

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50403124 (CHEMBL2115571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated to inactivate the bacterial AdoMet-DC; value ranges from 3.8 to 39.6 uM | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50281293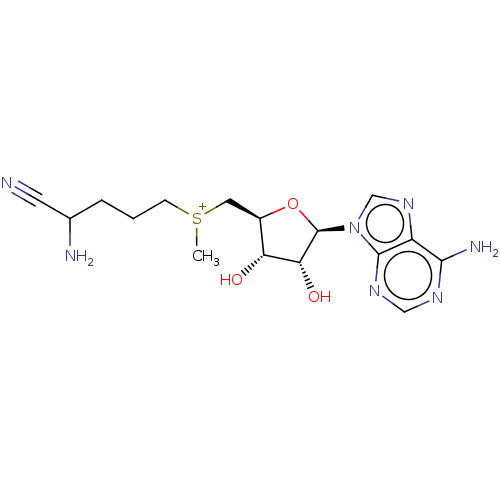 (2-amino-5-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated to inactivate the human AdoMet-DC | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50403124 (CHEMBL2115571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated to inactivate the human AdoMet-DC; value ranges from 10.7 to 62.7 uM | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50403124 (CHEMBL2115571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated to inactivate the bacterial AdoMet-DC; value ranges from 3.8 to 39.6 uM | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366321 (CHEMBL3392203 | CHEMBL606101) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli | Bioorg Med Chem Lett 3: 147-152 (1993) Article DOI: 10.1016/S0960-894X(01)80865-9 BindingDB Entry DOI: 10.7270/Q2PR7WG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50281292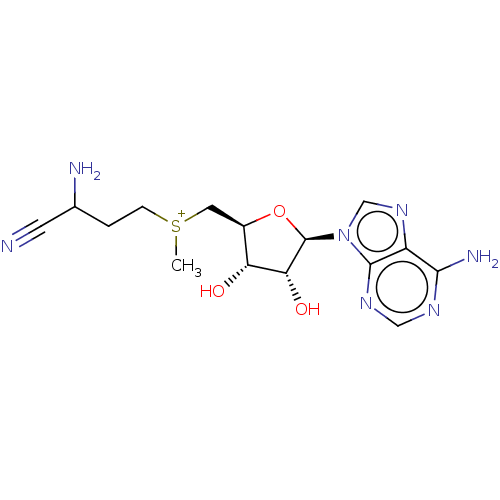 (2-amino-4-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 3.