

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897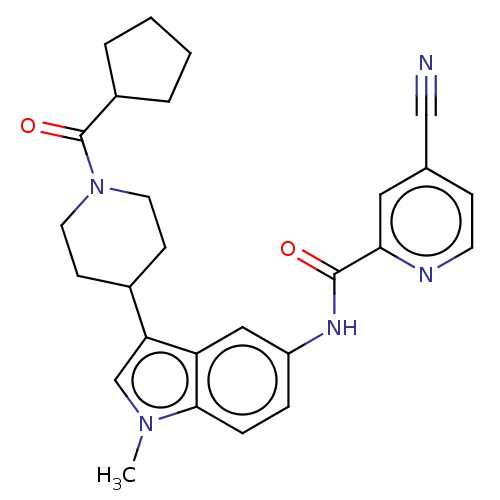 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891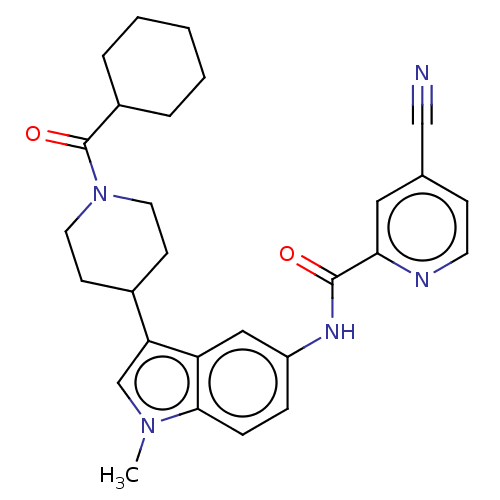 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893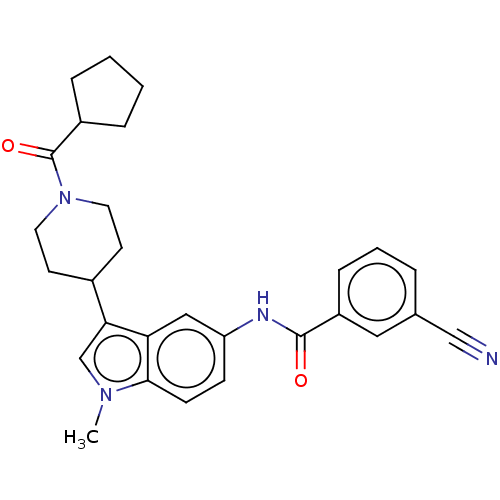 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466892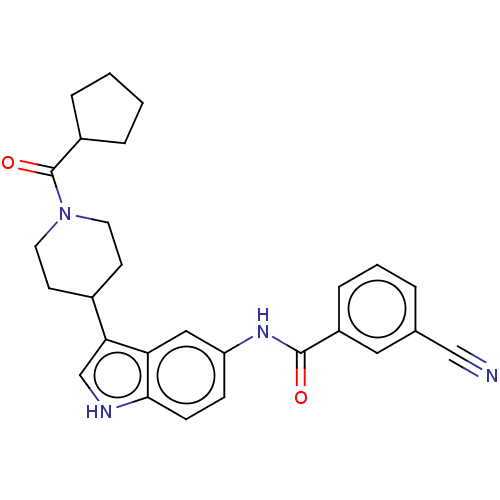 (CHEMBL4283871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466913 (CHEMBL4289304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324200 (4-[(5R)-1-(3-Chloro-4-cyanophenyl)-5-cyclopentyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at Gal4-tagged mineralocorticoid receptor expressed in human Huh7 cells by luciferase reporter gene assay | J Med Chem 53: 5979-6002 (2010) Article DOI: 10.1021/jm100505n BindingDB Entry DOI: 10.7270/Q2T43V2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM254948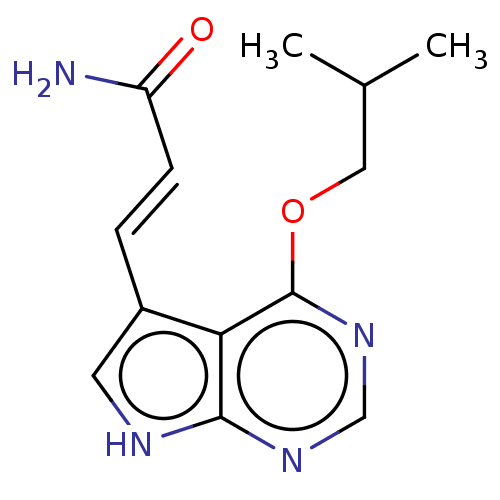 (US9505765, 38) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CONFLUENCE LIFE SCIENCES INC. US Patent | Assay Description TAK1-TAB1 Binding Inhibitory Potency: The ability of candidate compounds to interact with TAK1-TAB1 is quantitated by a competitive binding assay usi... | US Patent US9505765 (2016) BindingDB Entry DOI: 10.7270/Q2T43S1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466890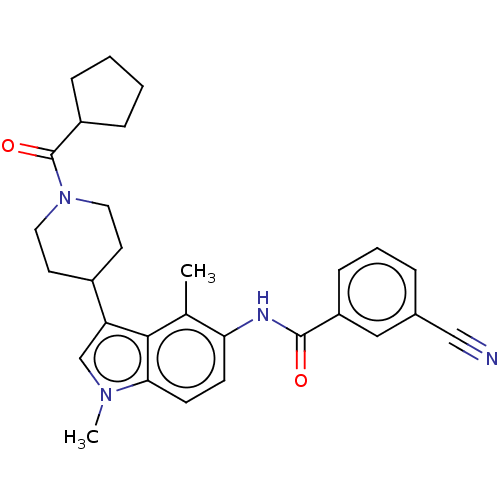 (CHEMBL4290728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50318295 (CHEMBL1097751 | tert-Butyl (4R)-4-(2-Chloro-4-fluo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Antagonist activity at Gal4-fused mineralocorticoid receptor expressed in human HuH7 cells assessed as inhibition of aldosterone-induced receptor act... | J Med Chem 53: 4300-4 (2010) Article DOI: 10.1021/jm1002827 BindingDB Entry DOI: 10.7270/Q22R3RV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324215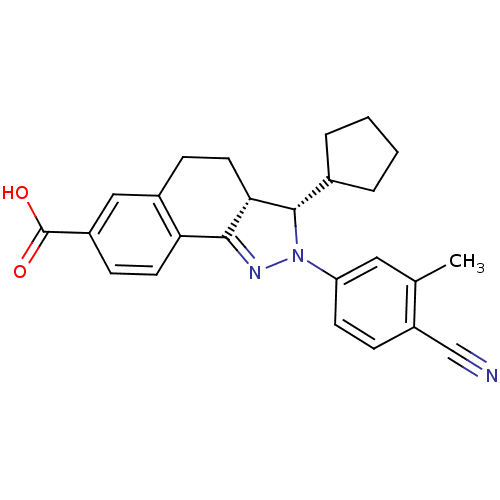 ((+/-)-(3SR,3aRS)-2-(4-Cyano-3-methylphenyl)-3-cycl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at Gal4-tagged mineralocorticoid receptor expressed in human Huh7 cells by luciferase reporter gene assay | J Med Chem 53: 5979-6002 (2010) Article DOI: 10.1021/jm100505n BindingDB Entry DOI: 10.7270/Q2T43V2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189877 (US10227346, Example 5 | US10426135, Example 5 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM254963 (US9505765, 187) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
CONFLUENCE LIFE SCIENCES INC. US Patent | Assay Description TAK1-TAB1 Binding Inhibitory Potency: The ability of candidate compounds to interact with TAK1-TAB1 is quantitated by a competitive binding assay usi... | US Patent US9505765 (2016) BindingDB Entry DOI: 10.7270/Q2T43S1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM254964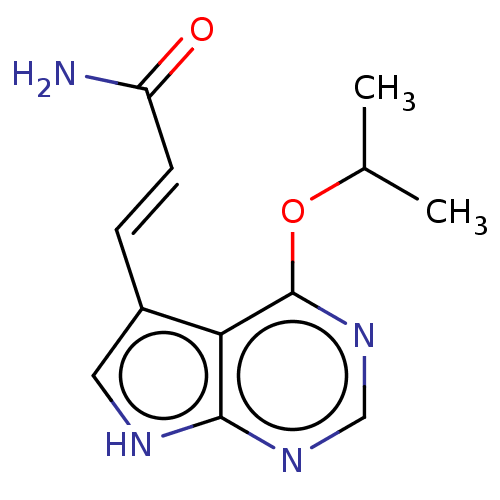 (US9505765, 188) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
CONFLUENCE LIFE SCIENCES INC. US Patent | Assay Description TAK1-TAB1 Binding Inhibitory Potency: The ability of candidate compounds to interact with TAK1-TAB1 is quantitated by a competitive binding assay usi... | US Patent US9505765 (2016) BindingDB Entry DOI: 10.7270/Q2T43S1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466912 (CHEMBL4283051) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466890 (CHEMBL4290728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM254968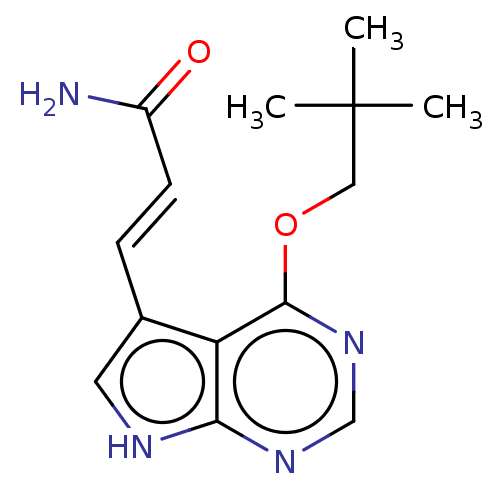 (US9505765, 192) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
CONFLUENCE LIFE SCIENCES INC. US Patent | Assay Description TAK1-TAB1 Binding Inhibitory Potency: The ability of candidate compounds to interact with TAK1-TAB1 is quantitated by a competitive binding assay usi... | US Patent US9505765 (2016) BindingDB Entry DOI: 10.7270/Q2T43S1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324201 (4-[(5R)-1-(3-Chloro-4-cyanophenyl)-5-cyclopentyl-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at Gal4-tagged mineralocorticoid receptor expressed in human Huh7 cells by luciferase reporter gene assay | J Med Chem 53: 5979-6002 (2010) Article DOI: 10.1021/jm100505n BindingDB Entry DOI: 10.7270/Q2T43V2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324216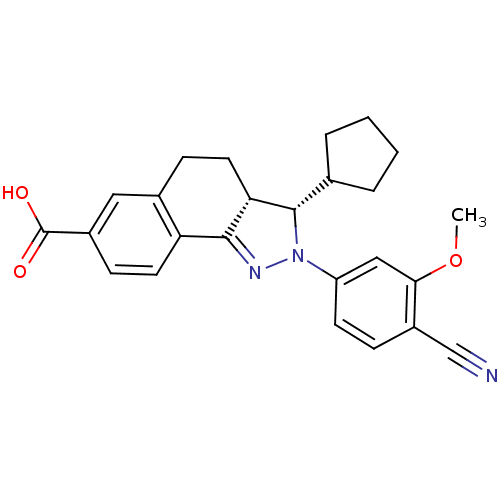 ((+/-)-(3SR,3aRS)-2-(4-Cyano-3-methoxyphenyl)-3-cyc...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at Gal4-tagged mineralocorticoid receptor expressed in human Huh7 cells by luciferase reporter gene assay | J Med Chem 53: 5979-6002 (2010) Article DOI: 10.1021/jm100505n BindingDB Entry DOI: 10.7270/Q2T43V2T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase 7/TGF-beta-activated kinase 1 and MAP3K7-binding protein 1 (Homo sapiens (Human)) | BDBM254962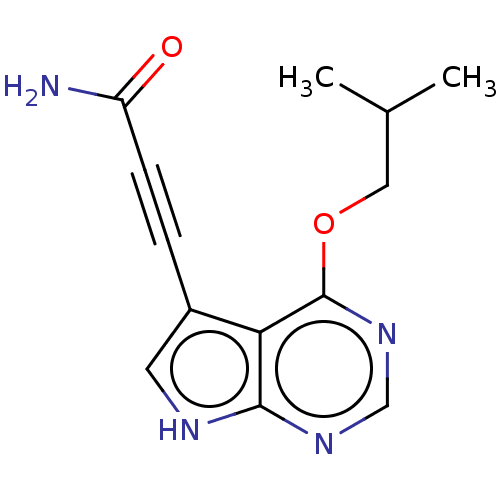 (US9505765, 186) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
CONFLUENCE LIFE SCIENCES INC. US Patent | Assay Description TAK1-TAB1 Binding Inhibitory Potency: The ability of candidate compounds to interact with TAK1-TAB1 is quantitated by a competitive binding assay usi... | US Patent US9505765 (2016) BindingDB Entry DOI: 10.7270/Q2T43S1J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466914 (CHEMBL4283312) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466915 (CHEMBL4282848) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50324117 (CHEMBL1215552 | Methyl 2-((1H-Imidazol-1-yl)methyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonist activity at mineralocorticoid receptor LBD expressed in human HUH7 cells coexpressing GAL4 by luciferase reporter gene assay | J Med Chem 53: 5970-8 (2010) Article DOI: 10.1021/jm100506y BindingDB Entry DOI: 10.7270/Q2XW4KST | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189877 (US10227346, Example 5 | US10426135, Example 5 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM493009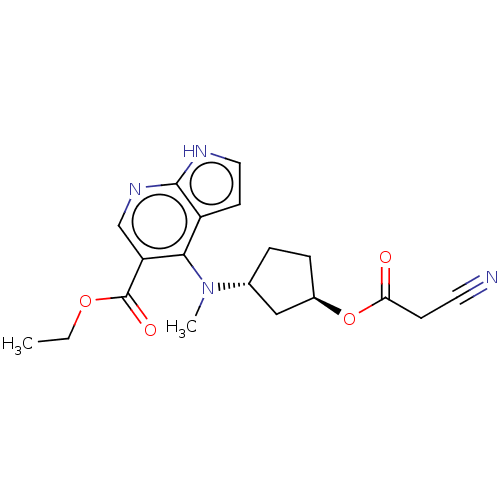 (US10981906, Example 69 | ethyl 4-(((1R,3R)-3-(2- c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 [781-1124] (Homo sapiens (Human)) | BDBM493009 (US10981906, Example 69 | ethyl 4-(((1R,3R)-3-(2- c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 [781-1124] (Homo sapiens (Human)) | BDBM493010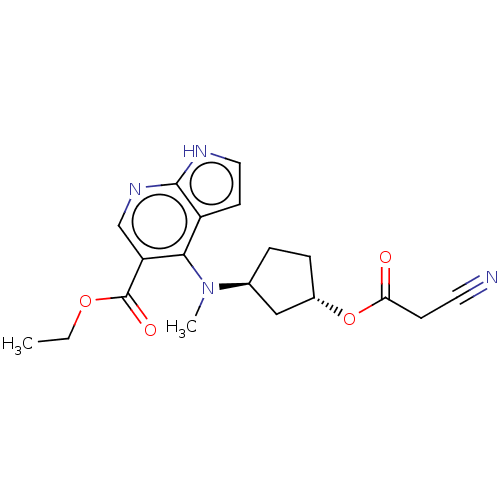 (US10981906, Example 70 | ethyl 4-(((1S,3S)-3-(2- c...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM493020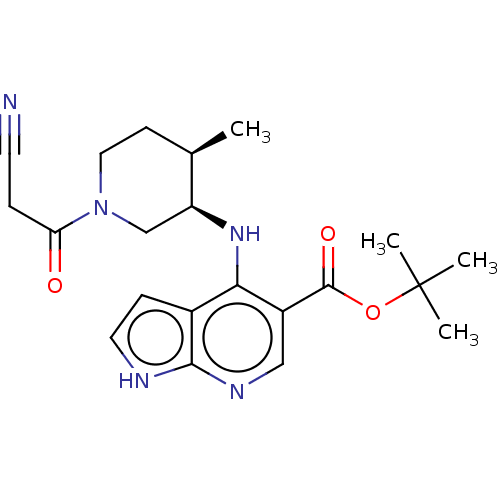 (US10981906, Example 119 | tert-butyl 4-(((3R,4R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 [781-1124] (Homo sapiens (Human)) | BDBM493020 (US10981906, Example 119 | tert-butyl 4-(((3R,4R)-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM493021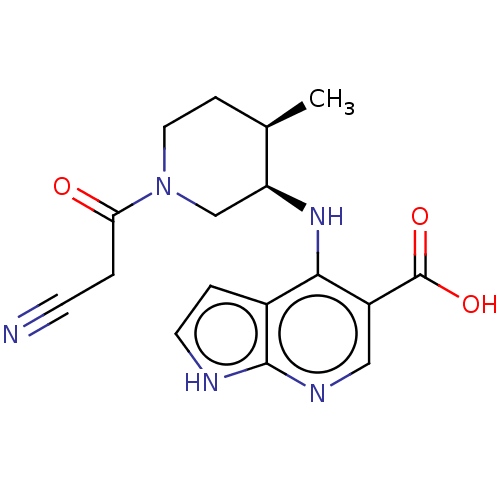 (4-(((3R,4R)-1-(2-cyanoacetyl)-4-methylpiperidin-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 [781-1124] (Homo sapiens (Human)) | BDBM493021 (4-(((3R,4R)-1-(2-cyanoacetyl)-4-methylpiperidin-3-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM493022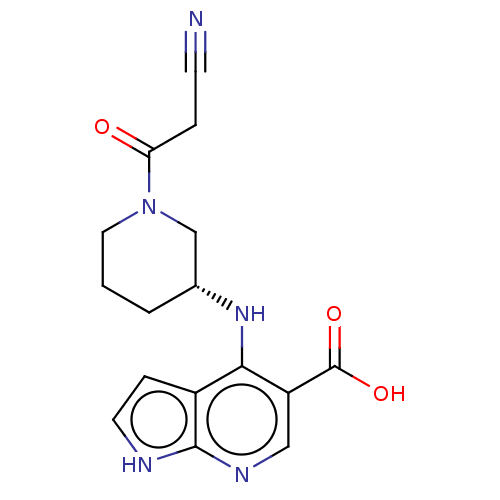 ((R)-4-((1-(2-cyanoacetyl)piperidin-3-yl)amino)-1H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 [781-1124] (Homo sapiens (Human)) | BDBM493022 ((R)-4-((1-(2-cyanoacetyl)piperidin-3-yl)amino)-1H-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM493043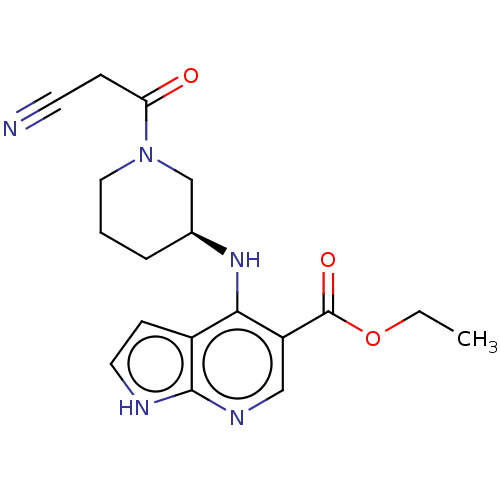 (US10981906, Example 143 | ethyl (S)-4-((1-(2-cyano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM493047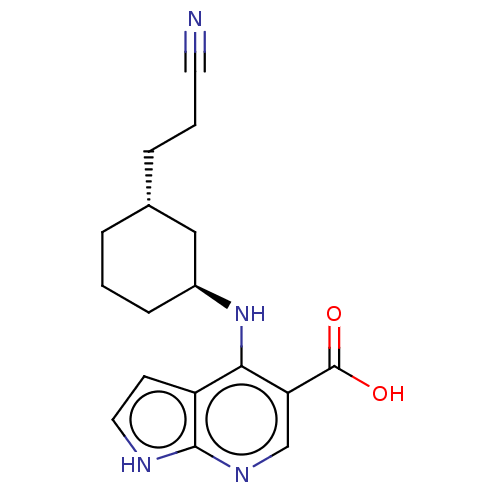 (4-(((1S,3R)-3-(2-cyanoethyl)cyclohexyl)amino)-1H- ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 [781-1124] (Homo sapiens (Human)) | BDBM493047 (4-(((1S,3R)-3-(2-cyanoethyl)cyclohexyl)amino)-1H- ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM493049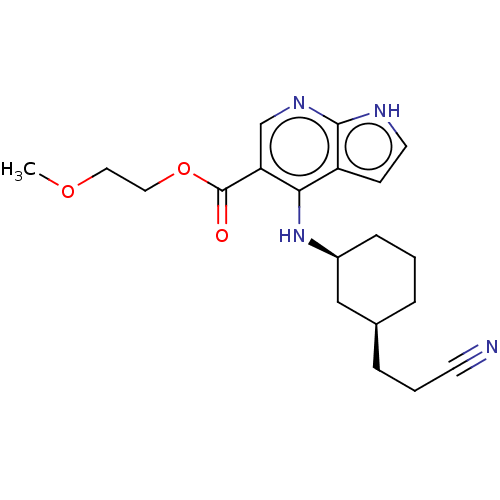 (US10981906, Example 149 | cis-2-methoxyethyl 4-((3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM493053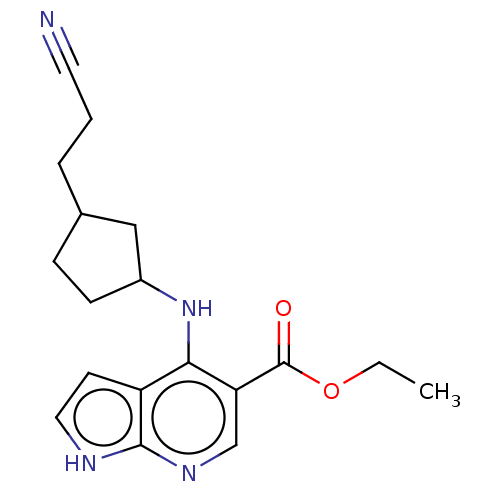 (US10981906, Example 153 | US10981906, Example 154 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM493053 (US10981906, Example 153 | US10981906, Example 154 ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10981906 (2021) BindingDB Entry DOI: 10.7270/Q2H70JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189922 (US10227346, Example 109 | US10426135, Example 109 ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466908 (CHEMBL4283530) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM466934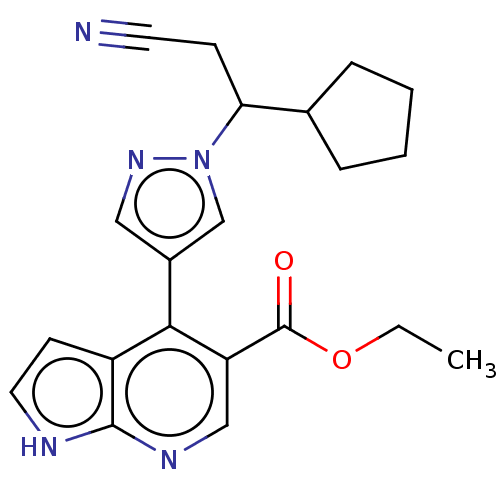 (US10800775, Example 1 | ethyl 4-(1-(2-cyano-1-cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10800775 (2020) BindingDB Entry DOI: 10.7270/Q2NV9NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM466935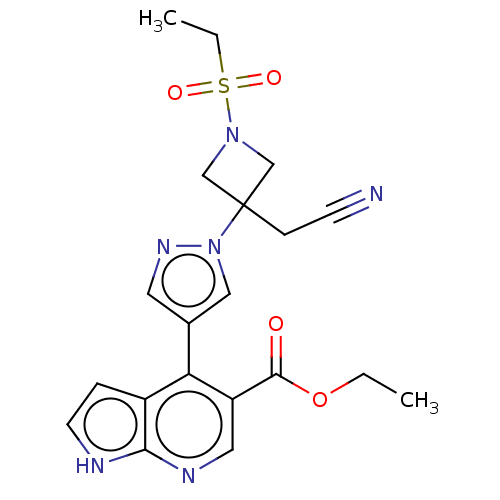 (US10800775, Example 9 | ethyl 4-(1-(3-(cyanomethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10800775 (2020) BindingDB Entry DOI: 10.7270/Q2NV9NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM466936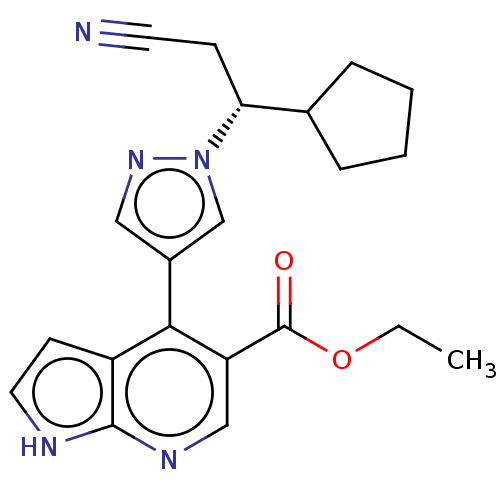 (US10800775, Example 16 | ethyl 4-(1-(2-cyano-1-cyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10800775 (2020) BindingDB Entry DOI: 10.7270/Q2NV9NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM466938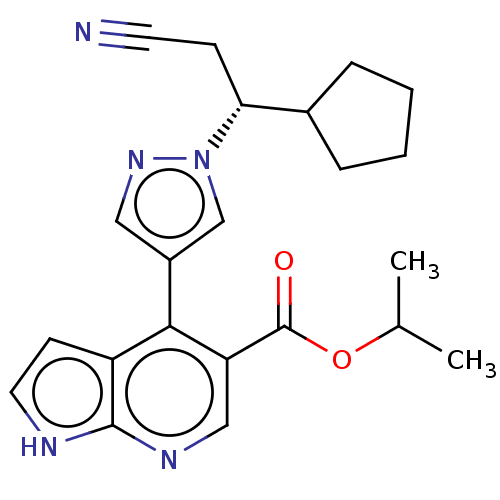 (US10800775, Example 18 | isopropyl 4-(1-(2-cyano-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10800775 (2020) BindingDB Entry DOI: 10.7270/Q2NV9NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM466940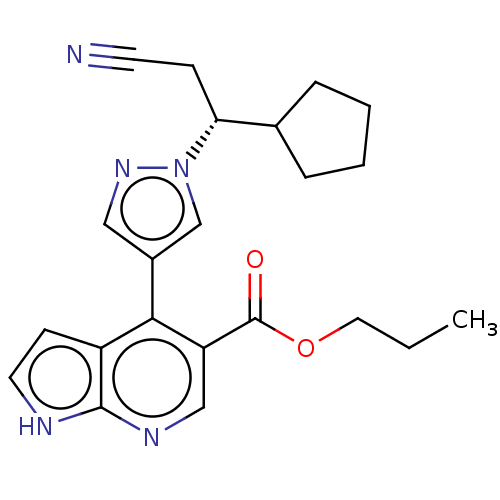 (US10800775, Example 20 | propyl (R)-4-(1-(2-cyano-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
ACLARIS THERAPEUTICS, INC. US Patent | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | US Patent US10800775 (2020) BindingDB Entry DOI: 10.7270/Q2NV9NBW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50318296 ((R)-Ethyl 5-Cyano-6-methyl-2-propyl-4-quinolin-4-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development Curated by ChEMBL | Assay Description Antagonist activity at Gal4-fused mineralocorticoid receptor expressed in human HuH7 cells assessed as inhibition of aldosterone-induced receptor act... | J Med Chem 53: 4300-4 (2010) Article DOI: 10.1021/jm1002827 BindingDB Entry DOI: 10.7270/Q22R3RV8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK1 (Homo sapiens (Human)) | BDBM567150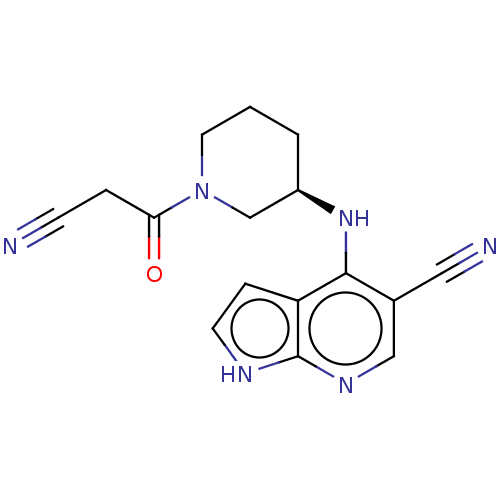 ((R)-4-((1-(2-cyanoacetyl)piperidin-3- yl)amino)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The activity of JAK3 (a.a. 781-1124, ThermoFisher) was quantified by measuring the phosphorylation of SRCtide (FAM-GEEPLYWSFPAKKK-NH2). Kinase reacti... | Citation and Details BindingDB Entry DOI: 10.7270/Q2GH9N63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 958 total ) | Next | Last >> |