 Found 74 hits with Last Name = 'reid' and Initial = 'jc'
Found 74 hits with Last Name = 'reid' and Initial = 'jc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50460673
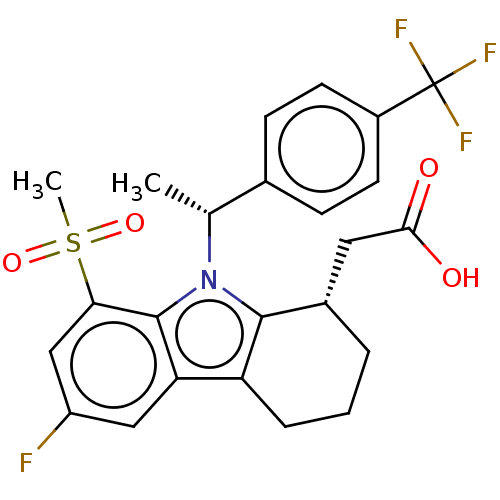
(CHEMBL4229054)Show SMILES C[C@H](c1ccc(cc1)C(F)(F)F)n1c2[C@H](CC(O)=O)CCCc2c2cc(F)cc(c12)S(C)(=O)=O |r| Show InChI InChI=1S/C24H23F4NO4S/c1-13(14-6-8-16(9-7-14)24(26,27)28)29-22-15(10-21(30)31)4-3-5-18(22)19-11-17(25)12-20(23(19)29)34(2,32)33/h6-9,11-13,15H,3-5,10H2,1-2H3,(H,30,31)/t13-,15+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 674 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50460670
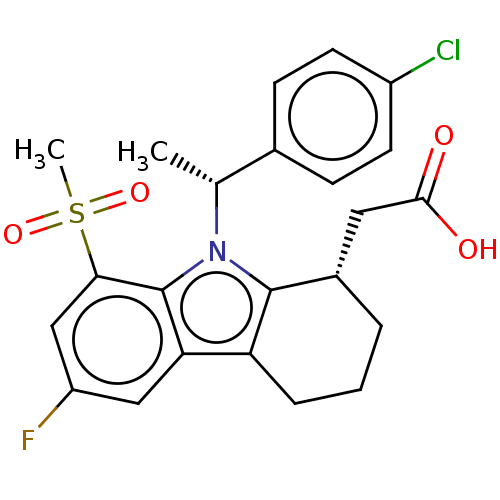
(CHEMBL4228792)Show SMILES C[C@H](c1ccc(Cl)cc1)n1c2[C@H](CC(O)=O)CCCc2c2cc(F)cc(c12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H23ClFNO4S/c1-13(14-6-8-16(24)9-7-14)26-22-15(10-21(27)28)4-3-5-18(22)19-11-17(25)12-20(23(19)26)31(2,29)30/h6-9,11-13,15H,3-5,10H2,1-2H3,(H,27,28)/t13-,15+/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 873 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479470

(CHEMBL489586 | MK-4965)Show SMILES Nc1ccc2c(COc3ccc(Cl)c(Oc4cc(Cl)cc(c4)C#N)c3)n[nH]c2n1 Show InChI InChI=1S/C20H13Cl2N5O2/c21-12-5-11(9-23)6-14(7-12)29-18-8-13(1-3-16(18)22)28-10-17-15-2-4-19(24)25-20(15)27-26-17/h1-8H,10H2,(H3,24,25,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 RT polymerase by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479471
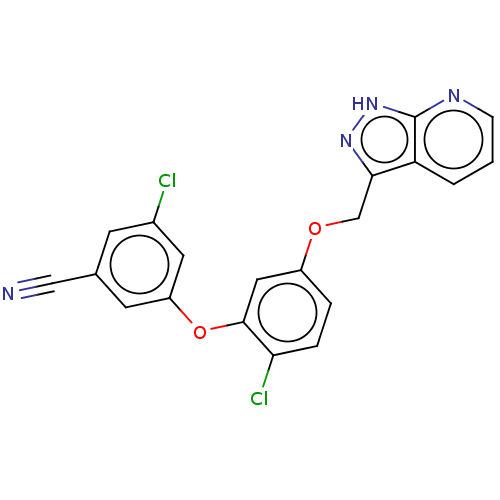
(CHEMBL491019 | MK-1107)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ncccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-6-12(10-23)7-15(8-13)28-19-9-14(3-4-17(19)22)27-11-18-16-2-1-5-24-20(16)26-25-18/h1-9H,11H2,(H,24,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase Y181C mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479471

