

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535447 (CHEMBL4453318) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86282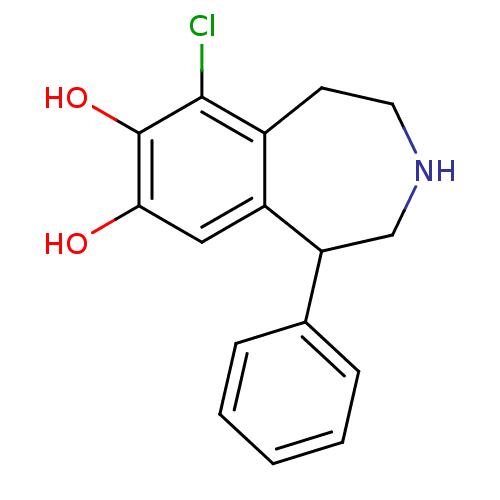 (6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535448 (CHEMBL4469983) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160875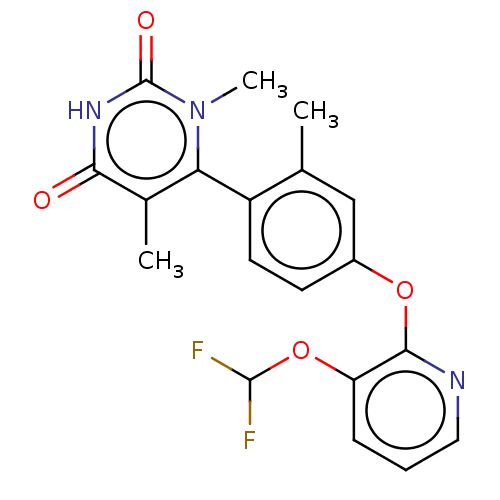 (US10093655, Example 45 | US11014909, Example 45 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Displacement of [3H]-SCH23390 from wild type human D1R expressed in HEK293 cell membranes incubated for 90 mins by scintillation counting based compe... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50554937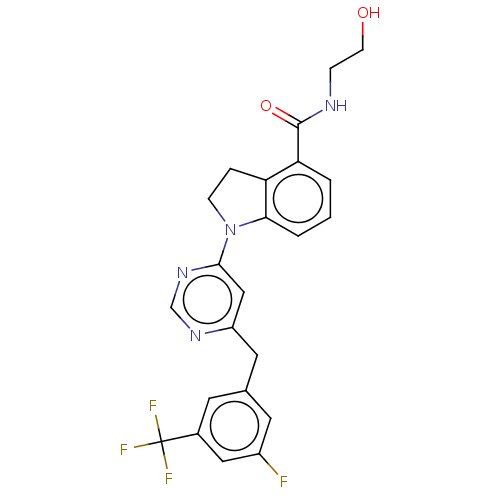 (CHEMBL4780654 | US20240059655, Compound PW0787) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to 5-HT2C (unknown origin) assessed as inhibition of ligand binding | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D2R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gi-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50018958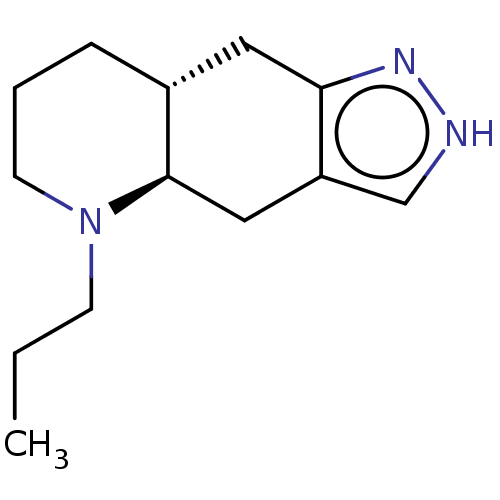 (CHEBI:75401 | QUINPIROLE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D4R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gi-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM86282 (6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D5R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM160875 (US10093655, Example 45 | US11014909, Example 45 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D5R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535455 (CHEMBL4538358) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 450 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535447 (CHEMBL4453318) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 35 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D5R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935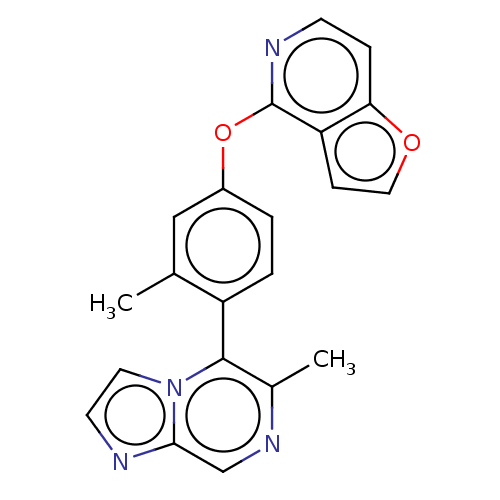 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86282 (6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50523450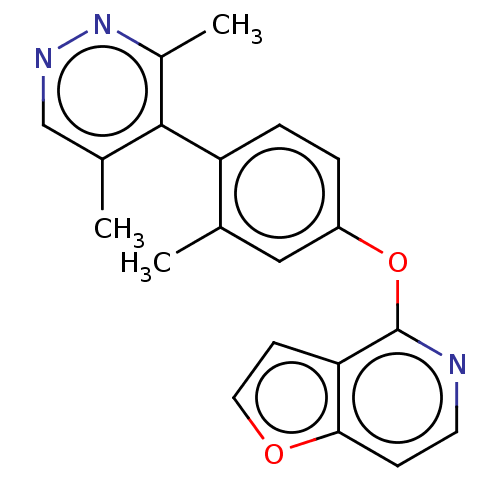 (CHEMBL4437012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 81 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535456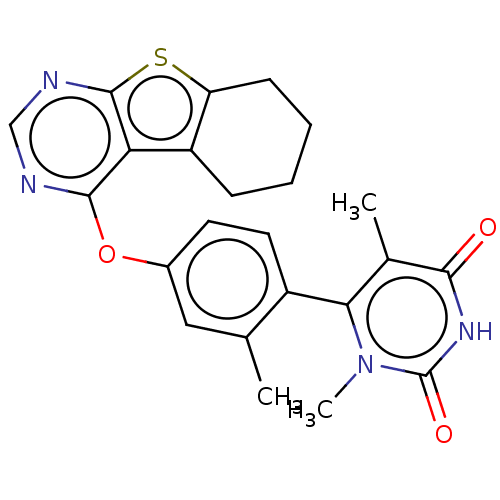 (CHEMBL4475914) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535457 (CHEMBL4449782) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 410 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535448 (CHEMBL4469983) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 490 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D4R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gi-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1B) dopamine receptor (Homo sapiens (Human)) | BDBM50535447 (CHEMBL4453318) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D5R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50018958 (CHEBI:75401 | QUINPIROLE) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at D2R (unknown origin) expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gi-cAMP Glosensor... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535446 (CHEMBL4565185) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 580 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86282 (6-Chloro-1-phenyl-2,3,4,5-tetrahydro-1H-benzo[d]az...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535448 (CHEMBL4469983) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535449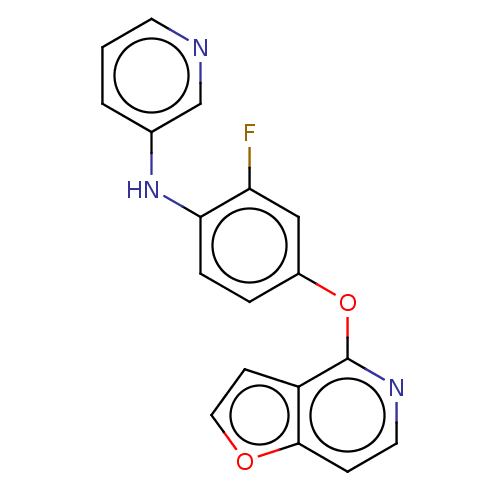 (CHEMBL4573462) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535447 (CHEMBL4453318) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535450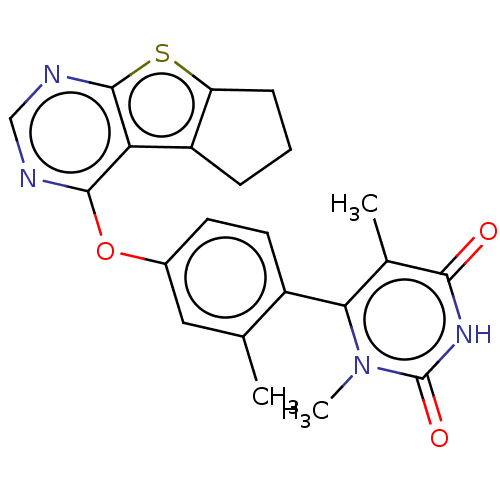 (CHEMBL4458646) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535451 (CHEMBL4470808) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 610 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM160875 (US10093655, Example 45 | US11014909, Example 45 | ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535452 (CHEMBL4445861) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535453 (CHEMBL4475239) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50535454 (CHEMBL4559240) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 950 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on cAMP accumulation incubated for 10 mins by Gs-cAMP Glosensor ... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50465935 (CHEMBL4277264) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 320 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50523450 (CHEMBL4437012) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a |
University of Texas Medical Branch Curated by ChEMBL | Assay Description Agonist activity at wild type human D1R expressed in HEK293 cells assessed as effect on beta-arrestin2 recruitment by PRESTO-Tango beta-arrestin2 rec... | ACS Med Chem Lett 10: 792-799 (2019) Article DOI: 10.1021/acsmedchemlett.9b00050 BindingDB Entry DOI: 10.7270/Q26W9FKV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554904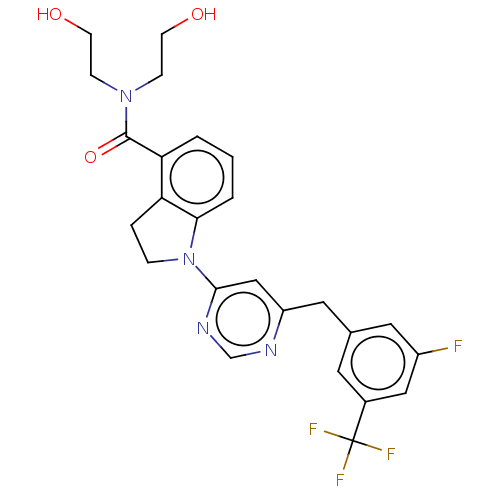 (CHEMBL4761550 | US20240059655, Compound PW0955) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 673 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554905 (CHEMBL4741318 | US20240059655, Compound PW0948) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 431 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554906 (CHEMBL4788591 | US20240059655, Compound PW0940) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 760 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554907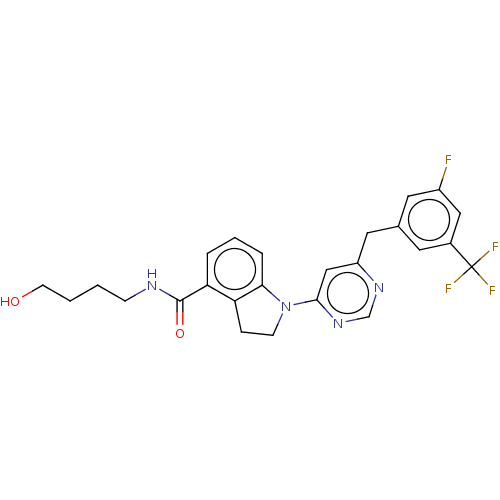 (CHEMBL4754346 | US20240059655, Compound PW0939) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 399 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554908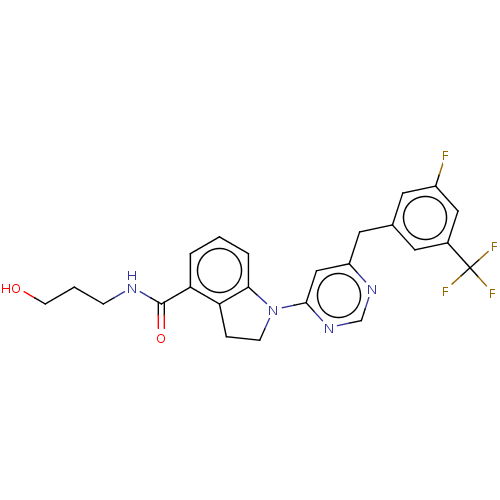 (CHEMBL4740473 | US20240059655, Compound PW0938) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 275 