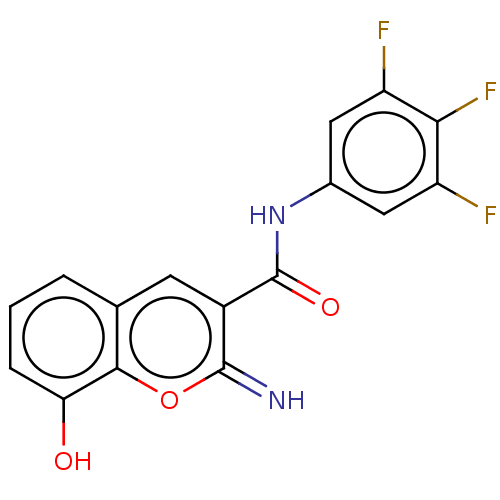 (CHEMBL4634140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543410 (CHEMBL4633866) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543414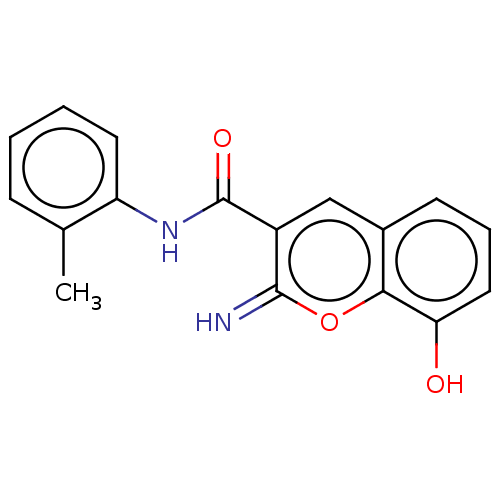 (CHEMBL4636345) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543415 (CHEMBL4641799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543413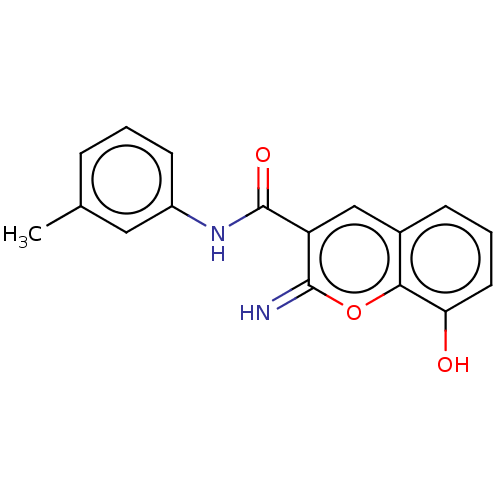 (CHEMBL4649502) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543400 (CHEMBL4640248) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543408 (CHEMBL4640782) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50213370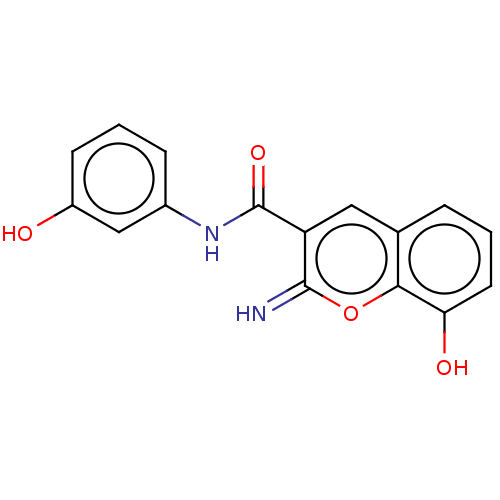 (CHEMBL82085) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543403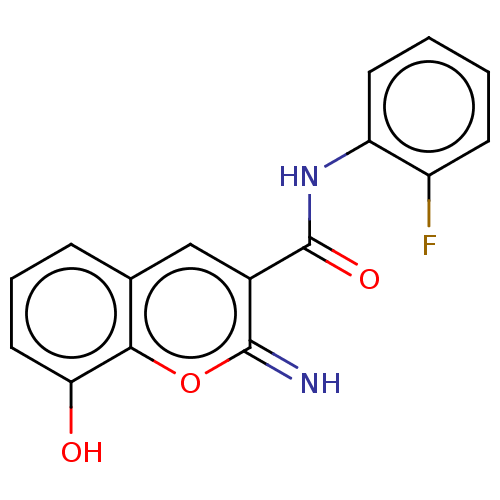 (CHEMBL4642678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50543404 (CHEMBL4640154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of CBR1 (unknown origin) | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50543405 (CHEMBL4639417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of CBR1 (unknown origin) | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543412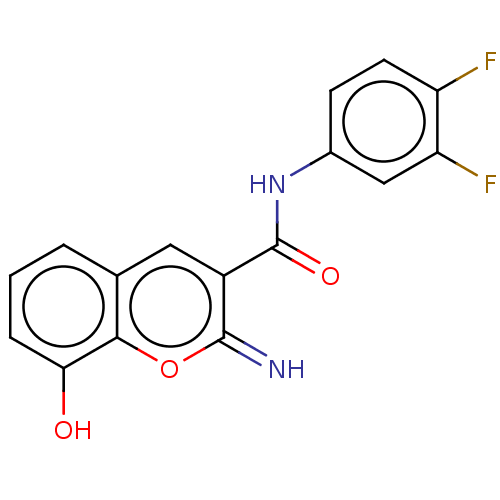 (CHEMBL4648687) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM35909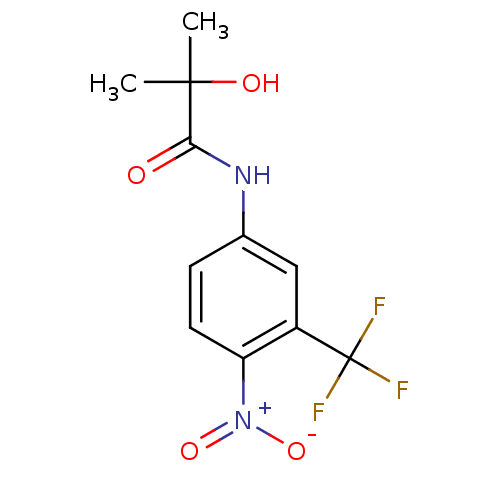 (2-Hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50543397 (CHEMBL4646593) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of CBR1 (unknown origin) | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50543399 (CHEMBL4648793) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of CBR1 (unknown origin) | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50543402 (CHEMBL4634960) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of CBR1 (unknown origin) | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543420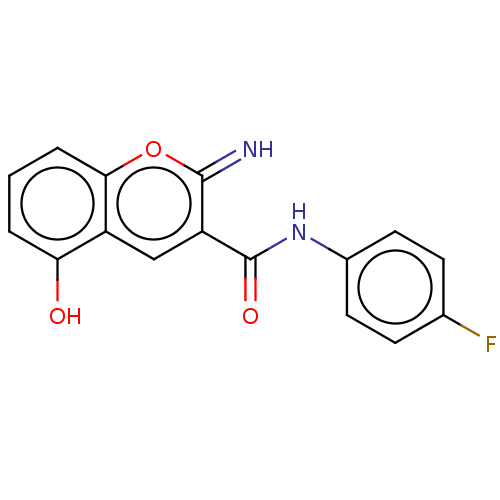 (CHEMBL4638008) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50543401 (CHEMBL4644092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of CBR1 (unknown origin) | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483935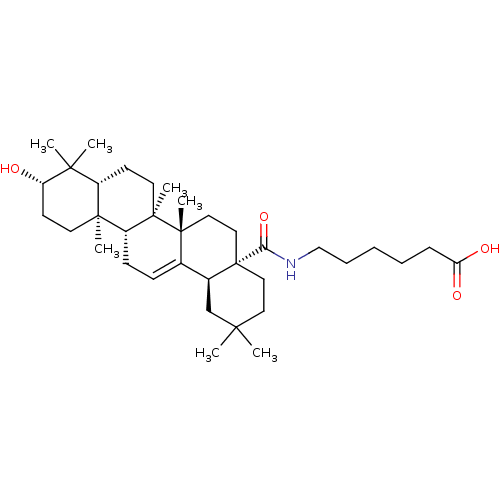 (CHEMBL1783812) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50483937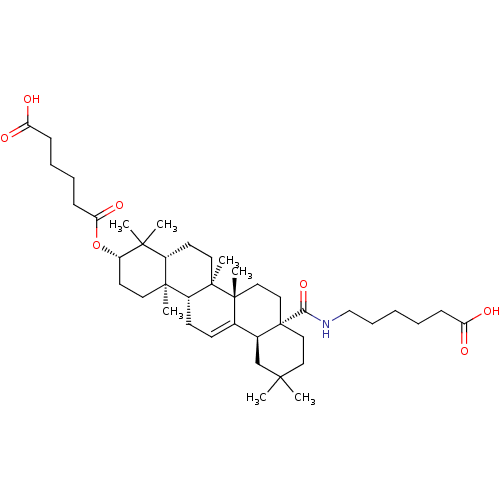 (CHEMBL1783813) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50543399 (CHEMBL4648793) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483932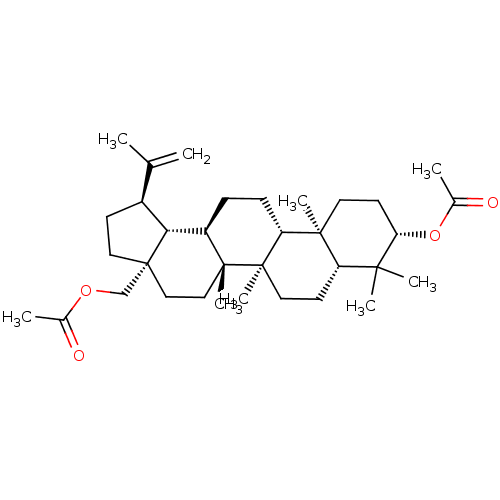 (Betulin 3,28-Diacetate | Betulin Diacetate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483934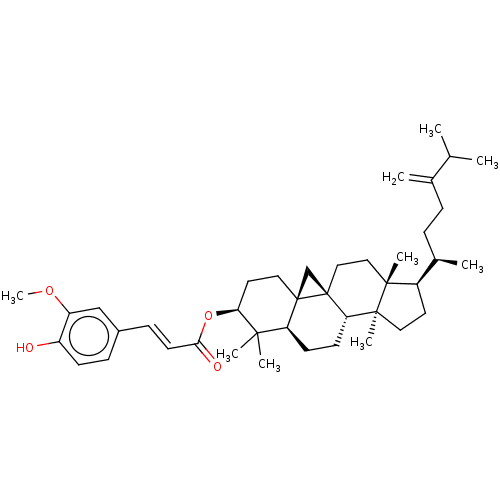 (24-Methylenecycloartanol Ferulate | 24-Methylenecy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50543400 (CHEMBL4640248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50543402 (CHEMBL4634960) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50543396 (CHEMBL4641012) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483936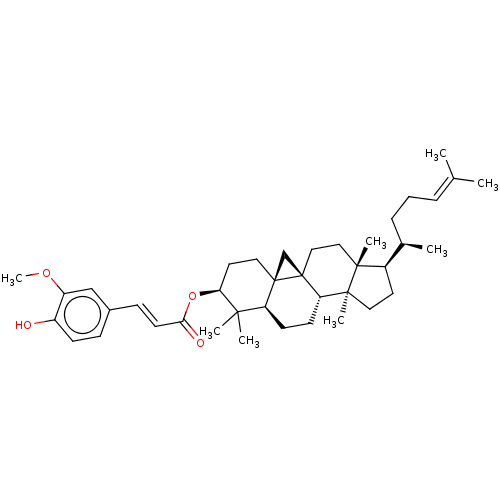 (3-O-Ferulylcycloartenol | Cycloartenol Ferulate | ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50346601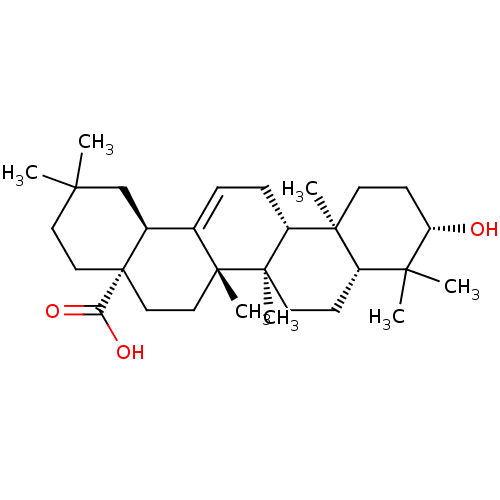 (NSC-114945 | OLEANOLIC_ACID | Oleanolic acid | Ole...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50148911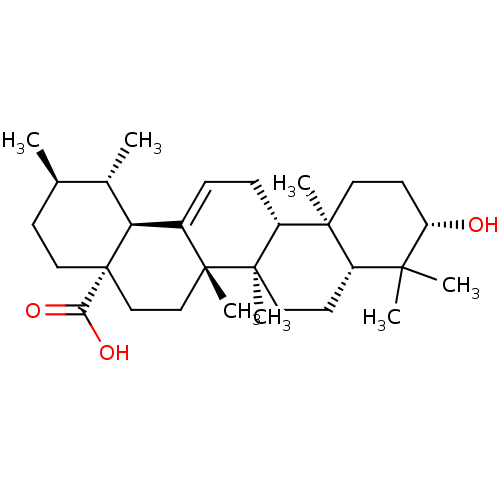 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM23208 ((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-17-hydroxy-1,...