

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50098551 ((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.339 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50098551 ((R)-3-(2-(2-(4-methylpiperidin-1-yl)ethyl)pyrrolid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of 5-HT2A receptor | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of SERT | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313283 (1-{2-[4-(6-Fluoro-1H-indol-3-yl)-3,6-dihydro-2H-py...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to SERT | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50069447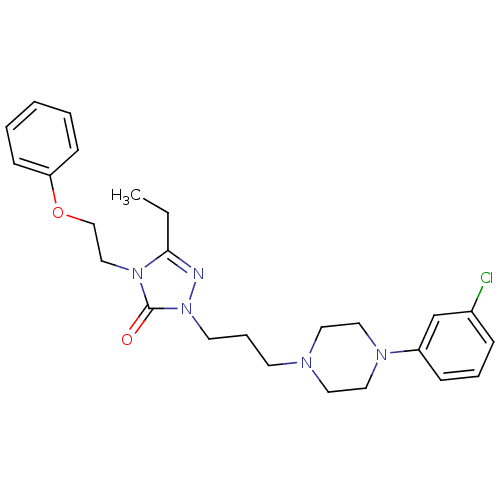 (1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase A (Homo sapiens (Human)) | BDBM50022815 ((3S,6S,9S,12R,15S,18S,21S,24S,30S,33S)-30-ethyl-33...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00211 BindingDB Entry DOI: 10.7270/Q2RF603J | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458557 (CHEMBL4215875) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458557 (CHEMBL4215875) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458557 (CHEMBL4215875) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology US Patent | Assay Description Human gene recombinant 5-HT7 receptor expressed in Chinese hamster ovary (CHO) cells was used. [3H]LSD 1 nM, the 5-HT7 receptor membrane (15 μg/... | US Patent US10435408 (2019) BindingDB Entry DOI: 10.7270/Q2ZS2ZXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50458557 (CHEMBL4215875) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2C receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of SERT | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to SERT | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458555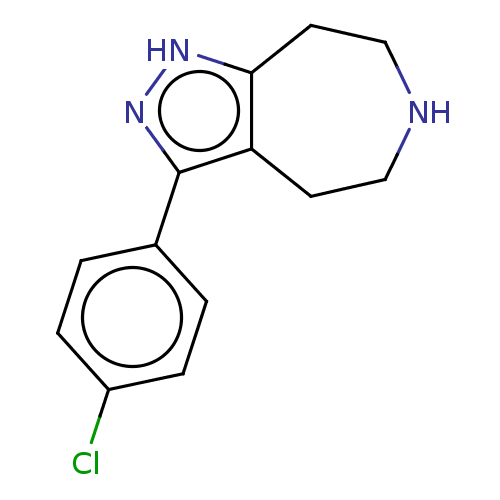 (CHEMBL4207884) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458555 (CHEMBL4207884) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology US Patent | Assay Description Human gene recombinant 5-HT7 receptor expressed in Chinese hamster ovary (CHO) cells was used. [3H]LSD 1 nM, the 5-HT7 receptor membrane (15 μg/... | US Patent US10435408 (2019) BindingDB Entry DOI: 10.7270/Q2ZS2ZXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458555 (CHEMBL4207884) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458558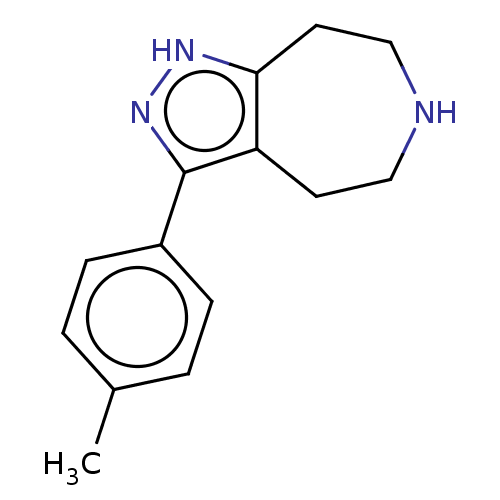 (CHEMBL4210782) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology US Patent | Assay Description Human gene recombinant 5-HT7 receptor expressed in Chinese hamster ovary (CHO) cells was used. [3H]LSD 1 nM, the 5-HT7 receptor membrane (15 μg/... | US Patent US10435408 (2019) BindingDB Entry DOI: 10.7270/Q2ZS2ZXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458558 (CHEMBL4210782) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458558 (CHEMBL4210782) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458553 (CHEMBL4218672) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458553 (CHEMBL4218672) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458553 (CHEMBL4218672) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology US Patent | Assay Description Human gene recombinant 5-HT7 receptor expressed in Chinese hamster ovary (CHO) cells was used. [3H]LSD 1 nM, the 5-HT7 receptor membrane (15 μg/... | US Patent US10435408 (2019) BindingDB Entry DOI: 10.7270/Q2ZS2ZXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458554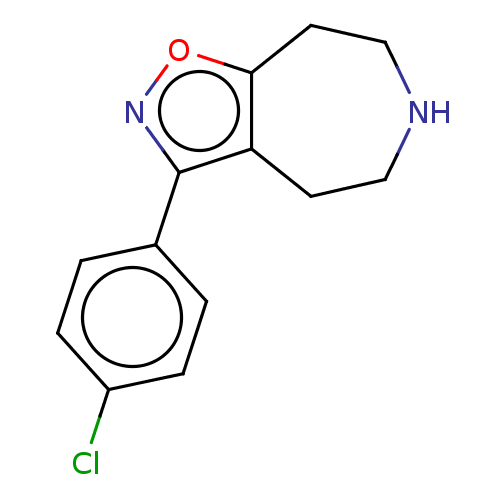 (CHEMBL4218801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458554 (CHEMBL4218801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology US Patent | Assay Description Human gene recombinant 5-HT7 receptor expressed in Chinese hamster ovary (CHO) cells was used. [3H]LSD 1 nM, the 5-HT7 receptor membrane (15 μg/... | US Patent US10435408 (2019) BindingDB Entry DOI: 10.7270/Q2ZS2ZXP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458554 (CHEMBL4218801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50313284 ((S)-2-((7-fluoro-2,3-dihydro-1H-inden-4-yloxy)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Patents Similars | Article PubMed | 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Inhibition of 5-HT2A receptor | Bioorg Med Chem Lett 20: 1705-11 (2010) Article DOI: 10.1016/j.bmcl.2010.01.093 BindingDB Entry DOI: 10.7270/Q2SN093C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50458550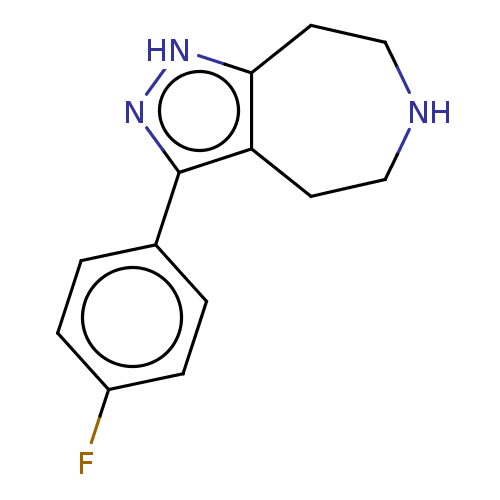 (CHEMBL4209274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to SERT | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50458555 (CHEMBL4207884) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458550 (CHEMBL4209274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 7 (Homo sapiens (Human)) | BDBM50458550 (CHEMBL4209274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT7 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50458558 (CHEMBL4210782) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50458558 (CHEMBL4210782) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM21962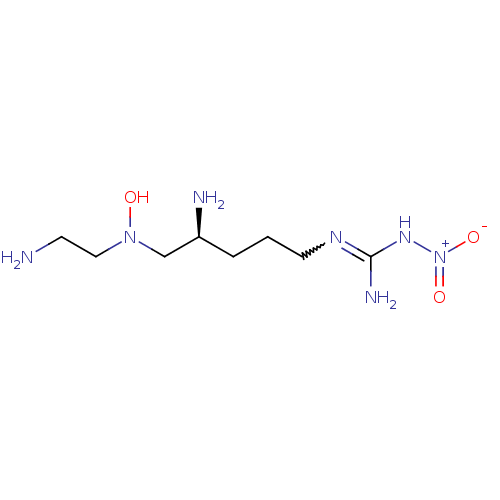 (3-[(4S)-4-amino-5-[(2-aminoethyl)(hydroxy)amino]pe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 120 | -40.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Northwestern University | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 50: 2089-99 (2007) Article DOI: 10.1021/jm061305c BindingDB Entry DOI: 10.7270/Q2W0947W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50458557 (CHEMBL4215875) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2A receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50458557 (CHEMBL4215875) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 147 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT1D receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50458557 (CHEMBL4215875) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50458558 (CHEMBL4210782) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2A receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50458558 (CHEMBL4210782) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 165 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT1D receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM21960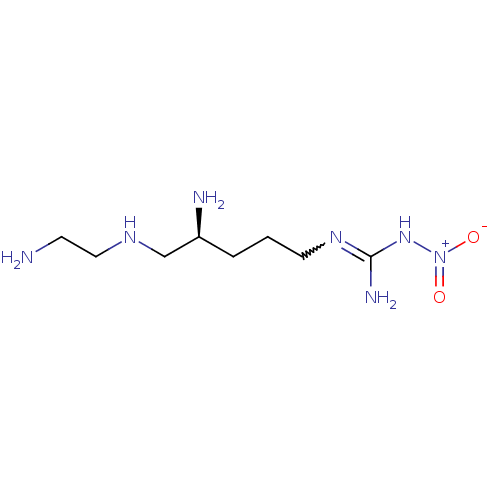 (3-[(4S)-4-amino-5-[(2-aminoethyl)amino]pentyl]-1-n...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 170 | -39.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Northwestern University | Assay Description Nitric oxide formation from NOS was monitored by the hemoglobin capture assay. The assay was initiated by addition of enzyme and was monitored at 401... | J Med Chem 50: 2089-99 (2007) Article DOI: 10.1021/jm061305c BindingDB Entry DOI: 10.7270/Q2W0947W | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50458550 (CHEMBL4209274) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 175 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2A receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50458555 (CHEMBL4207884) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2A receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50458550 (CHEMBL4209274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2C receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50203161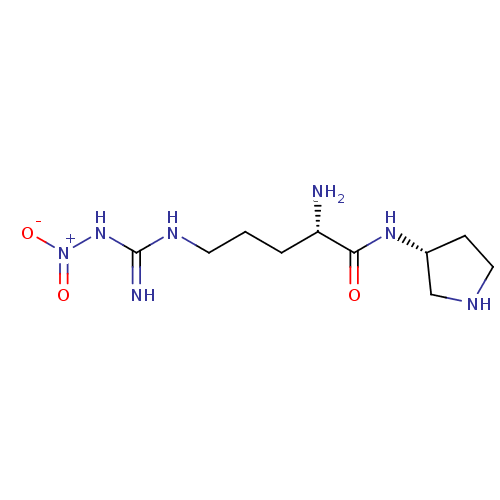 ((2S)-2-amino-5-nitroguanidinopentanoic acid ((3R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University Curated by ChEMBL | Assay Description Inhibition of rat recombinant nNOS expressed in Escherichia coli assessed as nitric oxide production by hemoglobin capture assay | Bioorg Med Chem 15: 1928-38 (2007) Article DOI: 10.1016/j.bmc.2007.01.001 BindingDB Entry DOI: 10.7270/Q2QF8SJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50458554 (CHEMBL4218801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT2B receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50458555 (CHEMBL4207884) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 248 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 receptor expressed in HEK cell membranes after 1.5 hrs by microbeta scintillation counting method | J Med Chem 61: 7218-7233 (2018) Article DOI: 10.1021/acs.jmedchem.8b00642 BindingDB Entry DOI: 10.7270/Q2BC424N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50069447 (1-(3-(4-(m-Chlorophenyl)-1-piperazinyl)propyl)-3-e...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Green Cross Corporation Curated by ChEMBL | Assay Description Binding affinity to SERT | J Med Chem 54: 6305-18 (2011) Article DOI: 10.1021/jm200682b BindingDB Entry DOI: 10.7270/Q2SX6DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 6602 total ) | Next | Last >> |