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated to inactivate the bacterial AdoMet-DC | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50281292 (2-amino-4-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.47E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated to inactivate the human AdoMet-DC | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366318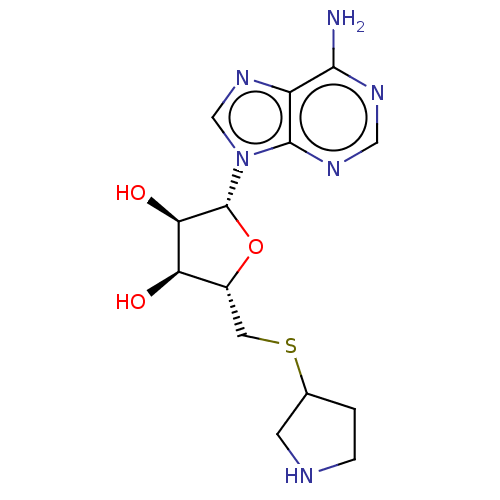 (CHEMBL3392208 | CHEMBL605698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli | Bioorg Med Chem Lett 3: 147-152 (1993) Article DOI: 10.1016/S0960-894X(01)80865-9 BindingDB Entry DOI: 10.7270/Q2PR7WG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366320 (CHEMBL3392206 | CHEMBL605899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 4.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli | Bioorg Med Chem Lett 3: 147-152 (1993) Article DOI: 10.1016/S0960-894X(01)80865-9 BindingDB Entry DOI: 10.7270/Q2PR7WG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366317 (CHEMBL3392204 | CHEMBL606102) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 6.69E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli | Bioorg Med Chem Lett 3: 147-152 (1993) Article DOI: 10.1016/S0960-894X(01)80865-9 BindingDB Entry DOI: 10.7270/Q2PR7WG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366316 (CHEMBL3392209 | CHEMBL605267) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 9.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli | Bioorg Med Chem Lett 3: 147-152 (1993) Article DOI: 10.1016/S0960-894X(01)80865-9 BindingDB Entry DOI: 10.7270/Q2PR7WG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366319 (CHEMBL1791405 | CHEMBL3392205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.07E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli | Bioorg Med Chem Lett 3: 147-152 (1993) Article DOI: 10.1016/S0960-894X(01)80865-9 BindingDB Entry DOI: 10.7270/Q2PR7WG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366319 (CHEMBL1791405 | CHEMBL3392205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.07E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for irreversible inhibitory activity against AdoMet-DC (S-adenosyl-methionine decarboxylase-) isolated from Escherichia coli | Bioorg Med Chem Lett 3: 147-152 (1993) Article DOI: 10.1016/S0960-894X(01)80865-9 BindingDB Entry DOI: 10.7270/Q2PR7WG3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50344821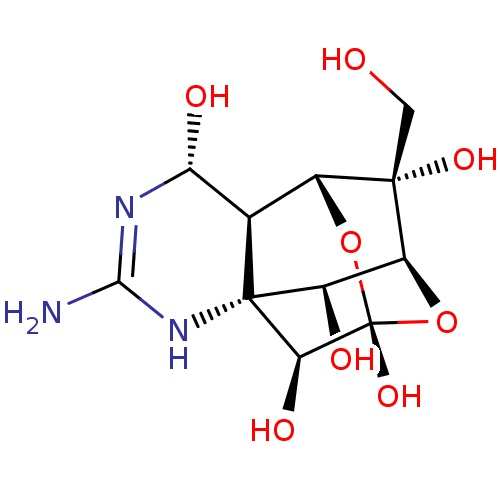 (10-hydroxymethyl-5-imino-(2S)-12,13-dioxa-4,6-diaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066062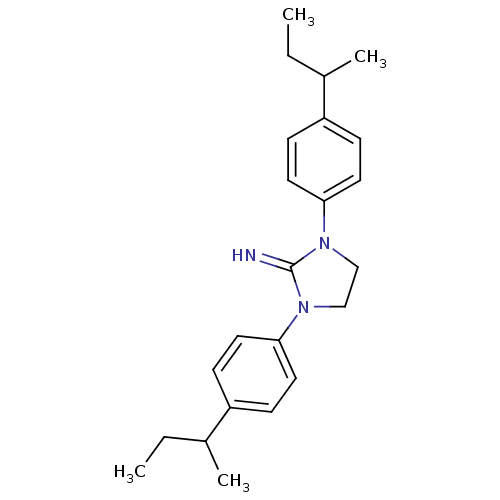 (1,3-Bis-(4-sec-butyl-phenyl)-imidazolidin-2-yliden...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066059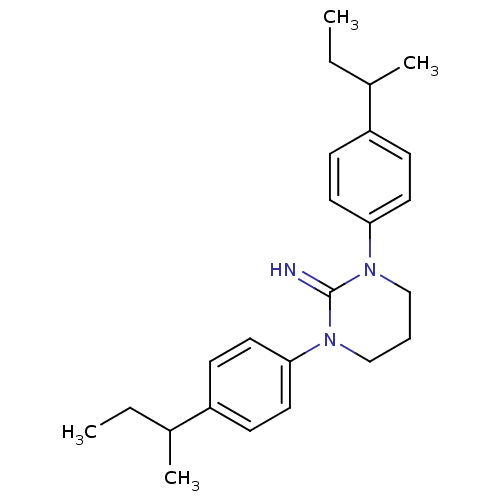 (1,3-Bis-(4-sec-butyl-phenyl)-tetrahydro-pyrimidin-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066066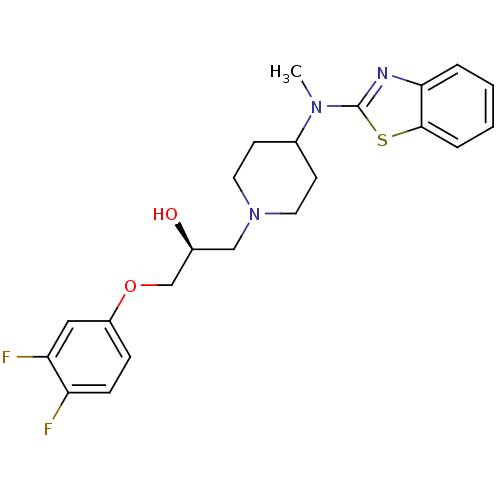 ((S)-1-[4-(Benzothiazol-2-yl-methyl-amino)-piperidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066067 (2-(4-tert-Butyl-phenyl)-6-isopropyl-3,4-dihydro-2H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066069 (CHEMBL103284 | N-(4-tert-Butyl-benzyl)-N-(4-sec-bu...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066052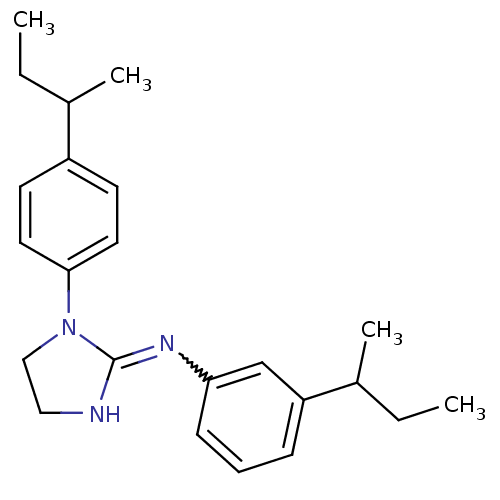 ((3-sec-Butyl-phenyl)-[1-(4-sec-butyl-phenyl)-imida...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066057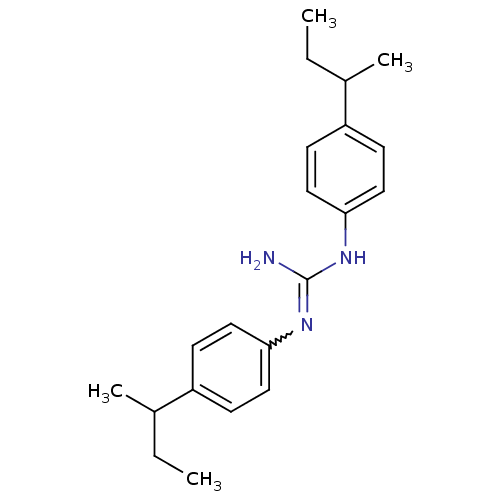 (CHEMBL110909 | CHEMBL320199 | N,N'-Bis-(4-sec-buty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066060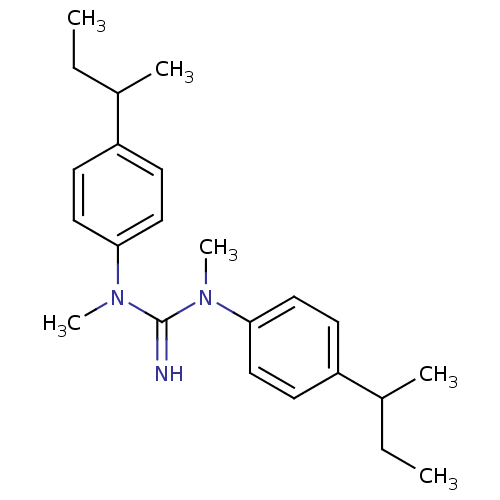 (CHEMBL545504 | N,N'-Bis-(4-sec-butyl-phenyl)-N,N'-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066068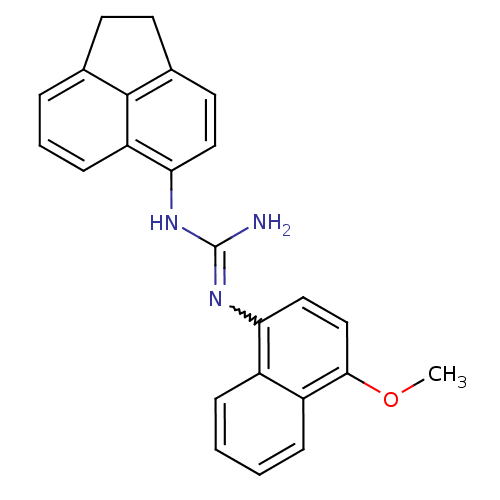 (CHEMBL28853 | N-Acenaphthen-5-yl-N'-(4-methoxy-nap...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066061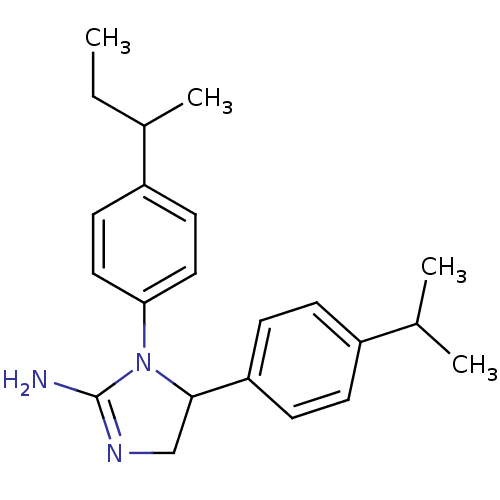 (1-(4-sec-Butyl-phenyl)-5-(4-isopropyl-phenyl)-imid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066063 (9,15-Dioxa-2,4-diaza-tricyclo[14.2.2.2*5,8*]docosa...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066053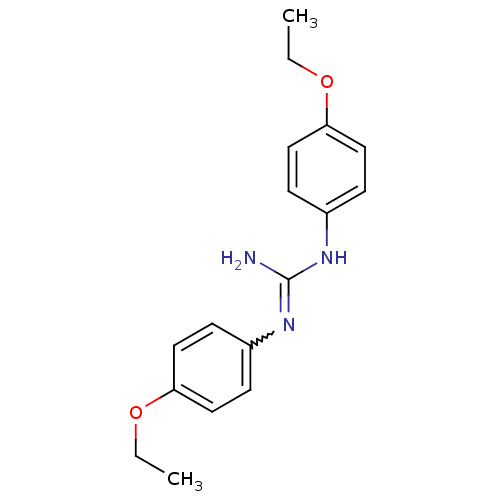 (CHEMBL544569 | N,N'-Bis-(4-ethoxy-phenyl)-guanidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50281292 (2-amino-4-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against AdoMet-DC from Escherichia coli | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066055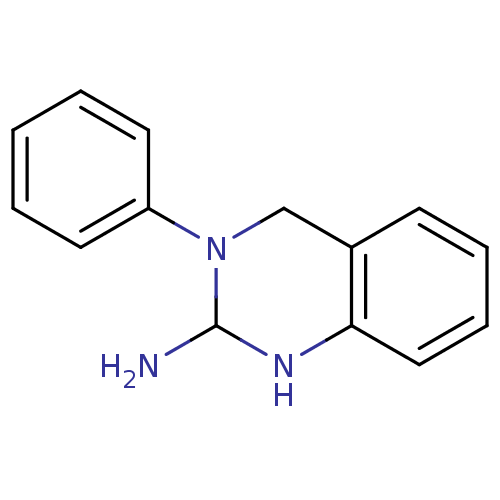 (3-Phenyl-1,2,3,4-tetrahydro-quinazolin-2-ylamine; ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50010769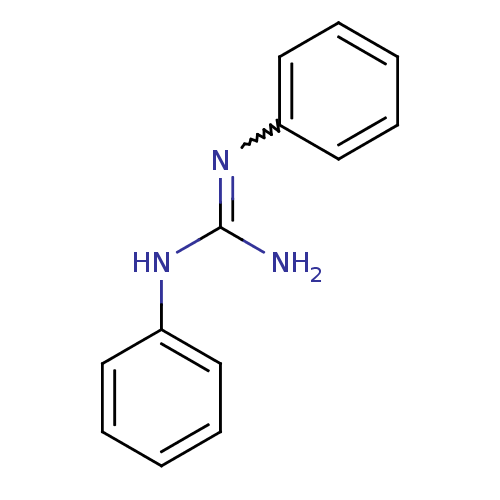 (CHEMBL556117 | CHEMBL77675 | N,N'-Diphenyl-guanidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50010804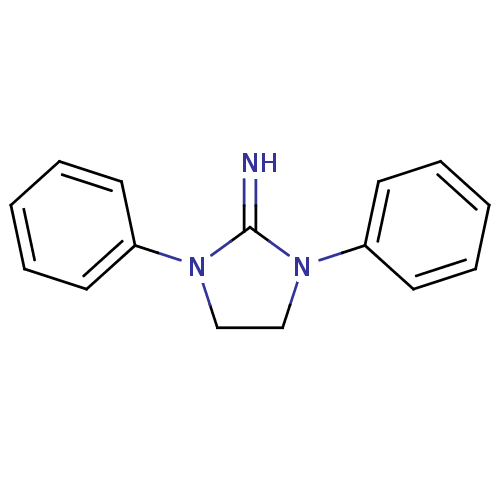 (1,3-Diphenyl-imidazolidin-2-ylideneamine | CHEMBL5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066070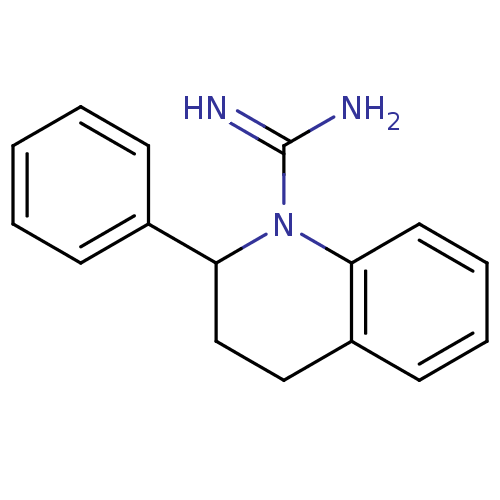 (2-Phenyl-3,4-dihydro-2H-quinoline-1-carboxamidine;...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066056 (1,5-Diphenyl-imidazolidin-(2Z)-ylideneamine; hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066064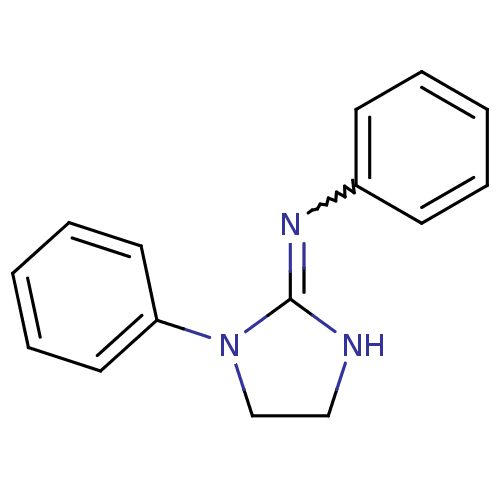 (CHEMBL103505 | Phenyl-[1-phenyl-imidazolidin-(2E)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066065 (1,3-Diphenyl-tetrahydro-pyrimidin-2-ylideneamine; ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066054 (11,12-Dihydro-6H-dibenzo[b,f]azocine-5-carboxamidi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50281293 (2-amino-5-({[(2S,3S,4R)-5-(6-amino-9H-purin-9-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated to inactivate the bacterial AdoMet-DC | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 2 subunit alpha (Rattus norvegicus) | BDBM50066071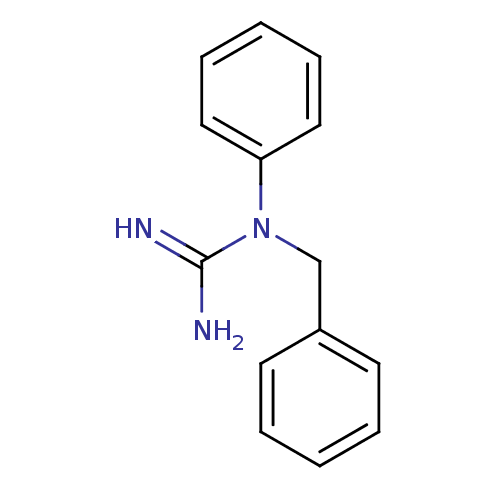 (CHEMBL543152 | N-Benzyl-N-phenyl-guanidine; hydroc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Cambridge NeuroScience Inc. Curated by ChEMBL | Assay Description In vitro inhibition of [14C]- guanidinium influx in Chinese hamster ovary (CHO) cells expressing rat brain sodium channel type IIA (CNaIIA-1) | J Med Chem 41: 3048-61 (1998) Article DOI: 10.1021/jm980124a BindingDB Entry DOI: 10.7270/Q2R49RF5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366314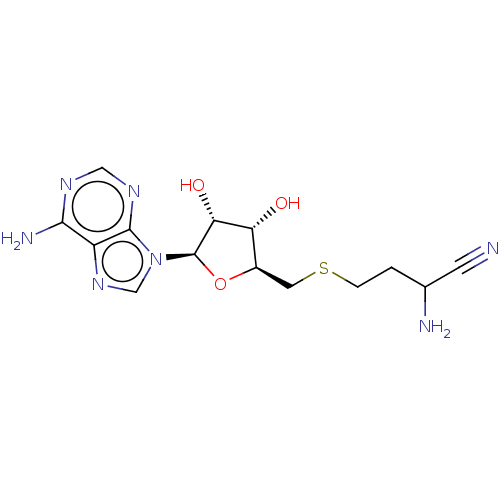 (CHEMBL3392211 | CHEMBL607699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against AdoMet-DC from Escherichia coli | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| S-adenosylmethionine decarboxylase proenzyme (Homo sapiens (Human)) | BDBM50366315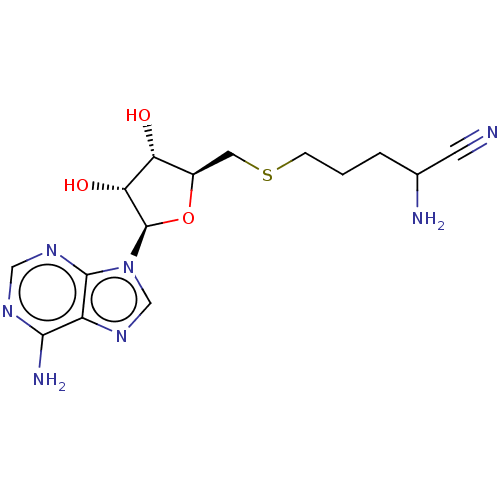 (CHEMBL1163096 | CHEMBL3392218) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for its inhibitory activity against AdoMet-DC from Escherichia coli | Bioorg Med Chem Lett 3: 2811-2816 (1993) Article DOI: 10.1016/S0960-894X(01)80770-8 BindingDB Entry DOI: 10.7270/Q2TH8N6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||