(CHEMBL491019 | MK-1107)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ncccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-6-12(10-23)7-15(8-13)28-19-9-14(3-4-17(19)22)27-11-18-16-2-1-5-24-20(16)26-25-18/h1-9H,11H2,(H,24,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 RT polymerase by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479470

(CHEMBL489586 | MK-4965)Show SMILES Nc1ccc2c(COc3ccc(Cl)c(Oc4cc(Cl)cc(c4)C#N)c3)n[nH]c2n1 Show InChI InChI=1S/C20H13Cl2N5O2/c21-12-5-11(9-23)6-14(7-12)29-18-8-13(1-3-16(18)22)28-10-17-15-2-4-19(24)25-20(15)27-26-17/h1-8H,10H2,(H3,24,25,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase Y181C mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479470

(CHEMBL489586 | MK-4965)Show SMILES Nc1ccc2c(COc3ccc(Cl)c(Oc4cc(Cl)cc(c4)C#N)c3)n[nH]c2n1 Show InChI InChI=1S/C20H13Cl2N5O2/c21-12-5-11(9-23)6-14(7-12)29-18-8-13(1-3-16(18)22)28-10-17-15-2-4-19(24)25-20(15)27-26-17/h1-8H,10H2,(H3,24,25,26,27) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase K103N mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479471

(CHEMBL491019 | MK-1107)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ncccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-6-12(10-23)7-15(8-13)28-19-9-14(3-4-17(19)22)27-11-18-16-2-1-5-24-20(16)26-25-18/h1-9H,11H2,(H,24,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase K103N mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479472
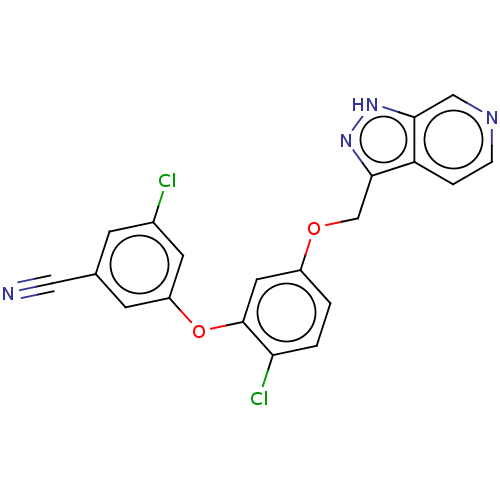
(CHEMBL523972)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4cnccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-5-12(9-23)6-15(7-13)28-20-8-14(1-2-17(20)22)27-11-19-16-3-4-24-10-18(16)25-26-19/h1-8,10H,11H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 RT polymerase by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 RT polymerase by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50478383

(CHEMBL402823)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ccccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C21H13Cl2N3O2/c22-14-7-13(11-24)8-16(9-14)28-21-10-15(5-6-18(21)23)27-12-20-17-3-1-2-4-19(17)25-26-20/h1-10H,12H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase K103N mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479473
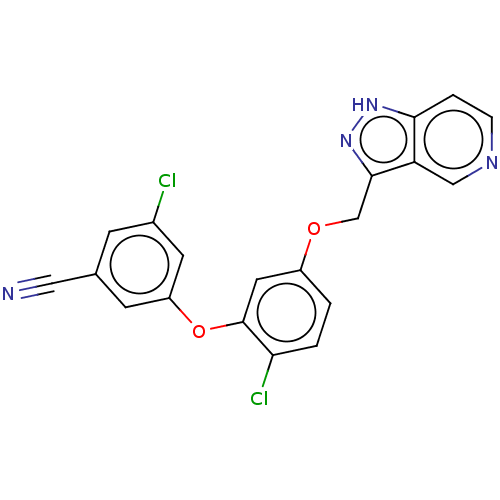
(CHEMBL491018)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ccncc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-5-12(9-23)6-15(7-13)28-20-8-14(1-2-17(20)22)27-11-19-16-10-24-4-3-18(16)25-26-19/h1-8,10H,11H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 RT polymerase by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479472

(CHEMBL523972)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4cnccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-5-12(9-23)6-15(7-13)28-20-8-14(1-2-17(20)22)27-11-19-16-3-4-24-10-18(16)25-26-19/h1-8,10H,11H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase K103N mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478383

