

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM8125 ((3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl N-[(2S...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498806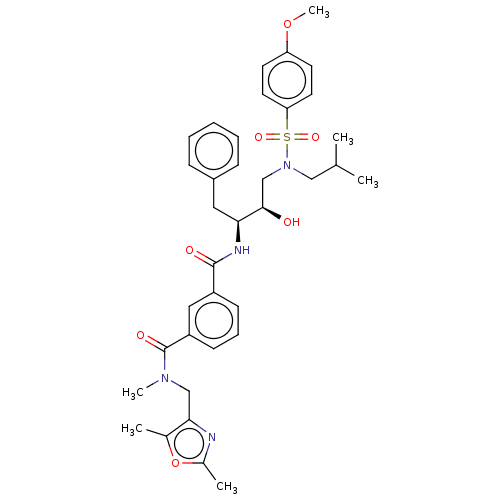 (CHEMBL3627879) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM171372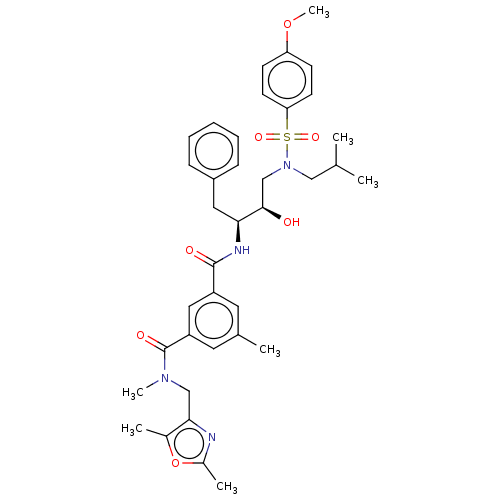 (US9085571, Table 1, Compound 20) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498814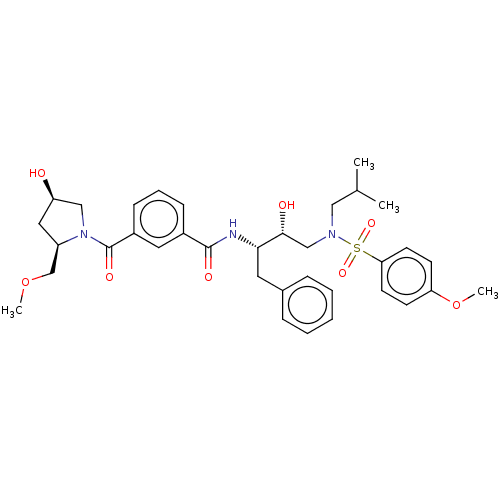 (CHEMBL3627876) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498808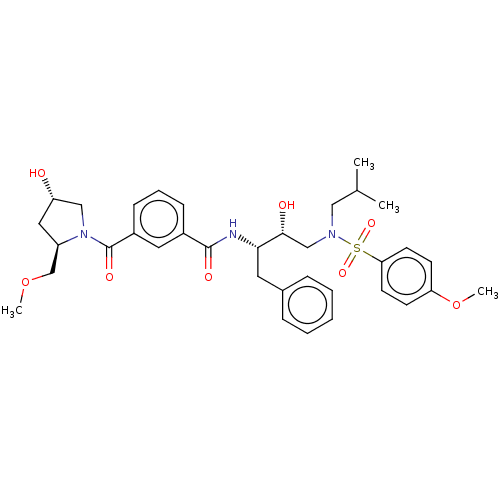 (CHEMBL3627874) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM171370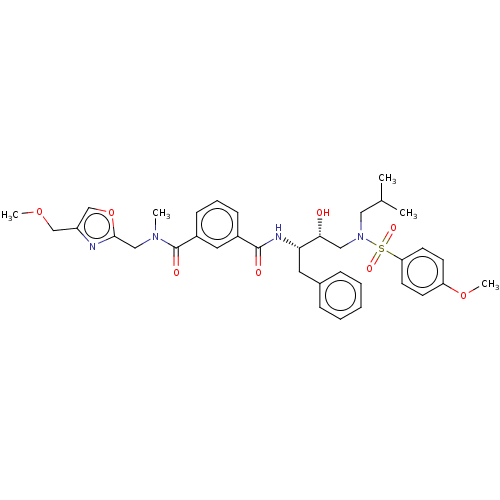 (US9085571, Table 1, Compound 18) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498812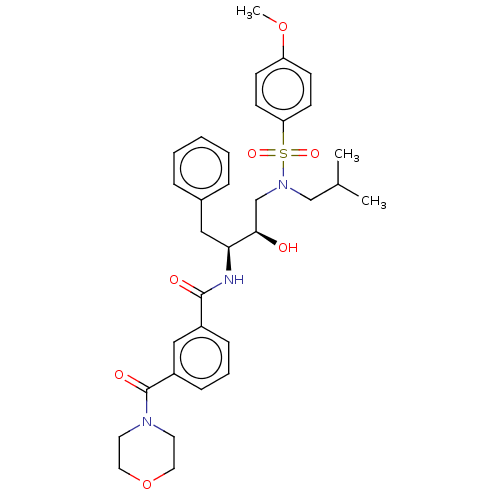 (CHEMBL3627855) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522184 (CHEMBL4444017) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498807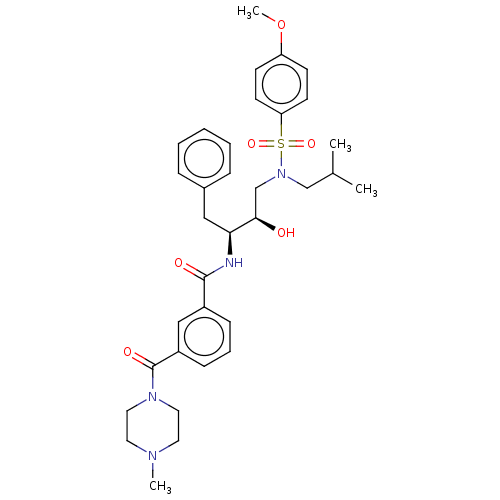 (CHEMBL3627856) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM171371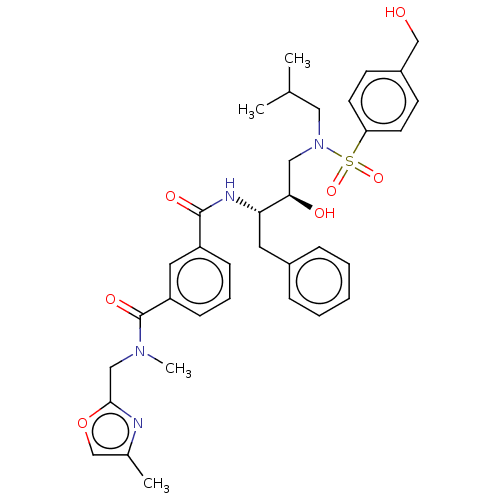 (US9085571, Table 1, Compound 19) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498813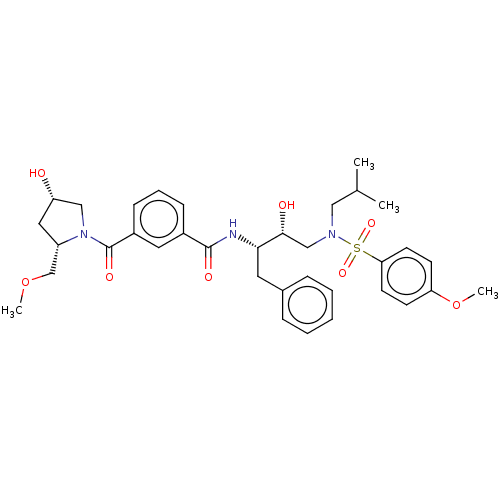 (CHEMBL3627875) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498810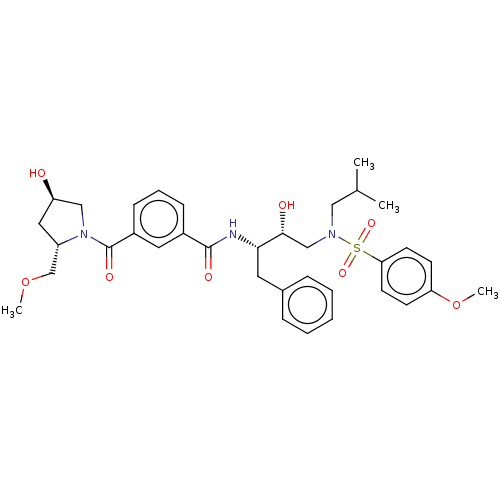 (CHEMBL3627857) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522181 (CHEMBL4456725) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498809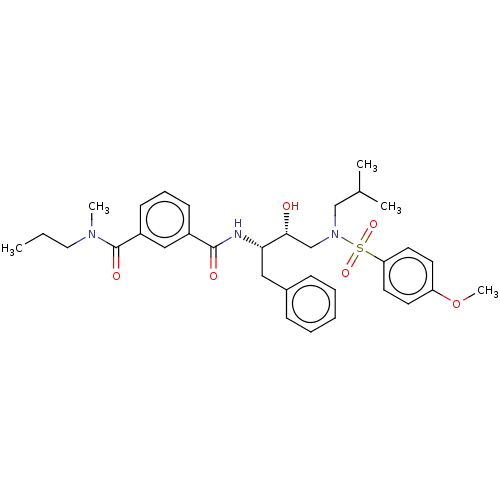 (CHEMBL3627852) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522183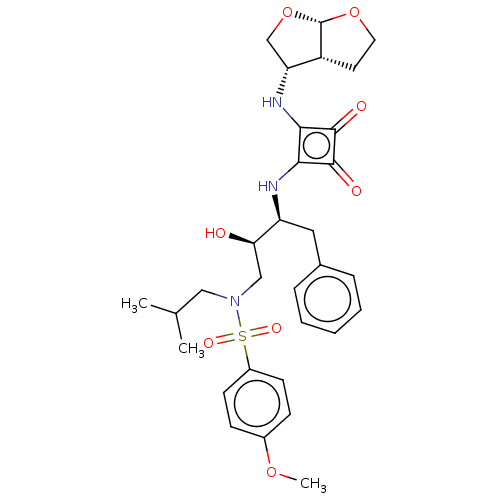 (CHEMBL4467544) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM171367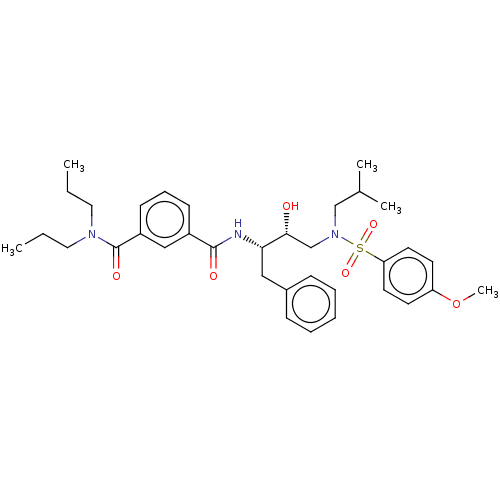 (US9085571, Table 1, Compound 15) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM171369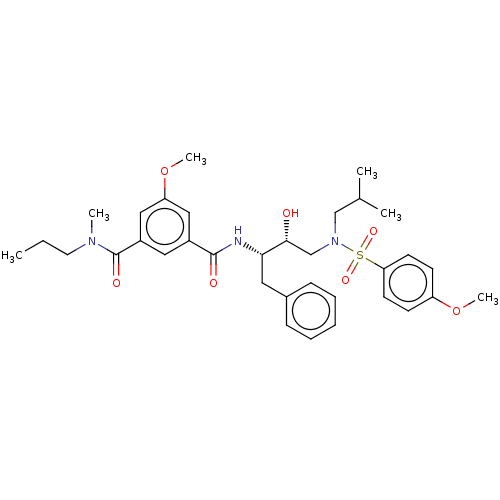 (US9085571, Table 1, Compound 17) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50498811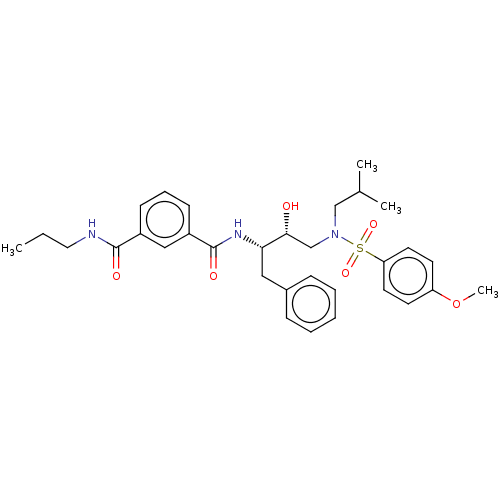 (CHEMBL3627851) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522186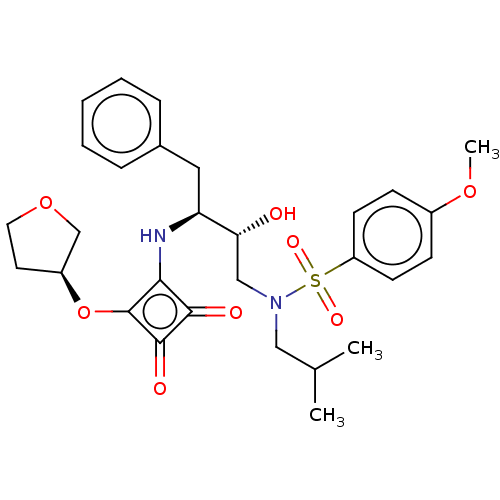 (CHEMBL4445644) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522189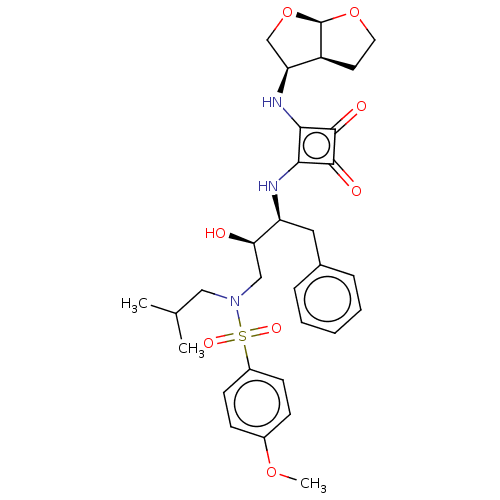 (CHEMBL4469790) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM171362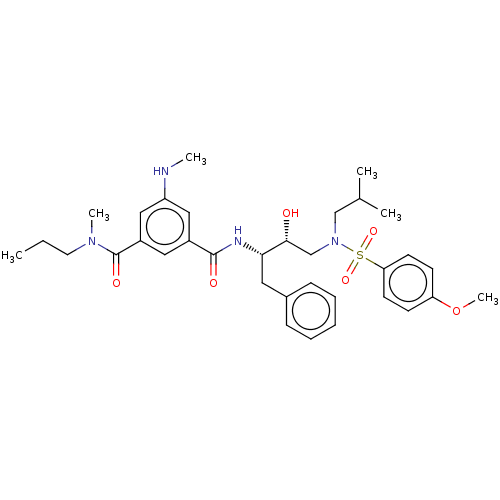 (US9085571, Table 1, Compound 10) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease by fluorometric assay | Bioorg Med Chem Lett 25: 4903-4909 (2015) Article DOI: 10.1016/j.bmcl.2015.05.052 BindingDB Entry DOI: 10.7270/Q2P84FW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522185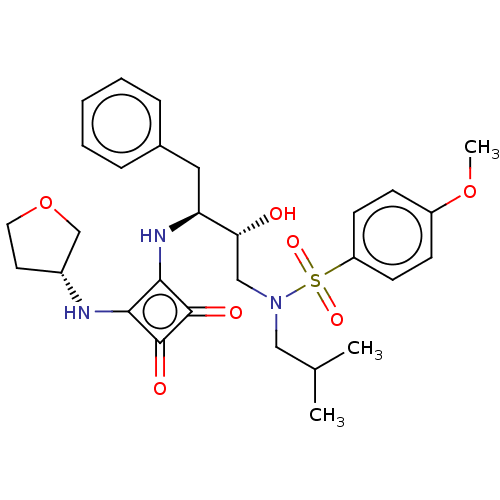 (CHEMBL4474819) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522192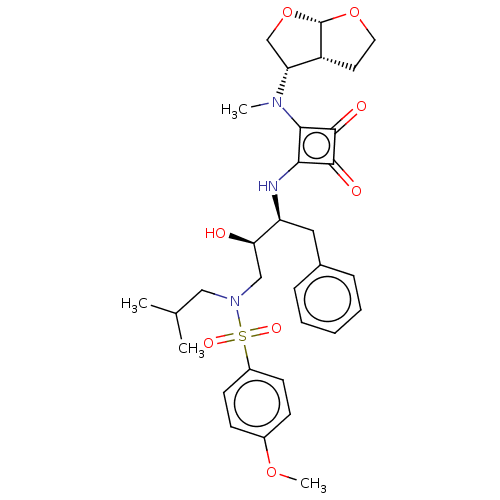 (CHEMBL4570048) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 201 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522190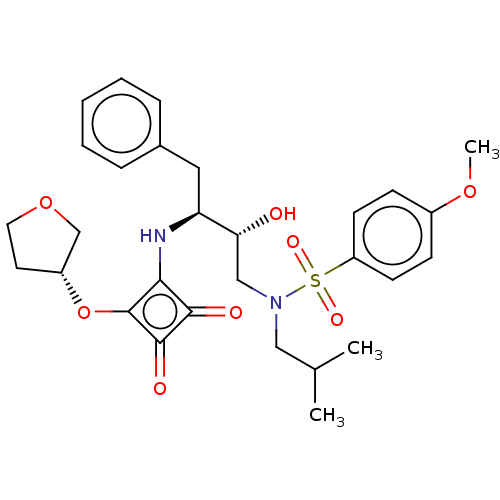 (CHEMBL4440087) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522191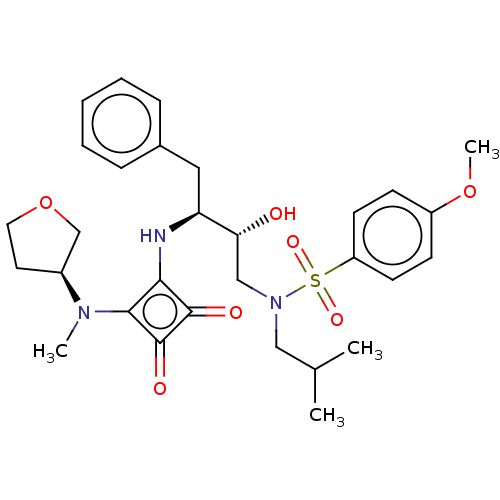 (CHEMBL4558365) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 397 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522187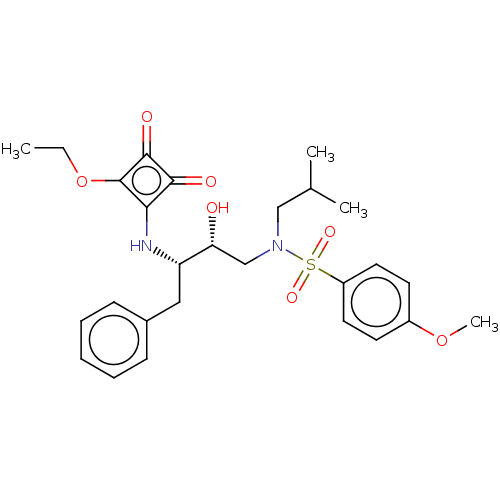 (CHEMBL4437581) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 463 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522182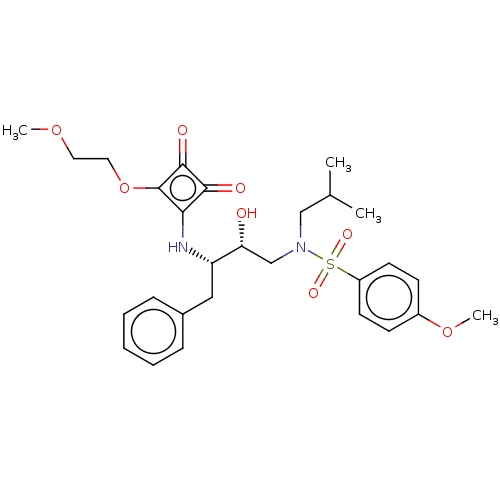 (CHEMBL4556792) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50522188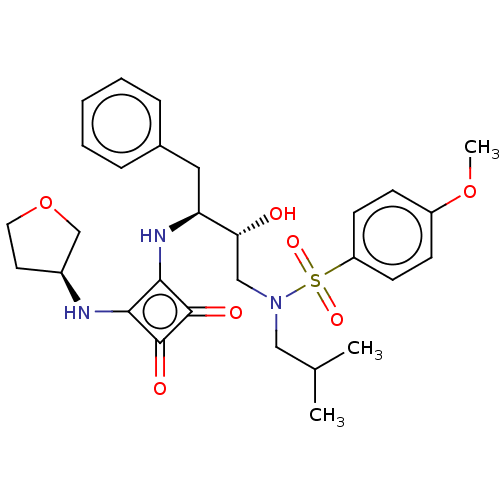 (CHEMBL4467187) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease using Ac-Thr-Ile-Nle-Nle-Gln-Arg-NH2 substrate by continuous fluorometric assay | Bioorg Med Chem Lett 29: 2565-2570 (2019) Article DOI: 10.1016/j.bmcl.2019.08.006 BindingDB Entry DOI: 10.7270/Q2JM2F1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31531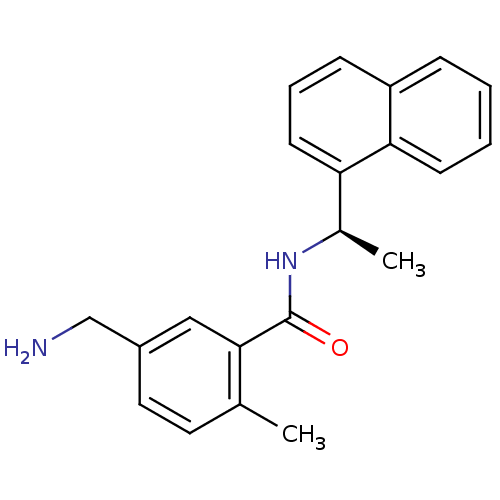 (Substituted Benzamide Derivative, 2 | med.21724, C...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31523 (Compound 6 | Naphthalene and Benzamide Derivative,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31530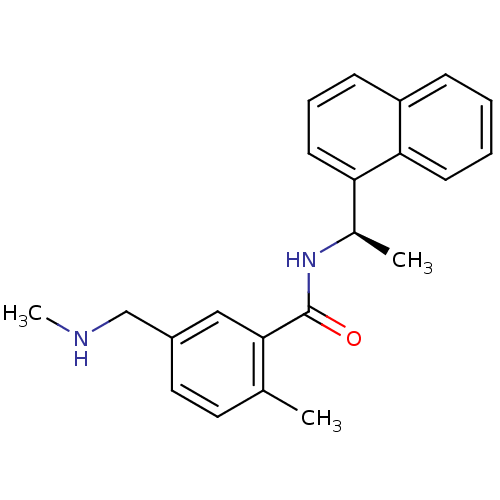 (2-methyl-5-[(methylamino)methyl]-N-[(1R)-1-(naphth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31528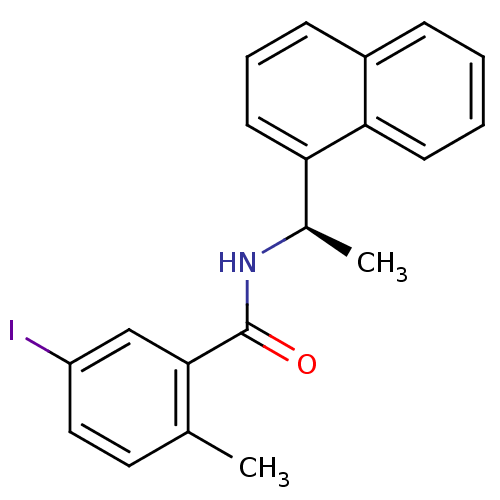 (Substituted Benzamide Derivative, 32) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31520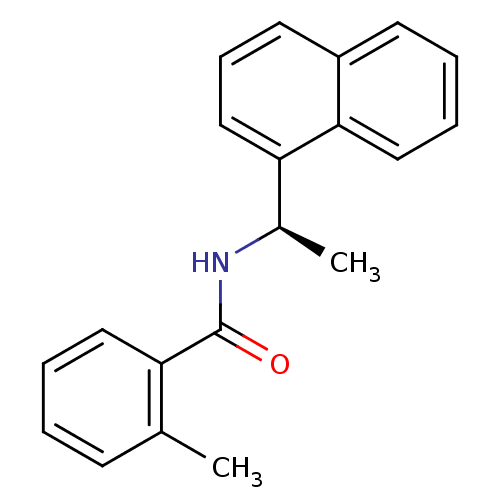 (Naphthalene and Benzamide Derivative, 