

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Rattus norvegicus (Rat)) | BDBM50474902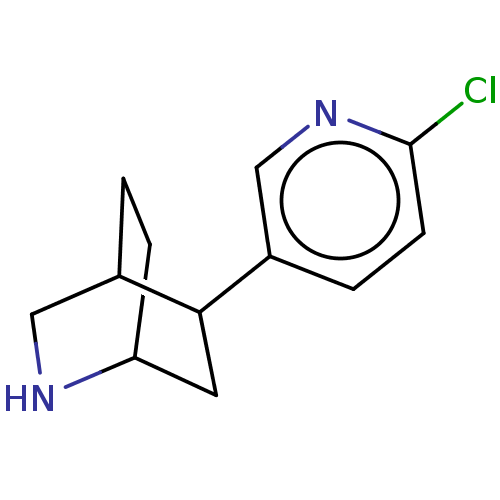 (CHEMBL187309) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibition of [3H]- cytisine binding to Nicotinic acetylcholine receptor alpha4-beta2 of rat cortical membranes | Bioorg Med Chem Lett 14: 5573-7 (2004) Article DOI: 10.1016/j.bmcl.2004.08.058 BindingDB Entry DOI: 10.7270/Q2XG9TWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50018174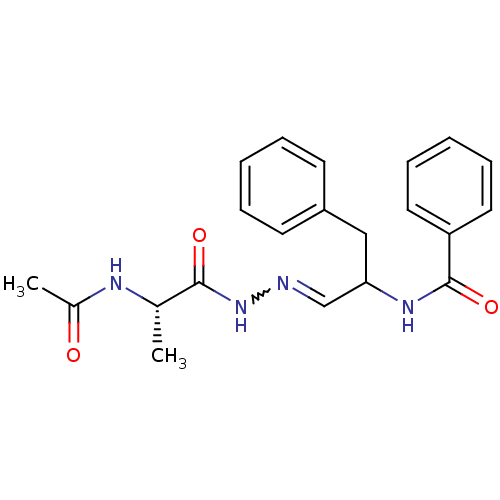 (CHEMBL283109 | N-{2-[(2-Acetylamino-propionyl)-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50018174 (CHEMBL283109 | N-{2-[(2-Acetylamino-propionyl)-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of papain-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 7 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50018173 (CHEMBL22745 | N-{2-[(2-Acetylamino-propionyl)-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of papain-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 7 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018174 (CHEMBL283109 | N-{2-[(2-Acetylamino-propionyl)-hyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.8 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50018173 (CHEMBL22745 | N-{2-[(2-Acetylamino-propionyl)-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of papain-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018174 (CHEMBL283109 | N-{2-[(2-Acetylamino-propionyl)-hyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Initial rate of reaction between Chymotrypsinogen and BzArg p-nitro-anilide in the presence of 400 microM of the compound at pH 7 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Papain (Carica papaya) | BDBM50018173 (CHEMBL22745 | N-{2-[(2-Acetylamino-propionyl)-hydr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of papain-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018175 (CHEMBL22389 | N-{1-Benzyl-2-[(pyridine-3-carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018175 (CHEMBL22389 | N-{1-Benzyl-2-[(pyridine-3-carbonyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 7.8 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018174 (CHEMBL283109 | N-{2-[(2-Acetylamino-propionyl)-hyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of papain-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5.5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-C (Homo sapiens (Human)) | BDBM50018173 (CHEMBL22745 | N-{2-[(2-Acetylamino-propionyl)-hydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | >8.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 5.0 | n/a |
Amherst College Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-catalyzed hydrolysis of BzArg p-nitroanilide by compound at pH 5 | J Med Chem 32: 1253-9 (1989) BindingDB Entry DOI: 10.7270/Q2K64H2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14776 (2-{2-ethoxy-5-[(4-ethylpiperazine-1-)sulfonyl]phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120086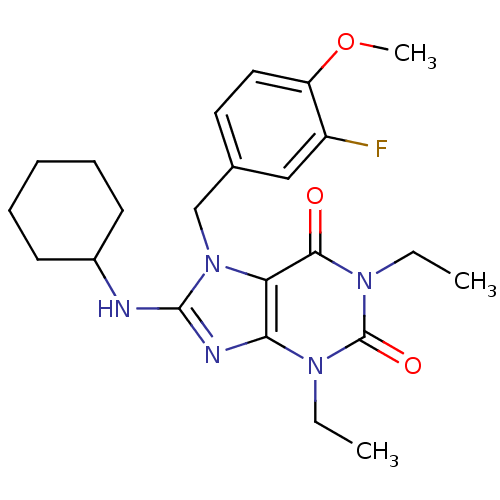 (8-Cyclohexylamino-1,3-diethyl-7-(3-fluoro-4-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120069 (8-Cyclohexylamino-1,3-diethyl-7-(3-fluoro-4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120079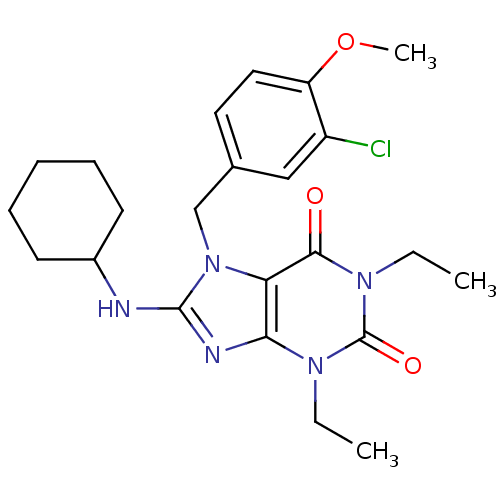 (7-(3-Chloro-4-methoxy-benzyl)-8-cyclohexylamino-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120074 (8-Cyclohexylamino-1,3-diethyl-7-(4-hydroxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120067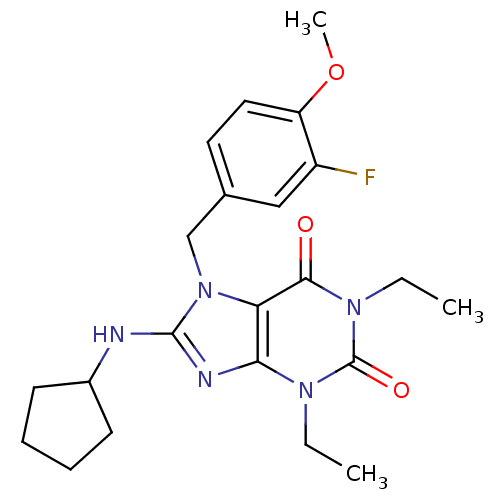 (8-Cyclopentylamino-1,3-diethyl-7-(3-fluoro-4-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120072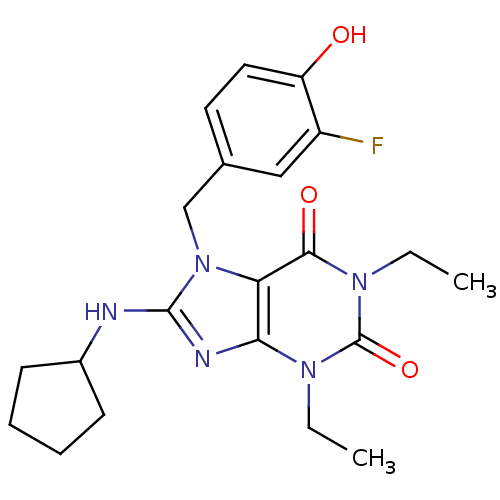 (8-Cyclopentylamino-1,3-diethyl-7-(3-fluoro-4-hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120071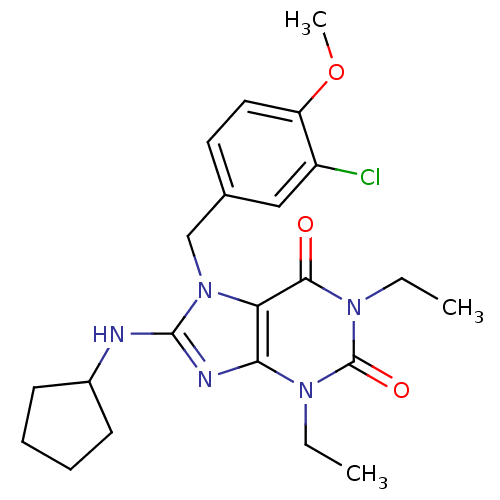 (7-(3-Chloro-4-methoxy-benzyl)-8-cyclopentylamino-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120080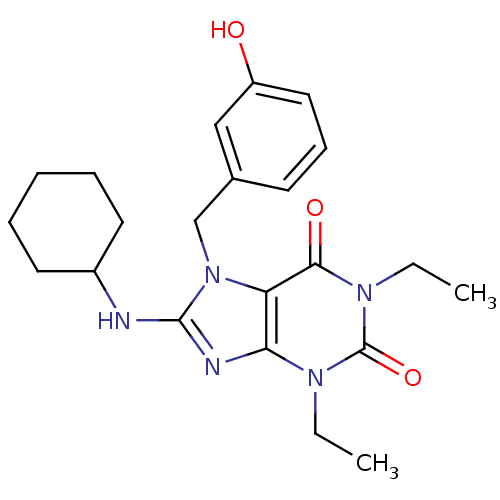 (8-Cyclohexylamino-1,3-diethyl-7-(3-hydroxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120070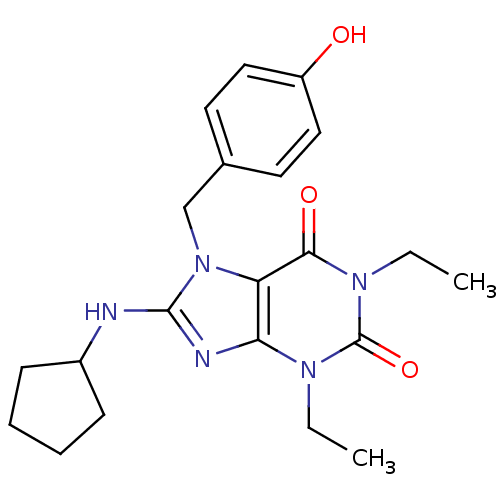 (8-Cyclopentylamino-1,3-diethyl-7-(4-methoxy-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120081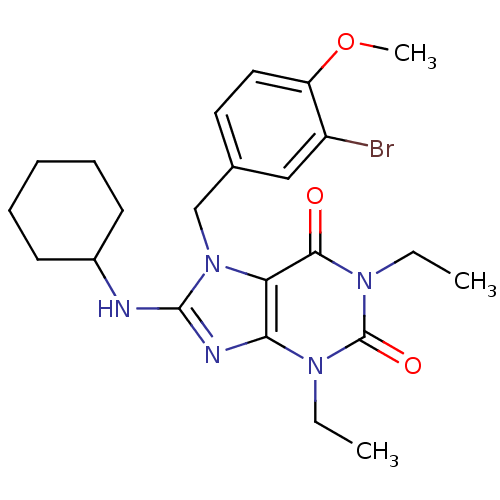 (7-(3-Bromo-4-methoxy-benzyl)-8-cyclohexylamino-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120078 (7-(3-Chloro-4-hydroxy-benzyl)-8-cyclopentylamino-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120085 (7-(3-Chloro-4-hydroxy-benzyl)-8-cyclohexylamino-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273951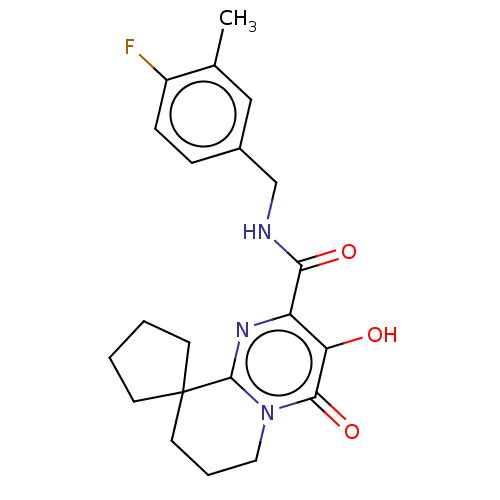 (CHEMBL4126165) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273914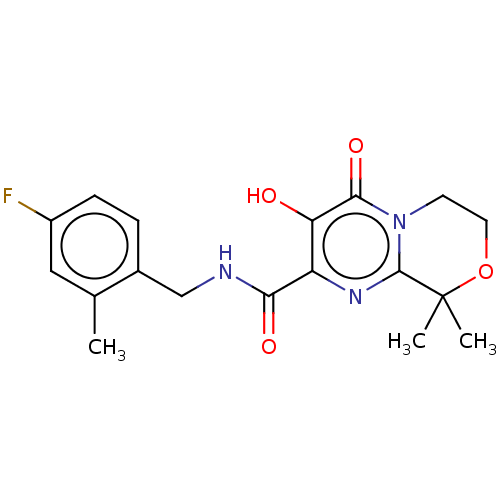 (CHEMBL4126686) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120068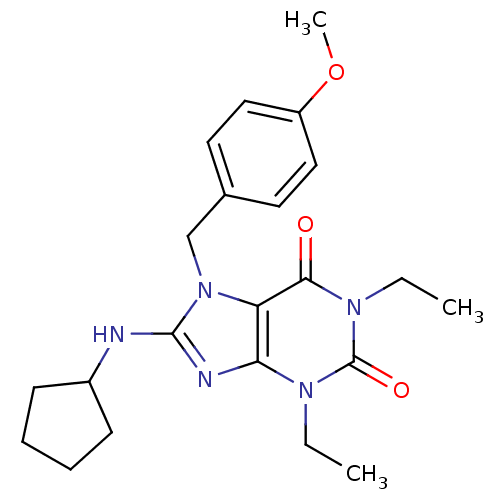 (8-Cyclopentylamino-1,3-diethyl-7-(4-methoxy-benzyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50165781 ((R)-7-Benzyl-2-chloro-5-ethyl-3-(4-hydroxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50165771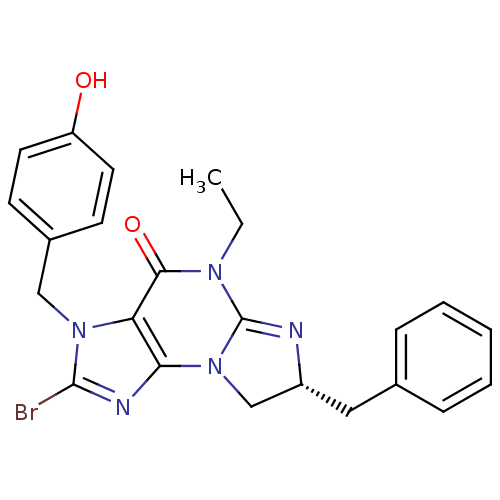 ((R)-7-Benzyl-2-bromo-5-ethyl-3-(4-hydroxy-benzyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120083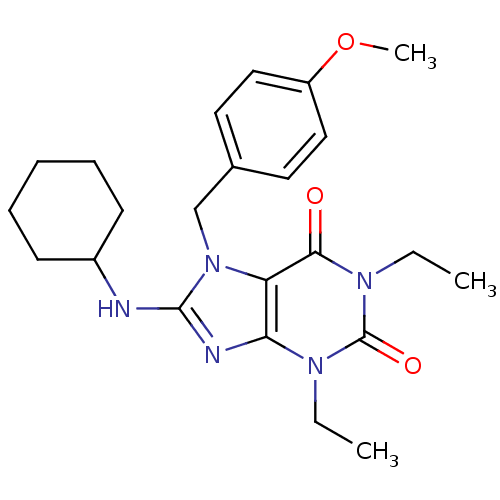 (8-Cyclohexylamino-1,3-diethyl-7-(4-methoxy-benzyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273903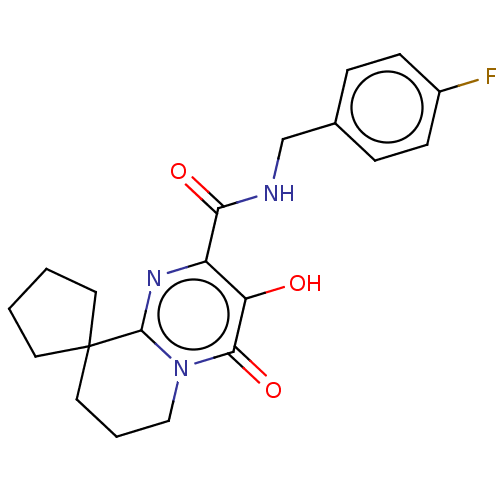 (CHEMBL4129717) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273915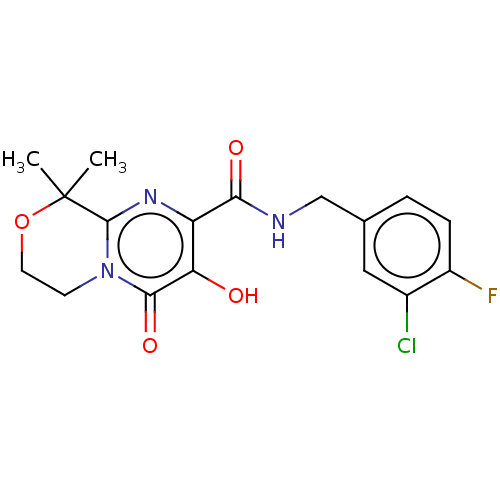 (CHEMBL4127891) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273913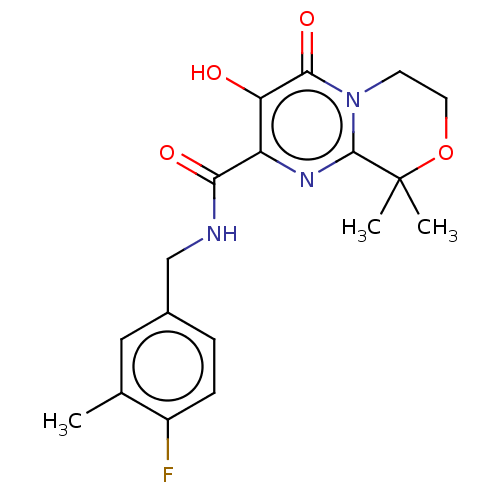 (CHEMBL4127663) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273916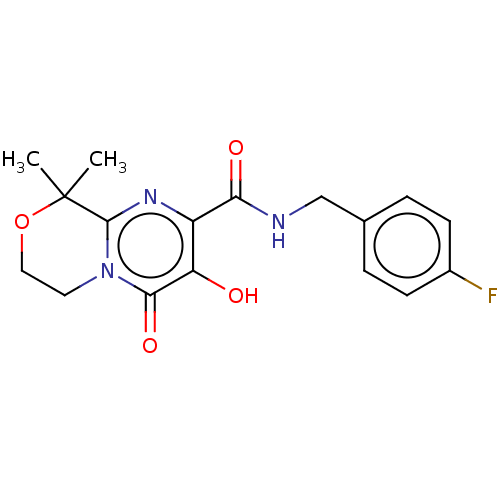 (CHEMBL4126289) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50165773 ((R)-7-Benzyl-5-ethyl-3-(4-hydroxy-benzyl)-2-iodo-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120075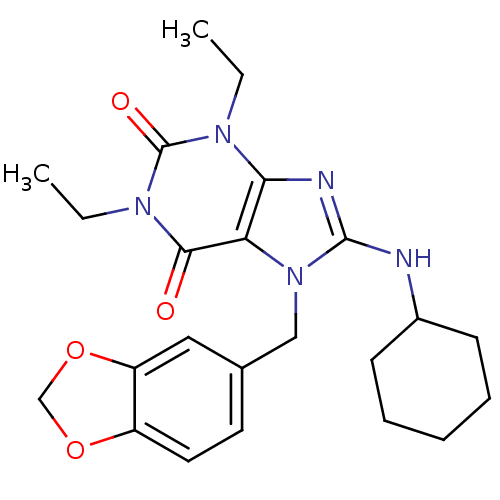 (7-Benzo[1,3]dioxol-5-ylmethyl-8-cyclohexylamino-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273950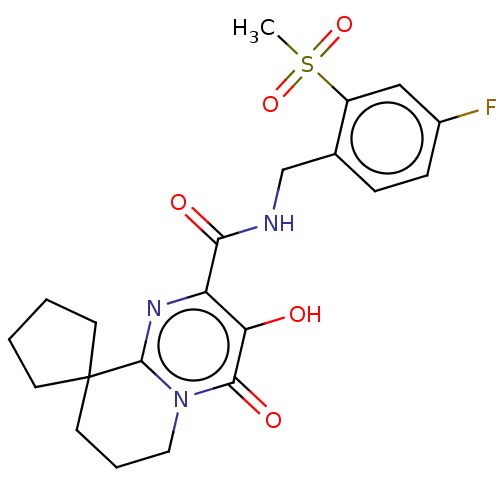 (CHEMBL4128240) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14777 ((2R,8R)-2-(2H-1,3-benzodioxol-5-yl)-6-methyl-3,6,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50120076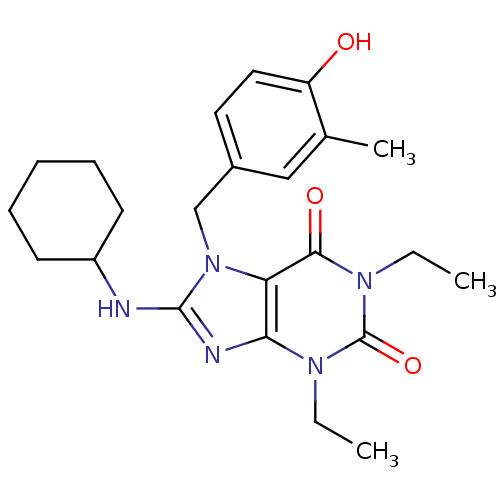 (8-Cyclohexylamino-1,3-diethyl-7-(4-hydroxy-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Concentration required to inhibit phosphodiesterase type 5 (PDE5) isolated from corpus cavernosum by 50% was determined | Bioorg Med Chem Lett 12: 3149-52 (2002) BindingDB Entry DOI: 10.7270/Q2S46R83 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50140595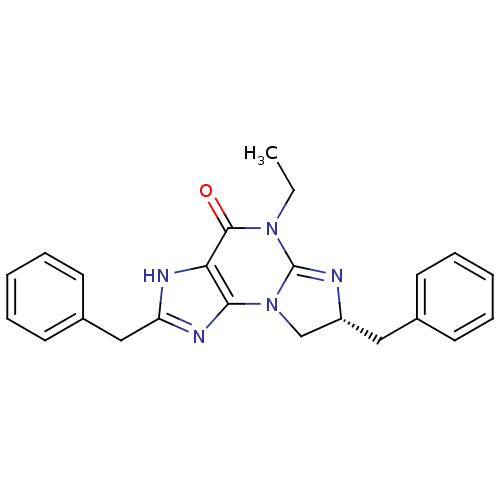 ((R)-2,7-Dibenzyl-5-ethyl-7,8-dihydro-1H,5H-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273965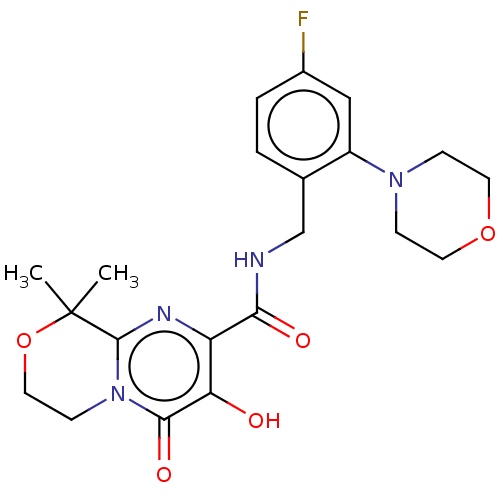 (CHEMBL4127923) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273948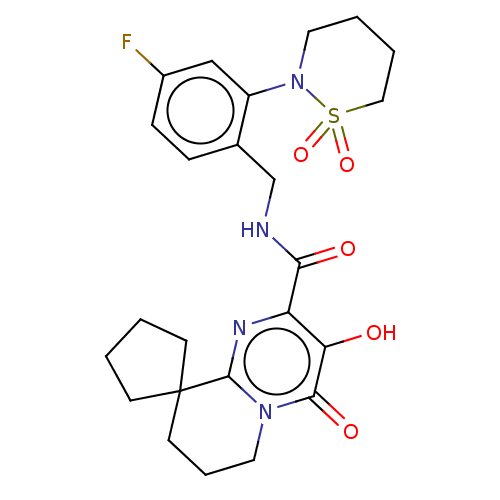 (CHEMBL4127873) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50241107 (1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Company