(CHEMBL402823)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ccccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C21H13Cl2N3O2/c22-14-7-13(11-24)8-16(9-14)28-21-10-15(5-6-18(21)23)27-12-20-17-3-1-2-4-19(17)25-26-20/h1-10H,12H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 RT polymerase by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50460667
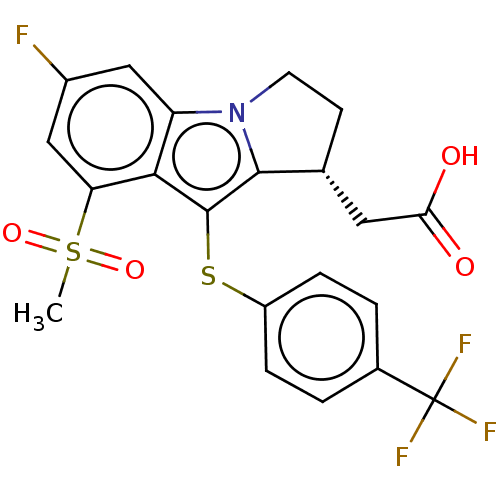
(CHEMBL4226404)Show SMILES CS(=O)(=O)c1cc(F)cc2n3CC[C@H](CC(O)=O)c3c(Sc3ccc(cc3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C21H17F4NO4S2/c1-32(29,30)16-10-13(22)9-15-18(16)20(19-11(8-17(27)28)6-7-26(15)19)31-14-4-2-12(3-5-14)21(23,24)25/h2-5,9-11H,6-8H2,1H3,(H,27,28)/t11-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478383

(CHEMBL402823)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ccccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C21H13Cl2N3O2/c22-14-7-13(11-24)8-16(9-14)28-21-10-15(5-6-18(21)23)27-12-20-17-3-1-2-4-19(17)25-26-20/h1-10H,12H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase Y181C mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479474

(CHEMBL507164)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4cccnc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-6-12(10-23)7-15(8-13)28-19-9-14(3-4-16(19)22)27-11-18-20-17(25-26-18)2-1-5-24-20/h1-9H,11H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of wild type HIV1 RT polymerase by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479473

(CHEMBL491018)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ccncc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-5-12(9-23)6-15(7-13)28-20-8-14(1-2-17(20)22)27-11-19-16-10-24-4-3-18(16)25-26-19/h1-8,10H,11H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase K103N mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479472

(CHEMBL523972)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4cnccc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-5-12(9-23)6-15(7-13)28-20-8-14(1-2-17(20)22)27-11-19-16-3-4-24-10-18(16)25-26-19/h1-8,10H,11H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase Y181C mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50460671
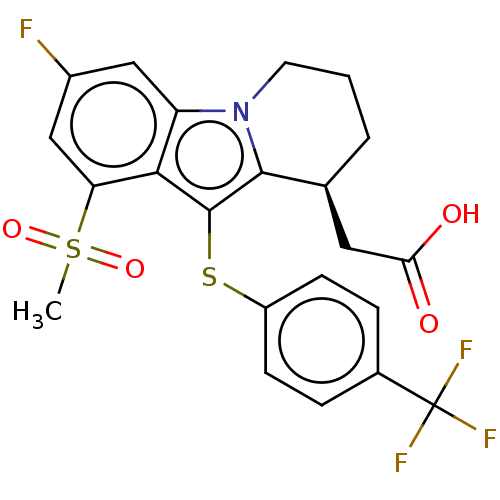
(CHEMBL4228478)Show SMILES CS(=O)(=O)c1cc(F)cc2n3CCC[C@@H](CC(O)=O)c3c(Sc3ccc(cc3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C22H19F4NO4S2/c1-33(30,31)17-11-14(23)10-16-19(17)21(20-12(9-18(28)29)3-2-8-27(16)20)32-15-6-4-13(5-7-15)22(24,25)26/h4-7,10-12H,2-3,8-9H2,1H3,(H,28,29)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
NACHT, LRR and PYD domains-containing protein 3
(Homo sapiens (Human)) | BDBM50155926

(CHEMBL3183703)Show SMILES CC(C)(O)c1coc(c1)S(=O)(=O)NC(=O)Nc1c2CCCc2cc2CCCc12 Show InChI InChI=1S/C20H24N2O5S/c1-20(2,24)14-10-17(27-11-14)28(25,26)22-19(23)21-18-15-7-3-5-12(15)9-13-6-4-8-16(13)18/h9-11,24H,3-8H2,1-2H3,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of NLRP3 in human monocyte derived macrophages assessed as reduction in LPS-induced IL-1beta production incubated for 30 mins followed by ... |
Bioorg Med Chem Lett 28: 793-795 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.054
BindingDB Entry DOI: 10.7270/Q26T0Q81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NACHT, LRR and PYD domains-containing protein 3
(Homo sapiens (Human)) | BDBM50155926