5h) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31524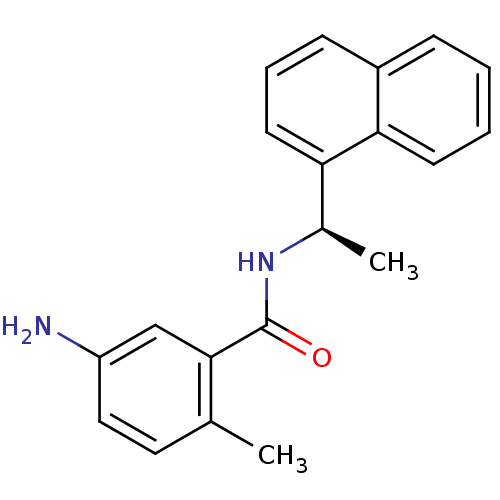 (5-amino-2-methyl-N-[(1R)-1-(naphthalen-1-yl)ethyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 2.64E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31527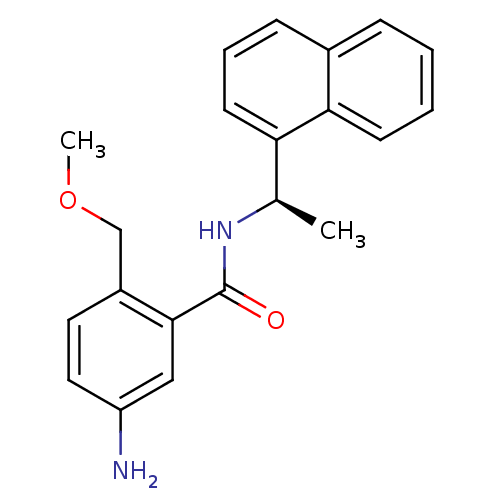 (Substituted Benzamide Derivative, 40) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31529 (Substituted Benzamide Derivative, 47) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31526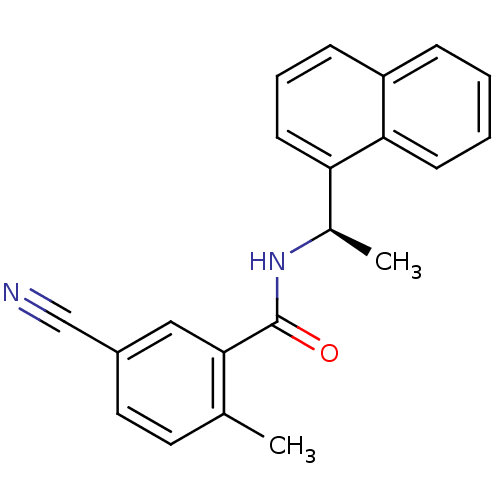 (Substituted Benzamide Derivative, 33) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31508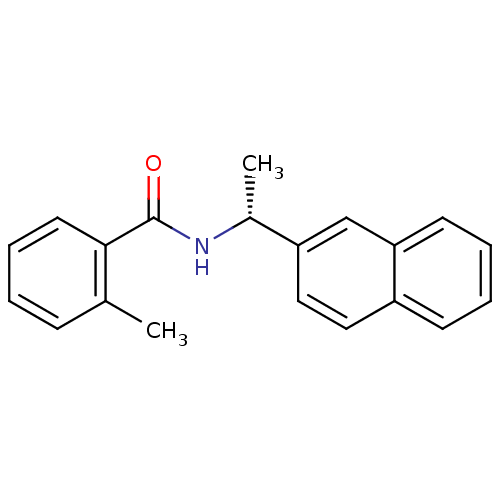 (Substituted Benzamide Derivative, lead | med.21724...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31525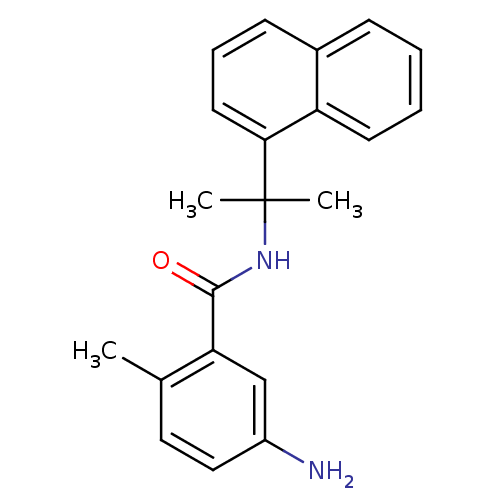 (Substituted Benzamide Derivative, 29 | med.21724, ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31514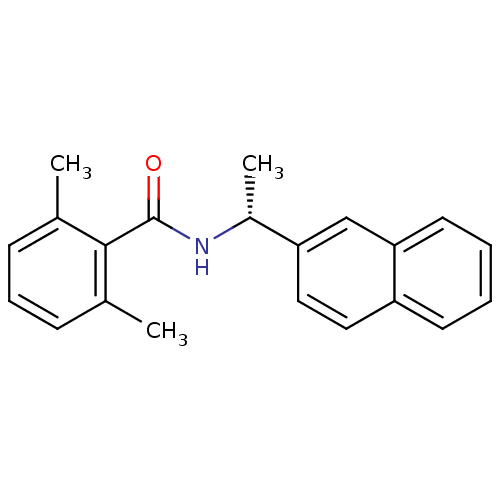 (Naphthyl and Benzamide Derivative, 5f) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31512 (Substituted Benzamide Derivative, 5d) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31509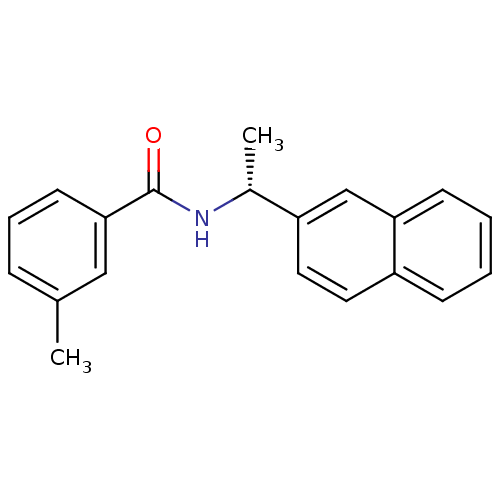 (Substituted Benzamide Derivative, 5a) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31521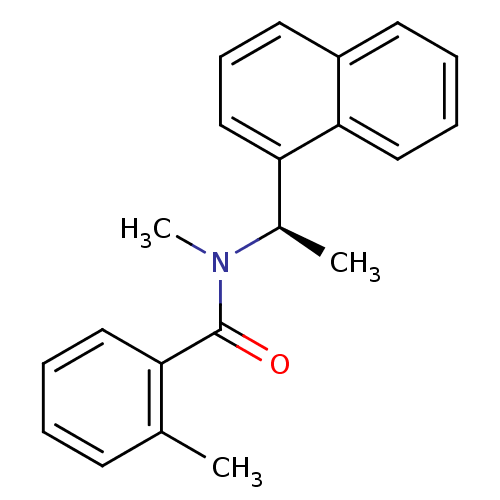 (Naphthalene and Benzamide Derivative, 21 | med.217...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31522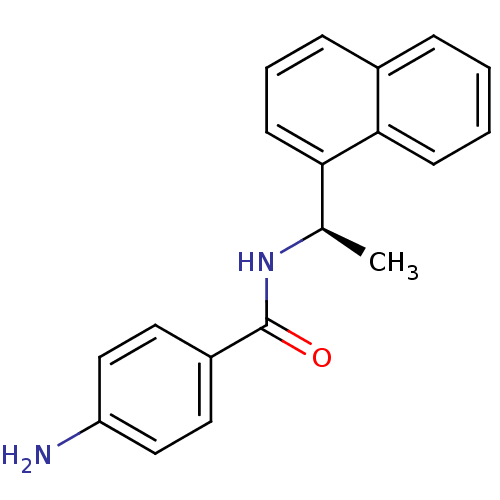 (Naphthalene and Benzamide Derivative, 23) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31510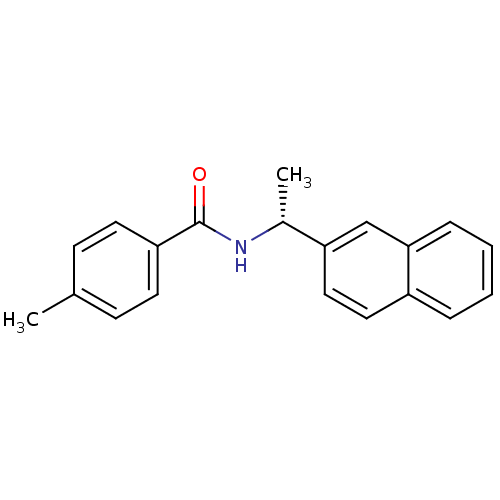 (Substituted Benzamide Derivative, 5b) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.91E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31516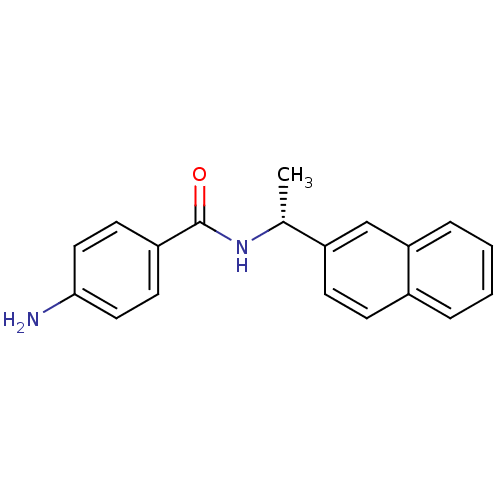 (Naphthyl and Benzamide