Curated by ChEMBL | Assay Description In vitro antagonistic activity against Dopamine receptor D2 was evaluated for the inhibition of [3H]spiperone binding | J Med Chem 31: 1548-58 (1988) BindingDB Entry DOI: 10.7270/Q25B032R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50165787 ((R)-7-Benzyl-5-ethyl-3-(4-hydroxy-benzyl)-2-phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273902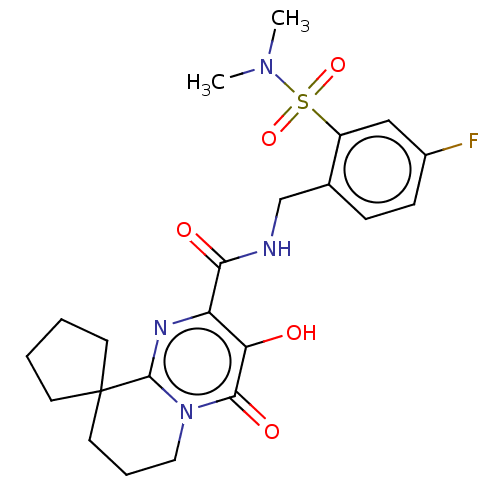 (CHEMBL4126659) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50273963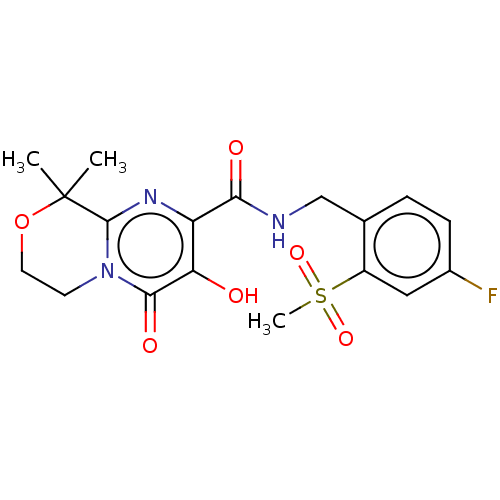 (CHEMBL4128890) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase strand transfer activity | Bioorg Med Chem Lett 28: 2124-2130 (2018) Article DOI: 10.1016/j.bmcl.2018.05.027 BindingDB Entry DOI: 10.7270/Q2BK1FVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50165782 (5-Ethyl-1-(4-hydroxy-benzyl)-2-phenylethynyl-7-((R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of human phosphodiesterase 5 | Bioorg Med Chem Lett 15: 2365-9 (2005) Article DOI: 10.1016/j.bmcl.2005.02.083 BindingDB Entry DOI: 10.7270/Q2TM79MQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 219 total ) | Next | Last >> |