(CHEMBL3183703)Show SMILES CC(C)(O)c1coc(c1)S(=O)(=O)NC(=O)Nc1c2CCCc2cc2CCCc12 Show InChI InChI=1S/C20H24N2O5S/c1-20(2,24)14-10-17(27-11-14)28(25,26)22-19(23)21-18-15-7-3-5-12(15)9-13-6-4-8-16(13)18/h9-11,24H,3-8H2,1-2H3,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of NLRP3 in human monocyte derived macrophages assessed as reduction in LPS-induced IL-1beta production incubated for 30 mins followed by ... |
Bioorg Med Chem Lett 28: 793-795 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.054
BindingDB Entry DOI: 10.7270/Q26T0Q81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NACHT, LRR and PYD domains-containing protein 3
(Homo sapiens (Human)) | BDBM50155926

(CHEMBL3183703)Show SMILES CC(C)(O)c1coc(c1)S(=O)(=O)NC(=O)Nc1c2CCCc2cc2CCCc12 Show InChI InChI=1S/C20H24N2O5S/c1-20(2,24)14-10-17(27-11-14)28(25,26)22-19(23)21-18-15-7-3-5-12(15)9-13-6-4-8-16(13)18/h9-11,24H,3-8H2,1-2H3,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of NLRP3 in human monocyte derived macrophages assessed as reduction in LPS-induced IL-1beta production incubated for 30 mins followed by ... |
Bioorg Med Chem Lett 28: 793-795 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.054
BindingDB Entry DOI: 10.7270/Q26T0Q81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NACHT, LRR and PYD domains-containing protein 3
(Homo sapiens (Human)) | BDBM50155926

(CHEMBL3183703)Show SMILES CC(C)(O)c1coc(c1)S(=O)(=O)NC(=O)Nc1c2CCCc2cc2CCCc12 Show InChI InChI=1S/C20H24N2O5S/c1-20(2,24)14-10-17(27-11-14)28(25,26)22-19(23)21-18-15-7-3-5-12(15)9-13-6-4-8-16(13)18/h9-11,24H,3-8H2,1-2H3,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of NLRP3 in human monocyte derived macrophages assessed as reduction in LPS-induced IL-1beta production incubated for 30 mins followed by ... |
Bioorg Med Chem Lett 28: 793-795 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.054
BindingDB Entry DOI: 10.7270/Q26T0Q81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479473

(CHEMBL491018)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4ccncc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-5-12(9-23)6-15(7-13)28-20-8-14(1-2-17(20)22)27-11-19-16-10-24-4-3-18(16)25-26-19/h1-8,10H,11H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase Y181C mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50460673

(CHEMBL4229054)Show SMILES C[C@H](c1ccc(cc1)C(F)(F)F)n1c2[C@H](CC(O)=O)CCCc2c2cc(F)cc(c12)S(C)(=O)=O |r| Show InChI InChI=1S/C24H23F4NO4S/c1-13(14-6-8-16(9-7-14)24(26,27)28)29-22-15(10-21(30)31)4-3-5-18(22)19-11-17(25)12-20(23(19)29)34(2,32)33/h6-9,11-13,15H,3-5,10H2,1-2H3,(H,30,31)/t13-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NACHT, LRR and PYD domains-containing protein 3
(Homo sapiens (Human)) | BDBM50155926

(CHEMBL3183703)Show SMILES CC(C)(O)c1coc(c1)S(=O)(=O)NC(=O)Nc1c2CCCc2cc2CCCc12 Show InChI InChI=1S/C20H24N2O5S/c1-20(2,24)14-10-17(27-11-14)28(25,26)22-19(23)21-18-15-7-3-5-12(15)9-13-6-4-8-16(13)18/h9-11,24H,3-8H2,1-2H3,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of NLRP3 in human monocyte derived macrophages assessed as reduction in LPS-induced IL-1beta production incubated for 30 mins followed by ... |
Bioorg Med Chem Lett 28: 793-795 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.054
BindingDB Entry DOI: 10.7270/Q26T0Q81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase Y181C mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
NACHT, LRR and PYD domains-containing protein 3
(Mus musculus) | BDBM50155926

(CHEMBL3183703)Show SMILES CC(C)(O)c1coc(c1)S(=O)(=O)NC(=O)Nc1c2CCCc2cc2CCCc12 Show InChI InChI=1S/C20H24N2O5S/c1-20(2,24)14-10-17(27-11-14)28(25,26)22-19(23)21-18-15-7-3-5-12(15)9-13-6-4-8-16(13)18/h9-11,24H,3-8H2,1-2H3,(H2,21,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of NLRP3 inflammasome activation in LPS-stimulated mouse BMDM assessed as reduction in NLRP3 inflammasome-induced IL-1beta production prei... |
ACS Med Chem Lett 7: 1034-1038 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00198
BindingDB Entry DOI: 10.7270/Q2HD7XKV |
More data for this
Ligand-Target Pair | |
NACHT, LRR and PYD domains-containing protein 3
(Homo sapiens (Human)) | BDBM50155926