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50483933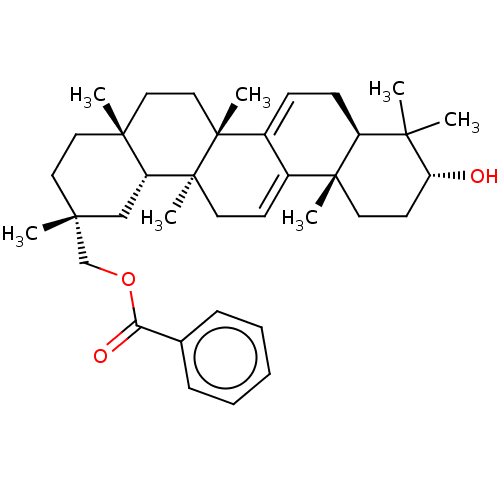 (CHEMBL1783816 | Karounidiol 29-Benzoate) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50543403 (CHEMBL4642678) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50382163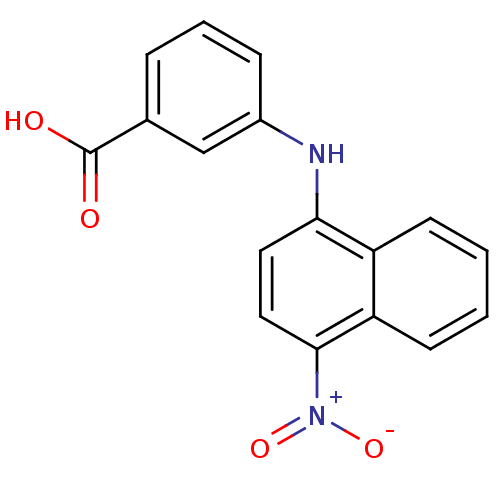 (CHEMBL2023820) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50241944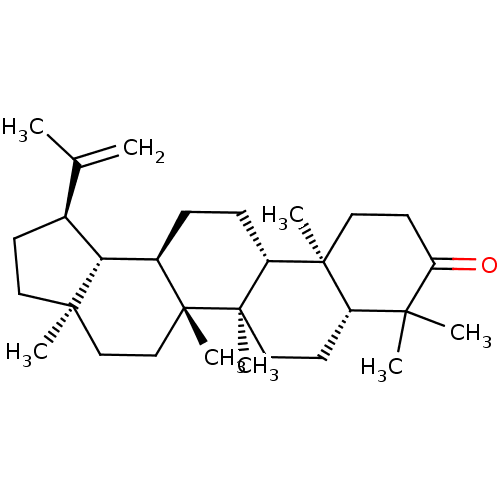 (CHEMBL486393 | Lupenone | lupeone) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Antiviral activity against HIV1 Reverse transcriptase activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50543397 (CHEMBL4646593) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assay | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50148911 ((3beta)-3-hydroxyurs-12-en-28-oic acid | 3beta-hyd...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Osaka University of Pharmaceutical Sciences Curated by ChEMBL | Assay Description Inhibition of HIV1 protease activity | Bioorg Med Chem 17: 5238-46 (2009) Article DOI: 10.1016/j.bmc.2009.05.049 BindingDB Entry DOI: 10.7270/Q2086856 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C1 (Homo sapiens (Human)) | BDBM50543401 (CHEMBL4644092) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C1 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 79 total ) | Next | Last >> |