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554909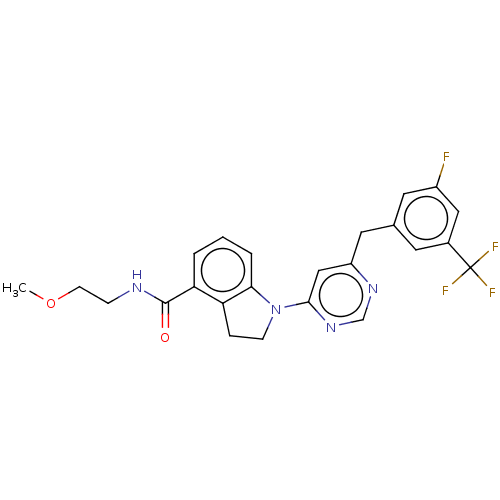 (CHEMBL4748048 | US20240059655, Compound PW0937) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 489 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554910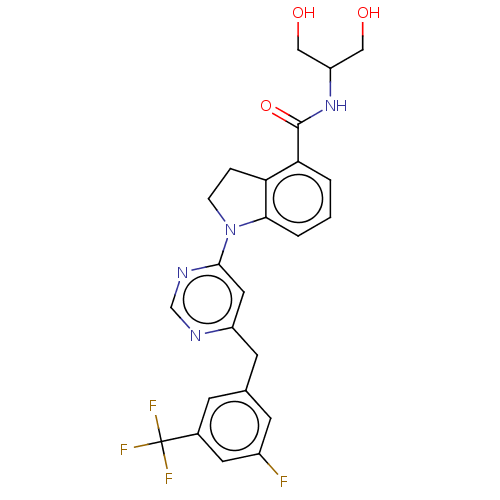 (CHEMBL4755614 | US20240059655, Compound PW0936) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 346 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554911 (CHEMBL4751311 | US20240059655, Compound PW0904) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 371 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554912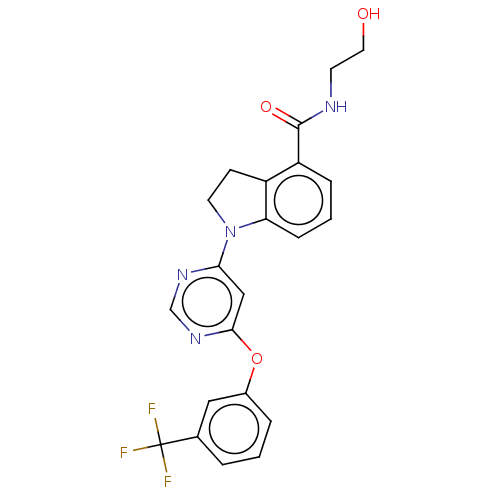 (CHEMBL4779168 | US20240059655, Compound PW0903) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554913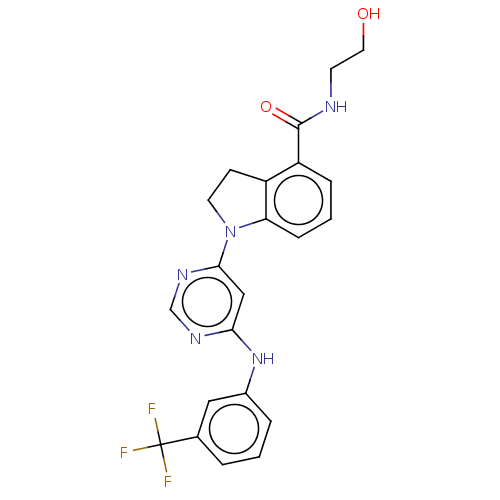 (CHEMBL4739868 | US20240059655, Compound PW0898) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.11E+3 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554914 (CHEMBL4790115 | US20240059655, Compound PW0875) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 557 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554915 (CHEMBL4797286) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 330 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554916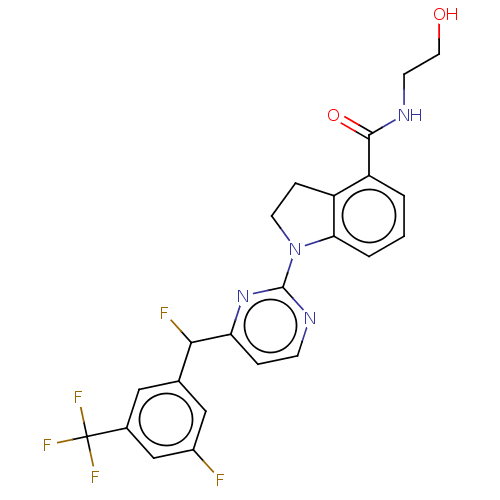 (CHEMBL4754435 | US20240059655, Compound PW0781) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 329 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 52 (Homo sapiens (Human)) | BDBM50554917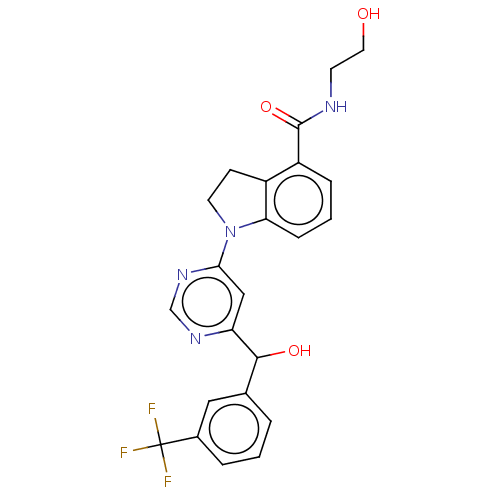 (CHEMBL4781520) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 560 | n/a | n/a | n/a | n/a |
TBA | Assay Description Agonist activity at human GPR52 expressed in HEK293 cells assessed as increase in cAMP levels incubated for 15 mins by Glosensor cAMP assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01498 BindingDB Entry DOI: 10.7270/Q22N55XV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 176 total ) | Next | Last >> |