(CHEMBL3183703)Show SMILES CC(C)(O)c1coc(c1)S(=O)(=O)NC(=O)Nc1c2CCCc2cc2CCCc12 Show InChI InChI=1S/C20H24N2O5S/c1-20(2,24)14-10-17(27-11-14)28(25,26)22-19(23)21-18-15-7-3-5-12(15)9-13-6-4-8-16(13)18/h9-11,24H,3-8H2,1-2H3,(H2,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of NLRP3 in human monocyte derived macrophages assessed as reduction in LPS-induced IL-1beta production by ELISA |
Bioorg Med Chem Lett 28: 793-795 (2018)
Article DOI: 10.1016/j.bmcl.2017.12.054
BindingDB Entry DOI: 10.7270/Q26T0Q81 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50460672

(CHEMBL4227417)Show SMILES C[C@H](c1ccc(cc1)C(F)(F)F)n1c2[C@@H](CC(O)=O)CCCc2c2cc(F)cc(c12)S(C)(=O)=O |r| Show InChI InChI=1S/C24H23F4NO4S/c1-13(14-6-8-16(9-7-14)24(26,27)28)29-22-15(10-21(30)31)4-3-5-18(22)19-11-17(25)12-20(23(19)29)34(2,32)33/h6-9,11-13,15H,3-5,10H2,1-2H3,(H,30,31)/t13-,15-/m1/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50460678
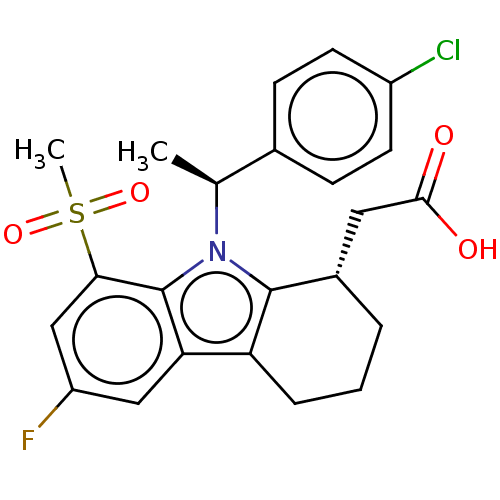
(CHEMBL4227971)Show SMILES C[C@@H](c1ccc(Cl)cc1)n1c2[C@H](CC(O)=O)CCCc2c2cc(F)cc(c12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H23ClFNO4S/c1-13(14-6-8-16(24)9-7-14)26-22-15(10-21(27)28)4-3-5-18(22)19-11-17(25)12-20(23(19)26)31(2,29)30/h6-9,11-13,15H,3-5,10H2,1-2H3,(H,27,28)/t13-,15-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50460679

(CHEMBL4224708)Show SMILES CS(=O)(=O)c1cc(F)cc2n3CC[C@@H](CC(O)=O)c3c(Sc3ccc(cc3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C21H17F4NO4S2/c1-32(29,30)16-10-13(22)9-15-18(16)20(19-11(8-17(27)28)6-7-26(15)19)31-14-4-2-12(3-5-14)21(23,24)25/h2-5,9-11H,6-8H2,1H3,(H,27,28)/t11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM2483

((4S)-6-chloro-4-(2-cyclopropylethynyl)-4-(trifluor...)Show SMILES FC(F)(F)[C@]1(OC(=O)Nc2ccc(Cl)cc12)C#CC1CC1 |r| Show InChI InChI=1S/C14H9ClF3NO2/c15-9-3-4-11-10(7-9)13(14(16,17)18,21-12(20)19-11)6-5-8-1-2-8/h3-4,7-8H,1-2H2,(H,19,20)/t13-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase K103N mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50460670

(CHEMBL4228792)Show SMILES C[C@H](c1ccc(Cl)cc1)n1c2[C@H](CC(O)=O)CCCc2c2cc(F)cc(c12)S(C)(=O)=O |r| Show InChI InChI=1S/C23H23ClFNO4S/c1-13(14-6-8-16(24)9-7-14)26-22-15(10-21(27)28)4-3-5-18(22)19-11-17(25)12-20(23(19)26)31(2,29)30/h6-9,11-13,15H,3-5,10H2,1-2H3,(H,27,28)/t13-,15+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50460667

(CHEMBL4226404)Show SMILES CS(=O)(=O)c1cc(F)cc2n3CC[C@H](CC(O)=O)c3c(Sc3ccc(cc3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C21H17F4NO4S2/c1-32(29,30)16-10-13(22)9-15-18(16)20(19-11(8-17(27)28)6-7-26(15)19)31-14-4-2-12(3-5-14)21(23,24)25/h2-5,9-11H,6-8H2,1H3,(H,27,28)/t11-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50460679

(CHEMBL4224708)Show SMILES CS(=O)(=O)c1cc(F)cc2n3CC[C@@H](CC(O)=O)c3c(Sc3ccc(cc3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C21H17F4NO4S2/c1-32(29,30)16-10-13(22)9-15-18(16)20(19-11(8-17(27)28)6-7-26(15)19)31-14-4-2-12(3-5-14)21(23,24)25/h2-5,9-11H,6-8H2,1H3,(H,27,28)/t11-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Reverse transcriptase protein
(Human immunodeficiency virus 1) | BDBM50479474

(CHEMBL507164)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4cccnc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-6-12(10-23)7-15(8-13)28-19-9-14(3-4-16(19)22)27-11-18-20-17(25-26-18)2-1-5-24-20/h1-9H,11H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase K103N mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50460676

(CHEMBL4225707)Show SMILES CS(=O)(=O)c1cc(F)cc2c3CCCC(CC(O)=O)c3n(Cc3ccc(cc3)C(F)(F)F)c12 Show InChI InChI=1S/C23H21F4NO4S/c1-33(31,32)19-11-16(24)10-18-17-4-2-3-14(9-20(29)30)21(17)28(22(18)19)12-13-5-7-15(8-6-13)23(25,26)27/h5-8,10-11,14H,2-4,9,12H2,1H3,(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50460682
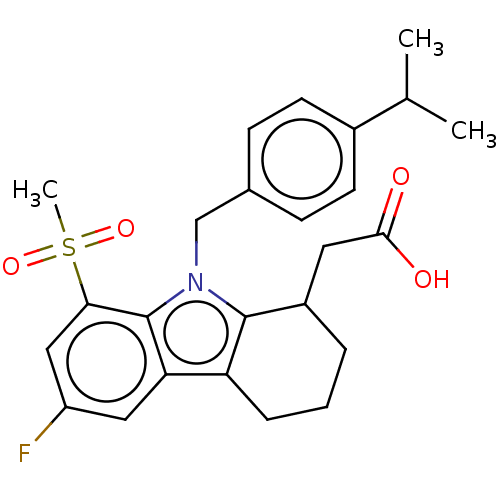
(CHEMBL4225177)Show SMILES CC(C)c1ccc(Cn2c3C(CC(O)=O)CCCc3c3cc(F)cc(c23)S(C)(=O)=O)cc1 Show InChI InChI=1S/C25H28FNO4S/c1-15(2)17-9-7-16(8-10-17)14-27-24-18(11-23(28)29)5-4-6-20(24)21-12-19(26)13-22(25(21)27)32(3,30)31/h7-10,12-13,15,18H,4-6,11,14H2,1-3H3,(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Prostaglandin D2 receptor
(Homo sapiens (Human)) | BDBM50460671

(CHEMBL4228478)Show SMILES CS(=O)(=O)c1cc(F)cc2n3CCC[C@@H](CC(O)=O)c3c(Sc3ccc(cc3)C(F)(F)F)c12 |r| Show InChI InChI=1S/C22H19F4NO4S2/c1-33(30,31)17-11-14(23)10-16-19(17)21(20-12(9-18(28)29)3-2-8-27(16)20)32-15-6-4-13(5-7-15)22(24,25)26/h4-7,10-12H,2-3,8-9H2,1H3,(H,28,29)/t12-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at DP1 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM2580
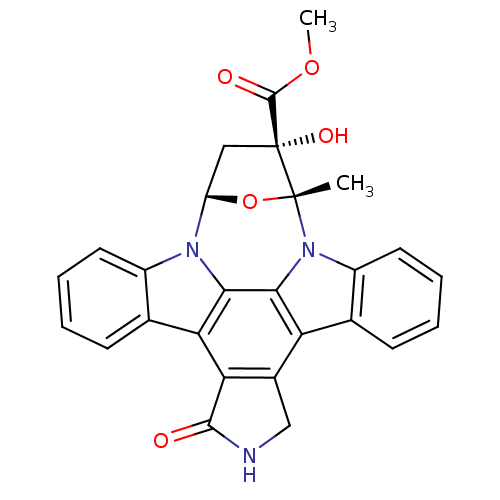
(K252a | methyl (15R,16S,18S)-16-hydroxy-15-methyl-...)Show SMILES COC(=O)[C@]1(O)C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate.HTRF is based on the proximity of europium cryptate (donor fluorophore... |
Biochemistry 44: 9430-40 (2005)
Article DOI: 10.1021/bi0500628
BindingDB Entry DOI: 10.7270/Q2BV7DV5 |
More data for this
Ligand-Target Pair | |
NACHT, LRR and PYD domains-containing protein 3
(Homo sapiens (Human)) | BDBM50199870

(CHEMBL3904593)Show SMILES CC(C)(O)c1coc(c1)S(=O)(=O)NC(=O)Nc1c2CCCc2cc2CCC(O)c12 Show InChI InChI=1S/C20H24N2O6S/c1-20(2,25)13-9-16(28-10-13)29(26,27)22-19(24)21-18-14-5-3-4-11(14)8-12-6-7-15(23)17(12)18/h8-10,15,23,25H,3-7H2,1-2H3,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 232 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of NLRP3 inflammasome in LPS-stimulated human monocyte derived macrophages assessed as reduction in NLRP3 inflammasome-induced IL-1beta pr... |
ACS Med Chem Lett 7: 1034-1038 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00198
BindingDB Entry DOI: 10.7270/Q2HD7XKV |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50460675

(CHEMBL4227300)Show SMILES OC(=O)CC1CCCc2c1n(Cc1ccc(cc1)C(F)(F)F)c1c(cc(F)cc21)C(F)(F)F Show InChI InChI=1S/C23H18F7NO2/c24-15-9-17-16-3-1-2-13(8-19(32)33)20(16)31(21(17)18(10-15)23(28,29)30)11-12-4-6-14(7-5-12)22(25,26)27/h4-7,9-10,13H,1-3,8,11H2,(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 233 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50460680
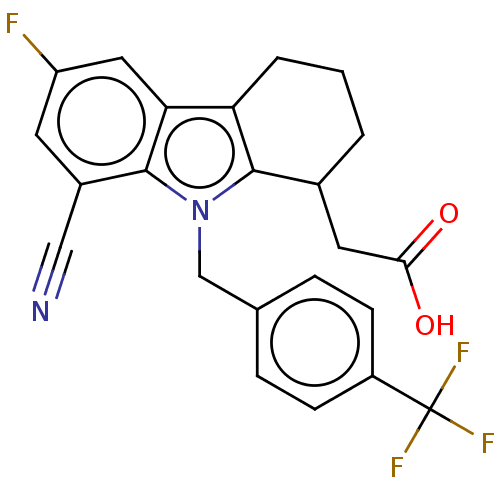
(CHEMBL4225978)Show SMILES OC(=O)CC1CCCc2c1n(Cc1ccc(cc1)C(F)(F)F)c1c(cc(F)cc21)C#N Show InChI InChI=1S/C23H18F4N2O2/c24-17-8-15(11-28)22-19(10-17)18-3-1-2-14(9-20(30)31)21(18)29(22)12-13-4-6-16(7-5-13)23(25,26)27/h4-8,10,14H,1-3,9,12H2,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 422 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
cGMP-dependent 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50184225
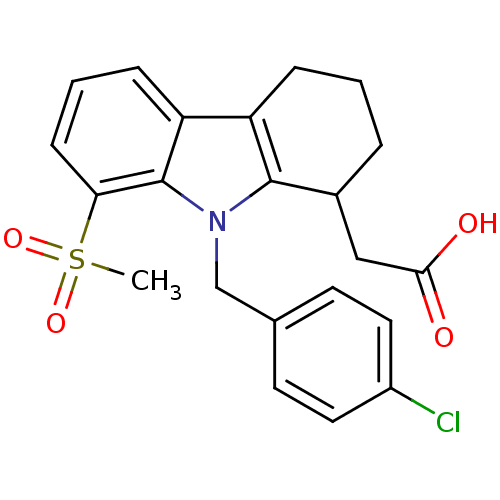
(2-(9-(4-chlorobenzyl)-8-(methylsulfonyl)-2,3,4,9-t...)Show SMILES CS(=O)(=O)c1cccc2c3CCCC(CC(O)=O)c3n(Cc3ccc(Cl)cc3)c12 Show InChI InChI=1S/C22H22ClNO4S/c1-29(27,28)19-7-3-6-18-17-5-2-4-15(12-20(25)26)21(17)24(22(18)19)13-14-8-10-16(23)11-9-14/h3,6-11,15H,2,4-5,12-13H2,1H3,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDE2 (unknown origin) |
Bioorg Med Chem Lett 28: 1122-1126 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.039
BindingDB Entry DOI: 10.7270/Q2QV3Q4C |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM2580

(K252a | methyl (15R,16S,18S)-16-hydroxy-15-methyl-...)Show SMILES COC(=O)[C@]1(O)C[C@@H]2O[C@@]1(C)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C27H21N3O5/c1-26-27(33,25(32)34-2)11-18(35-26)29-16-9-5-3-7-13(16)20-21-15(12-28-24(21)31)19-14-8-4-6-10-17(14)30(26)23(19)22(20)29/h3-10,18,33H,11-12H2,1-2H3,(H,28,31)/t18?,26-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate.HTRF is based on the proximity of europium cryptate (donor fluorophore... |
Biochemistry 44: 9430-40 (2005)
Article DOI: 10.1021/bi0500628
BindingDB Entry DOI: 10.7270/Q2BV7DV5 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM15015
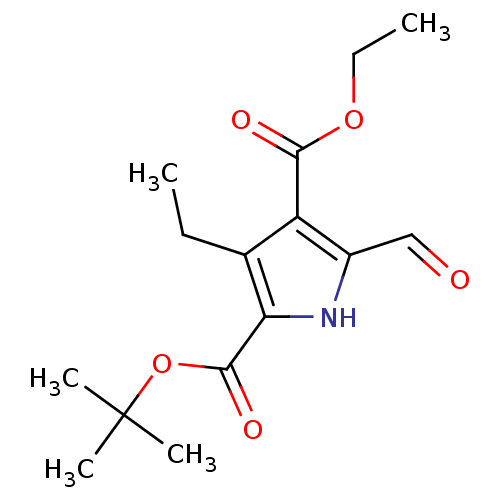
(2-tert-Butyl 4-Ethyl 3-Ethyl-5-formyl-1H-pyrrole-2...)Show InChI InChI=1S/C15H21NO5/c1-6-9-11(13(18)20-7-2)10(8-17)16-12(9)14(19)21-15(3,4)5/h8,16H,6-7H2,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 490 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate.HTRF is based on the proximity of europium cryptate (donor fluorophore... |
Biochemistry 44: 9430-40 (2005)
Article DOI: 10.1021/bi0500628
BindingDB Entry DOI: 10.7270/Q2BV7DV5 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50479474

(CHEMBL507164)Show SMILES Clc1cc(Oc2cc(OCc3n[nH]c4cccnc34)ccc2Cl)cc(c1)C#N Show InChI InChI=1S/C20H12Cl2N4O2/c21-13-6-12(10-23)7-15(8-13)28-19-9-14(3-4-16(19)22)27-11-18-20-17(25-26-18)2-1-5-24-20/h1-9H,11H2,(H,25,26) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 RT polymerase Y181C mutant by SPA |
J Med Chem 51: 6503-11 (2008)
Article DOI: 10.1021/jm800856c
BindingDB Entry DOI: 10.7270/Q2H41V7H |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM15012
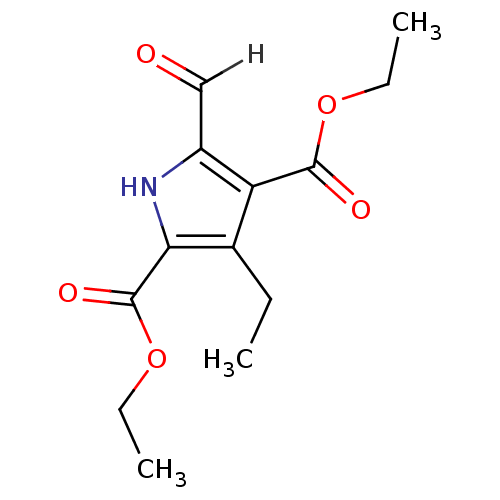
(2,4-diethyl 3-ethyl-5-formyl-1H-pyrrole-2,4-dicarb...)Show InChI InChI=1S/C13H17NO5/c1-4-8-10(12(16)18-5-2)9(7-15)14-11(8)13(17)19-6-3/h7,14H,4-6H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Merck Research Laboratories
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate.HTRF is based on the proximity of europium cryptate (donor fluorophore... |
Biochemistry 44: 9430-40 (2005)
Article DOI: 10.1021/bi0500628
BindingDB Entry DOI: 10.7270/Q2BV7DV5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data