 Found 77 hits with Last Name = 'vermeulen' and Initial = 'j'
Found 77 hits with Last Name = 'vermeulen' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214016

(CHEMBL243342 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES Cc1nc(N)sc1S(=O)(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C27H26FN7O4S2/c1-15-25(40-26(29)30-15)41(38,39)33-19-10-8-16(9-11-19)12-21-31-22-23(32-21)34(13-17-6-7-17)27(37)35(24(22)36)14-18-4-2-3-5-20(18)28/h2-5,8-11,17,33H,6-7,12-14H2,1H3,(H2,29,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214007

(CHEMBL394621 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES Cc1nn(C)cc1S(=O)(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C28H28FN7O4S/c1-17-23(16-34(2)32-17)41(39,40)33-21-11-9-18(10-12-21)13-24-30-25-26(31-24)35(14-19-7-8-19)28(38)36(27(25)37)15-20-5-3-4-6-22(20)29/h3-6,9-12,16,19,33H,7-8,13-15H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214001

(CHEMBL389494 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES Cc1nn(C)c(Cl)c1S(=O)(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C28H27ClFN7O4S/c1-16-24(25(29)35(2)33-16)42(40,41)34-20-11-9-17(10-12-20)13-22-31-23-26(32-22)36(14-18-7-8-18)28(39)37(27(23)38)15-19-5-3-4-6-21(19)30/h3-6,9-12,18,34H,7-8,13-15H2,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214008

(CHEMBL243343 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES Cc1nc(cn1C)S(=O)(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C28H28FN7O4S/c1-17-30-24(16-34(17)2)41(39,40)33-21-11-9-18(10-12-21)13-23-31-25-26(32-23)35(14-19-7-8-19)28(38)36(27(25)37)15-20-5-3-4-6-22(20)29/h3-6,9-12,16,19,33H,7-8,13-15H2,1-2H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214003

(CHEMBL243134 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES Cn1cc(cn1)S(=O)(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C27H26FN7O4S/c1-33-16-21(13-29-33)40(38,39)32-20-10-8-17(9-11-20)12-23-30-24-25(31-23)34(14-18-6-7-18)27(37)35(26(24)36)15-19-4-2-3-5-22(19)28/h2-5,8-11,13,16,18,32H,6-7,12,14-15H2,1H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214000

(CHEMBL243180 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES Fc1ccccc1Cn1c(=O)n(CC2CC2)c2nc(Cc3ccc(NS(=O)(=O)c4cccc5cccnc45)cc3)[nH]c2c1=O Show InChI InChI=1S/C32H27FN6O4S/c33-25-8-2-1-5-23(25)19-39-31(40)29-30(38(32(39)41)18-21-10-11-21)36-27(35-29)17-20-12-14-24(15-13-20)37-44(42,43)26-9-3-6-22-7-4-16-34-28(22)26/h1-9,12-16,21,37H,10-11,17-19H2,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214004

(CHEMBL243116 | N-(4-((1-(2-fluorobenzyl)-3-butyl-2...)Show SMILES CCCCn1c2nc(Cc3ccc(NS(=O)(=O)c4c(C)nn(C)c4Cl)cc3)[nH]c2c(=O)n(Cc2ccccc2F)c1=O Show InChI InChI=1S/C28H29ClFN7O4S/c1-4-5-14-36-26-23(27(38)37(28(36)39)16-19-8-6-7-9-21(19)30)31-22(32-26)15-18-10-12-20(13-11-18)34-42(40,41)24-17(2)33-35(3)25(24)29/h6-13,34H,4-5,14-16H2,1-3H3,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214014
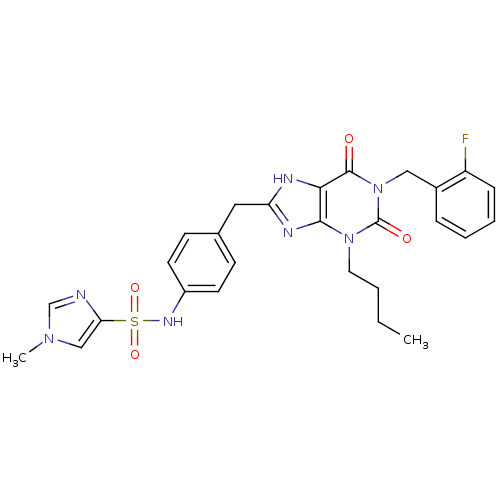
(CHEMBL245018 | N-(4-((1-(2-fluorobenzyl)-3-butyl-2...)Show SMILES CCCCn1c2nc(Cc3ccc(NS(=O)(=O)c4cn(C)cn4)cc3)[nH]c2c(=O)n(Cc2ccccc2F)c1=O Show InChI InChI=1S/C27H28FN7O4S/c1-3-4-13-34-25-24(26(36)35(27(34)37)15-19-7-5-6-8-21(19)28)30-22(31-25)14-18-9-11-20(12-10-18)32-40(38,39)23-16-33(2)17-29-23/h5-12,16-17,32H,3-4,13-15H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214010
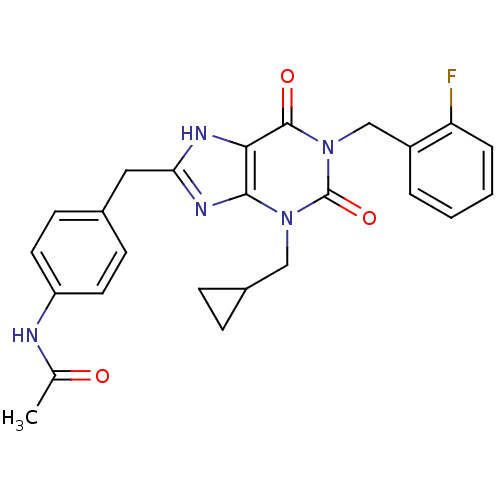
(CHEMBL244165 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES CC(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C25H24FN5O3/c1-15(32)27-19-10-8-16(9-11-19)12-21-28-22-23(29-21)30(13-17-6-7-17)25(34)31(24(22)33)14-18-4-2-3-5-20(18)26/h2-5,8-11,17H,6-7,12-14H2,1H3,(H,27,32)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214012
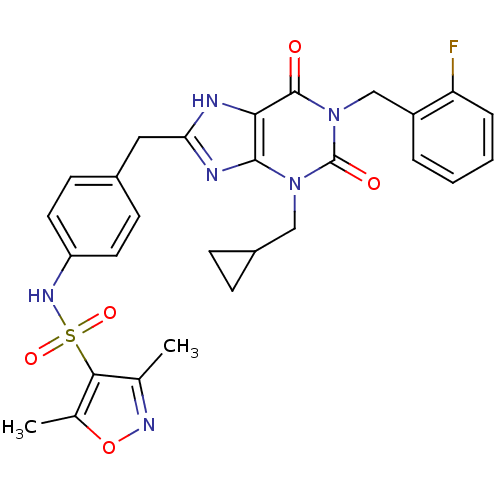
(CHEMBL394368 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES Cc1noc(C)c1S(=O)(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C28H27FN6O5S/c1-16-25(17(2)40-32-16)41(38,39)33-21-11-9-18(10-12-21)13-23-30-24-26(31-23)34(14-19-7-8-19)28(37)35(27(24)36)15-20-5-3-4-6-22(20)29/h3-6,9-12,19,33H,7-8,13-15H2,1-2H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 790 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214009

(3-(cyclopropylmethyl)-8-({4-[(dimethylsulfamoyl)am...)Show SMILES CN(C)S(=O)(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C25H27FN6O4S/c1-30(2)37(35,36)29-19-11-9-16(10-12-19)13-21-27-22-23(28-21)31(14-17-7-8-17)25(34)32(24(22)33)15-18-5-3-4-6-20(18)26/h3-6,9-12,17,29H,7-8,13-15H2,1-2H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214002
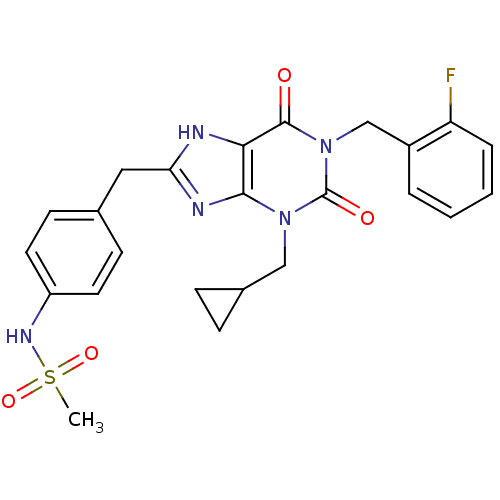
(CHEMBL244590 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES CS(=O)(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C24H24FN5O4S/c1-35(33,34)28-18-10-8-15(9-11-18)12-20-26-21-22(27-20)29(13-16-6-7-16)24(32)30(23(21)31)14-17-4-2-3-5-19(17)25/h2-5,8-11,16,28H,6-7,12-14H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214015
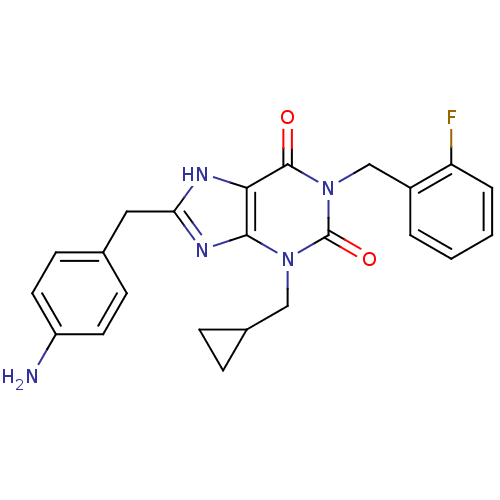
(1-(2-fluorobenzyl)-8-(4-aminobenzyl)-3-(cyclopropy...)Show SMILES Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C23H22FN5O2/c24-18-4-2-1-3-16(18)13-29-22(30)20-21(28(23(29)31)12-15-5-6-15)27-19(26-20)11-14-7-9-17(25)10-8-14/h1-4,7-10,15H,5-6,11-13,25H2,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214006

(CHEMBL397513 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES Fc1ccccc1Cn1c(=O)n(CC2CC2)c2nc(Cc3ccc(NC(=O)C(F)(F)F)cc3)[nH]c2c1=O Show InChI InChI=1S/C25H21F4N5O3/c26-18-4-2-1-3-16(18)13-34-22(35)20-21(33(24(34)37)12-15-5-6-15)32-19(31-20)11-14-7-9-17(10-8-14)30-23(36)25(27,28)29/h1-4,7-10,15H,5-6,11-13H2,(H,30,36)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214013

(CHEMBL242486 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES Fc1ccccc1Cn1c(=O)n(CC2CC2)c2nc(Cc3ccc(NS(=O)(=O)c4cccnc4)cc3)[nH]c2c1=O Show InChI InChI=1S/C28H25FN6O4S/c29-23-6-2-1-4-20(23)17-35-27(36)25-26(34(28(35)37)16-19-7-8-19)32-24(31-25)14-18-9-11-21(12-10-18)33-40(38,39)22-5-3-13-30-15-22/h1-6,9-13,15,19,33H,7-8,14,16-17H2,(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214017
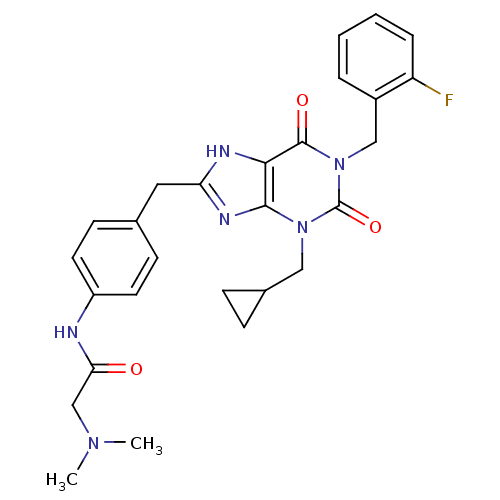
(CHEMBL244589 | N-(4-((1-(2-fluorobenzyl)-3-(cyclop...)Show SMILES CN(C)CC(=O)Nc1ccc(Cc2nc3n(CC4CC4)c(=O)n(Cc4ccccc4F)c(=O)c3[nH]2)cc1 Show InChI InChI=1S/C27H29FN6O3/c1-32(2)16-23(35)29-20-11-9-17(10-12-20)13-22-30-24-25(31-22)33(14-18-7-8-18)27(37)34(26(24)36)15-19-5-3-4-6-21(19)28/h3-6,9-12,18H,7-8,13-16H2,1-2H3,(H,29,35)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50133801

(CHEMBL120708 | N-(4-((1-(2-fluorobenzyl)-3-butyl-2...)Show SMILES CCCCn1c2nc(Cc3ccc(NC(C)=O)cc3)[nH]c2c(=O)n(Cc2ccccc2F)c1=O Show InChI InChI=1S/C25H26FN5O3/c1-3-4-13-30-23-22(24(33)31(25(30)34)15-18-7-5-6-8-20(18)26)28-21(29-23)14-17-9-11-19(12-10-17)27-16(2)32/h5-12H,3-4,13-15H2,1-2H3,(H,27,32)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214018
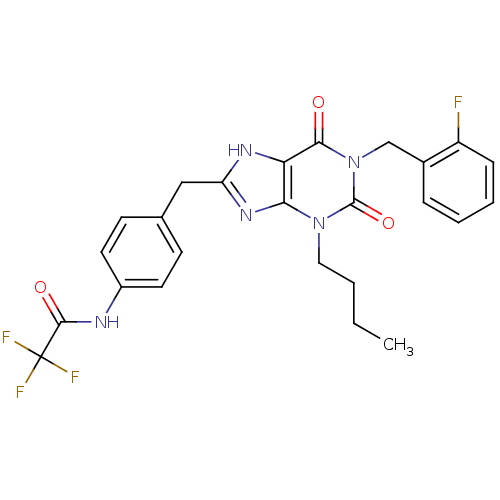
(CHEMBL428567 | N-(4-((1-(2-fluorobenzyl)-3-butyl-2...)Show SMILES CCCCn1c2nc(Cc3ccc(NC(=O)C(F)(F)F)cc3)[nH]c2c(=O)n(Cc2ccccc2F)c1=O Show InChI InChI=1S/C25H23F4N5O3/c1-2-3-12-33-21-20(22(35)34(24(33)37)14-16-6-4-5-7-18(16)26)31-19(32-21)13-15-8-10-17(11-9-15)30-23(36)25(27,28)29/h4-11H,2-3,12-14H2,1H3,(H,30,36)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214011

(1-(2-fluorobenzyl)-8-(4-aminobenzyl)-3-butyl-1H-pu...)Show SMILES CCCCn1c2nc(Cc3ccc(N)cc3)[nH]c2c(=O)n(Cc2ccccc2F)c1=O Show InChI InChI=1S/C23H24FN5O2/c1-2-3-12-28-21-20(26-19(27-21)13-15-8-10-17(25)11-9-15)22(30)29(23(28)31)14-16-6-4-5-7-18(16)24/h4-11H,2-3,12-14,25H2,1H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214019

(8-(2,4-diaminobenzyl)-1-(2-fluorobenzyl)-3-butyl-1...)Show SMILES CCCCn1c2nc(Cc3ccc(N)cc3N)[nH]c2c(=O)n(Cc2ccccc2F)c1=O Show InChI InChI=1S/C23H25FN6O2/c1-2-3-10-29-21-20(27-19(28-21)11-14-8-9-16(25)12-18(14)26)22(31)30(23(29)32)13-15-6-4-5-7-17(15)24/h4-9,12H,2-3,10-11,13,25-26H2,1H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Phosphoenolpyruvate carboxykinase, cytosolic [GTP]
(Homo sapiens (Human)) | BDBM50214005
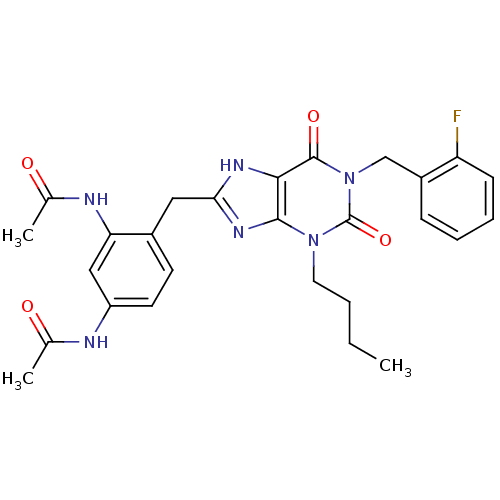
(CHEMBL242437 | N-{3-acetylamino-4-[3-butyl-1-(2-fl...)Show SMILES CCCCn1c2nc(Cc3ccc(NC(C)=O)cc3NC(C)=O)[nH]c2c(=O)n(Cc2ccccc2F)c1=O Show InChI InChI=1S/C27H29FN6O4/c1-4-5-12-33-25-24(26(37)34(27(33)38)15-19-8-6-7-9-21(19)28)31-23(32-25)13-18-10-11-20(29-16(2)35)14-22(18)30-17(3)36/h6-11,14H,4-5,12-13,15H2,1-3H3,(H,29,35)(H,30,36)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human cPEPCK |
Bioorg Med Chem Lett 17: 3835-9 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.013
BindingDB Entry DOI: 10.7270/Q2BV7HF1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50012905
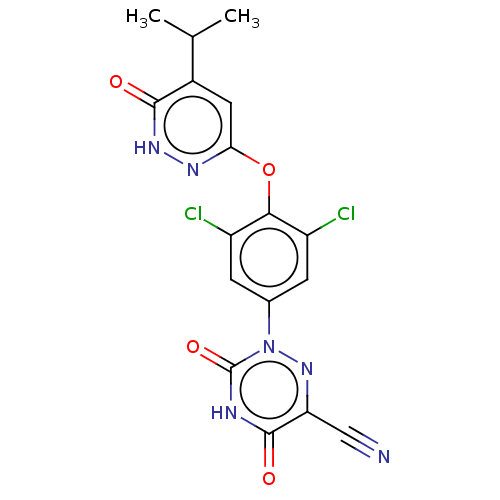
(CHEMBL3261331 | US20240059676, Compound MGL-3196 |...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc(cc2Cl)-n2nc(C#N)c(=O)[nH]c2=O)n[nH]c1=O Show InChI InChI=1S/C17H12Cl2N6O4/c1-7(2)9-5-13(22-23-15(9)26)29-14-10(18)3-8(4-11(14)19)25-17(28)21-16(27)12(6-20)24-25/h3-5,7H,1-2H3,(H,23,26)(H,21,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50012905

(CHEMBL3261331 | US20240059676, Compound MGL-3196 |...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc(cc2Cl)-n2nc(C#N)c(=O)[nH]c2=O)n[nH]c1=O Show InChI InChI=1S/C17H12Cl2N6O4/c1-7(2)9-5-13(22-23-15(9)26)29-14-10(18)3-8(4-11(14)19)25-17(28)21-16(27)12(6-20)24-25/h3-5,7H,1-2H3,(H,23,26)(H,21,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 (unknown origin) |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A5
(Homo sapiens (Human)) | BDBM50012905

(CHEMBL3261331 | US20240059676, Compound MGL-3196 |...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc(cc2Cl)-n2nc(C#N)c(=O)[nH]c2=O)n[nH]c1=O Show InChI InChI=1S/C17H12Cl2N6O4/c1-7(2)9-5-13(22-23-15(9)26)29-14-10(18)3-8(4-11(14)19)25-17(28)21-16(27)12(6-20)24-25/h3-5,7H,1-2H3,(H,23,26)(H,21,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A5 (unknown origin) |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50012905

(CHEMBL3261331 | US20240059676, Compound MGL-3196 |...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc(cc2Cl)-n2nc(C#N)c(=O)[nH]c2=O)n[nH]c1=O Show InChI InChI=1S/C17H12Cl2N6O4/c1-7(2)9-5-13(22-23-15(9)26)29-14-10(18)3-8(4-11(14)19)25-17(28)21-16(27)12(6-20)24-25/h3-5,7H,1-2H3,(H,23,26)(H,21,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50012817
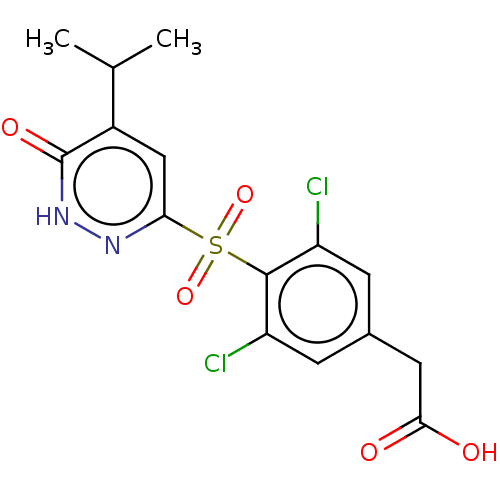
(CHEMBL3261330 | USRE46024, 19)Show SMILES CC(C)c1cc(n[nH]c1=O)S(=O)(=O)c1c(Cl)cc(CC(O)=O)cc1Cl Show InChI InChI=1S/C15H14Cl2N2O5S/c1-7(2)9-6-12(18-19-15(9)22)25(23,24)14-10(16)3-8(4-11(14)17)5-13(20)21/h3-4,6-7H,5H2,1-2H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.98E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50012859

(CHEMBL3261319 | USRE46024, 2)Show SMILES CC(C)c1cc(Oc2c(C)cc(CC(O)=O)cc2Cl)n[nH]c1=O Show InChI InChI=1S/C16H17ClN2O4/c1-8(2)11-7-13(18-19-16(11)22)23-15-9(3)4-10(5-12(15)17)6-14(20)21/h4-5,7-8H,6H2,1-3H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.01E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012901

(CHEMBL3261320 | USRE46024, 4)Show SMILES CC(C)c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)n[nH]c1=O Show InChI InChI=1S/C15H14Cl2N2O4/c1-7(2)9-6-12(18-19-15(9)22)23-14-10(16)3-8(4-11(14)17)5-13(20)21/h3-4,6-7H,5H2,1-2H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.01E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50012902
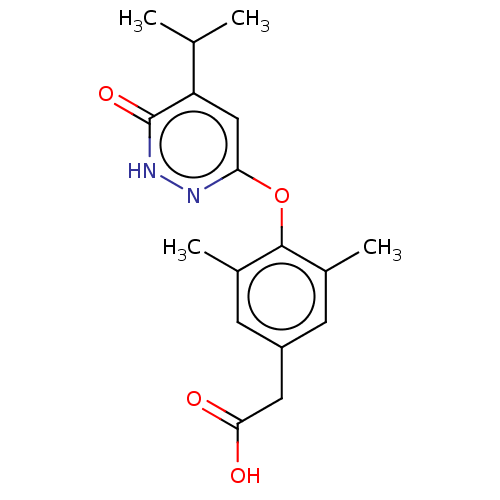
(CHEMBL3261318 | USRE46024, 1)Show InChI InChI=1S/C17H20N2O4/c1-9(2)13-8-14(18-19-17(13)22)23-16-10(3)5-12(6-11(16)4)7-15(20)21/h5-6,8-9H,7H2,1-4H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.75E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012903

(CHEMBL3261328 | USRE46024, 17)Show SMILES CC(C)c1cc(Sc2c(Cl)cc(CC(O)=O)cc2Cl)n[nH]c1=O Show InChI InChI=1S/C15H14Cl2N2O3S/c1-7(2)9-6-12(18-19-15(9)22)23-14-10(16)3-8(4-11(14)17)5-13(20)21/h3-4,6-7H,5H2,1-2H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 8.88E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50012904
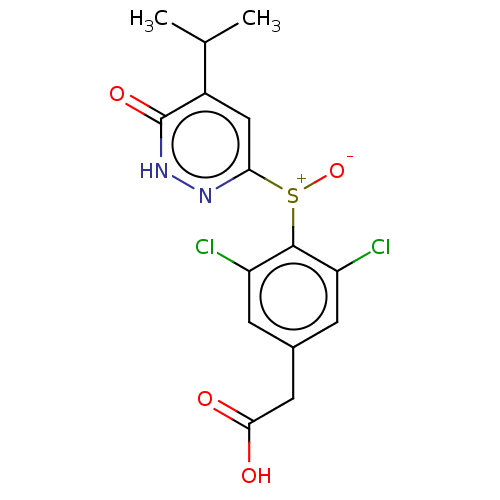
(CHEMBL3261329)Show SMILES CC(C)c1cc(n[nH]c1=O)[S+]([O-])c1c(Cl)cc(CC(O)=O)cc1Cl Show InChI InChI=1S/C15H14Cl2N2O4S/c1-7(2)9-6-12(18-19-15(9)22)24(23)14-10(16)3-8(4-11(14)17)5-13(20)21/h3-4,6-7H,5H2,1-2H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.17E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012859

(CHEMBL3261319 | USRE46024, 2)Show SMILES CC(C)c1cc(Oc2c(C)cc(CC(O)=O)cc2Cl)n[nH]c1=O Show InChI InChI=1S/C16H17ClN2O4/c1-8(2)11-7-13(18-19-16(11)22)23-15-9(3)4-10(5-12(15)17)6-14(20)21/h4-5,7-8H,6H2,1-3H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012817

(CHEMBL3261330 | USRE46024, 19)Show SMILES CC(C)c1cc(n[nH]c1=O)S(=O)(=O)c1c(Cl)cc(CC(O)=O)cc1Cl Show InChI InChI=1S/C15H14Cl2N2O5S/c1-7(2)9-6-12(18-19-15(9)22)25(23,24)14-10(16)3-8(4-11(14)17)5-13(20)21/h3-4,6-7H,5H2,1-2H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012902

(CHEMBL3261318 | USRE46024, 1)Show InChI InChI=1S/C17H20N2O4/c1-9(2)13-8-14(18-19-17(13)22)23-16-10(3)5-12(6-11(16)4)7-15(20)21/h5-6,8-9H,7H2,1-4H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012906
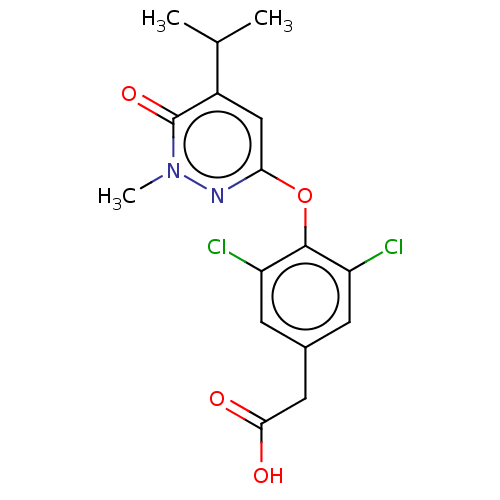
(CHEMBL3261323 | USRE46024, 10)Show SMILES CC(C)c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)nn(C)c1=O Show InChI InChI=1S/C16H16Cl2N2O4/c1-8(2)10-7-13(19-20(3)16(10)23)24-15-11(17)4-9(5-12(15)18)6-14(21)22/h4-5,7-8H,6H2,1-3H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 780 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012907

(CHEMBL3261336 | USRE46024, 13)Show SMILES CC(C)c1cc(Cc2c(Cl)cc(cc2Cl)-n2ncc(=O)[nH]c2=O)n[nH]c1=O Show InChI InChI=1S/C17H15Cl2N5O3/c1-8(2)11-3-9(22-23-16(11)26)4-12-13(18)5-10(6-14(12)19)24-17(27)21-15(25)7-20-24/h3,5-8H,4H2,1-2H3,(H,23,26)(H,21,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 820 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012908

(CHEMBL3261334 | USRE46024, 22)Show SMILES CC(C)c1cc(Cc2c(Cl)cc(cc2Cl)-n2nc(C#N)c(=O)[nH]c2=O)nn(C)c1=O Show InChI InChI=1S/C19H16Cl2N6O3/c1-9(2)12-4-10(24-26(3)18(12)29)5-13-14(20)6-11(7-15(13)21)27-19(30)23-17(28)16(8-22)25-27/h4,6-7,9H,5H2,1-3H3,(H,23,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50012903

(CHEMBL3261328 | USRE46024, 17)Show SMILES CC(C)c1cc(Sc2c(Cl)cc(CC(O)=O)cc2Cl)n[nH]c1=O Show InChI InChI=1S/C15H14Cl2N2O3S/c1-7(2)9-6-12(18-19-15(9)22)23-14-10(16)3-8(4-11(14)17)5-13(20)21/h3-4,6-7H,5H2,1-2H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.04E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50012910

(CHEMBL3261326 | USRE46024, 14)Show SMILES CC(C)c1cc(Cc2c(Cl)cc(CC(O)=O)cc2Cl)n[nH]c1=O Show InChI InChI=1S/C16H16Cl2N2O3/c1-8(2)11-6-10(19-20-16(11)23)7-12-13(17)3-9(4-14(12)18)5-15(21)22/h3-4,6,8H,5,7H2,1-2H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.99E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50012901

(CHEMBL3261320 | USRE46024, 4)Show SMILES CC(C)c1cc(Oc2c(Cl)cc(CC(O)=O)cc2Cl)n[nH]c1=O Show InChI InChI=1S/C15H14Cl2N2O4/c1-7(2)9-6-12(18-19-15(9)22)23-14-10(16)3-8(4-11(14)17)5-13(20)21/h3-4,6-7H,5H2,1-2H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012911

(CHEMBL3261332 | USRE46024, 12)Show SMILES CC(C)c1cc(Cc2c(Cl)cc(cc2Cl)-n2nc(C#N)c(=O)[nH]c2=O)n[nH]c1=O Show InChI InChI=1S/C18H14Cl2N6O3/c1-8(2)11-3-9(23-24-16(11)27)4-12-13(19)5-10(6-14(12)20)26-18(29)22-17(28)15(7-21)25-26/h3,5-6,8H,4H2,1-2H3,(H,24,27)(H,22,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012912

(CHEMBL3261321 | USRE46024, 3)Show SMILES CC(C)c1cc(Oc2c(Br)cc(CC(O)=O)cc2Br)n[nH]c1=O Show InChI InChI=1S/C15H14Br2N2O4/c1-7(2)9-6-12(18-19-15(9)22)23-14-10(16)3-8(4-11(14)17)5-13(20)21/h3-4,6-7H,5H2,1-2H3,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012905

(CHEMBL3261331 | US20240059676, Compound MGL-3196 |...)Show SMILES CC(C)c1cc(Oc2c(Cl)cc(cc2Cl)-n2nc(C#N)c(=O)[nH]c2=O)n[nH]c1=O Show InChI InChI=1S/C17H12Cl2N6O4/c1-7(2)9-5-13(22-23-15(9)26)29-14-10(18)3-8(4-11(14)19)25-17(28)21-16(27)12(6-20)24-25/h3-5,7H,1-2H3,(H,23,26)(H,21,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.74E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50012910

(CHEMBL3261326 | USRE46024, 14)Show SMILES CC(C)c1cc(Cc2c(Cl)cc(CC(O)=O)cc2Cl)n[nH]c1=O Show InChI InChI=1S/C16H16Cl2N2O3/c1-8(2)11-6-10(19-20-16(11)23)7-12-13(17)3-9(4-14(12)18)5-15(21)22/h3-4,6,8H,5,7H2,1-2H3,(H,20,23)(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.29E+3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM50115668
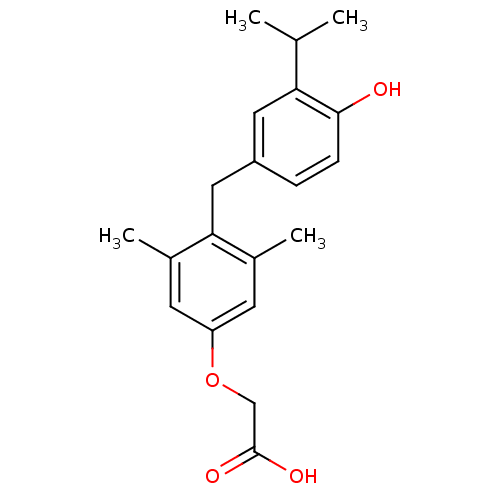
(3,5-dimethyl-4-(4'-hydroxy-3'-isopropylbenzyl)phen...)Show InChI InChI=1S/C20H24O4/c1-12(2)17-9-15(5-6-19(17)21)10-18-13(3)7-16(8-14(18)4)24-11-20(22)23/h5-9,12,21H,10-11H2,1-4H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18869
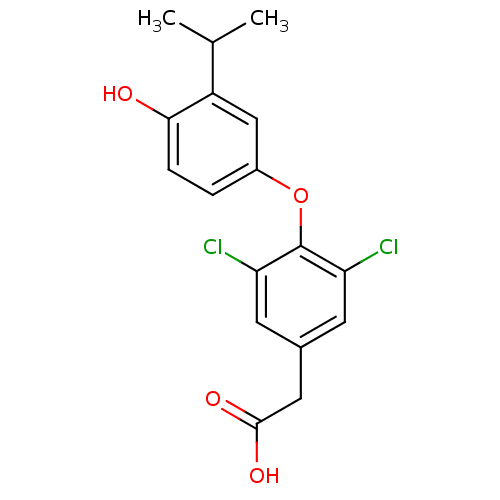
(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor alpha
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-alpha (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 ... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18860

((2R)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-di...)Show SMILES N[C@@H](Cc1cc(I)c(Oc2ccc(O)c(I)c2)c(I)c1)C(O)=O |r| Show InChI InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM50115668

(3,5-dimethyl-4-(4'-hydroxy-3'-isopropylbenzyl)phen...)Show InChI InChI=1S/C20H24O4/c1-12(2)17-9-15(5-6-19(17)21)10-18-13(3)7-16(8-14(18)4)24-11-20(22)23/h5-9,12,21H,10-11H2,1-4H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 18 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thyroid hormone receptor beta
(Homo sapiens (Human)) | BDBM18869

(2-{3,5-dichloro-4-[4-hydroxy-3-(propan-2-yl)phenox...)Show InChI InChI=1S/C17H16Cl2O4/c1-9(2)12-8-11(3-4-15(12)20)23-17-13(18)5-10(6-14(17)19)7-16(21)22/h3-6,8-9,20H,7H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Madrigal Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at recombinant His6-tagged THR-beta (unknown origin) expressed in Escherichia coli BL21(DE3) co-expressing RXR preincubated for 30 m... |
J Med Chem 57: 3912-23 (2014)
Article DOI: 10.1021/jm4019299
BindingDB Entry DOI: 10.7270/Q23T9JRQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data