Derivative, 9) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.61E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31511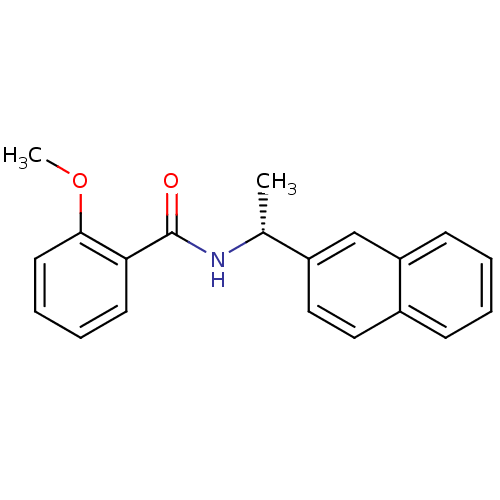 (Substituted Benzamide Derivative, 5c) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31513 (Substituted Benzamide Derivative, 5e) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.49E+5 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab [1541-1855] (Human SARS coronavirus (SARS-CoV)) | BDBM31518 (Naphthyl and Benzamide Derivative, 14 | racemic) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University | Assay Description IC50 values for all inhibitors were determined using a 96-well plate based assay. Reactions were performed in buffer containing RLRGG-AMC, 2% DMSO, a... | J Med Chem 52: 5228-40 (2009) Article DOI: 10.1021/jm900611t BindingDB Entry DOI: 10.7270/Q2P8497Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |