 Found 1707 hits with Last Name = 'flanagan' and Initial = 'ju'
Found 1707 hits with Last Name = 'flanagan' and Initial = 'ju' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50121975

((6-Methoxy-quinolin-4-yl)-(5-vinyl-1-aza-bicyclo[2...)Show SMILES COc1ccc2nccc([C@H](O)[C@H]3C[C@@H]4CCN3C[C@@H]4C=C)c2c1 |r,THB:20:19:12.13:16.15,10:12:18.19:16.15| Show InChI InChI=1S/C20H24N2O2/c1-3-13-12-22-9-7-14(13)10-19(22)20(23)16-6-8-21-18-5-4-15(24-2)11-17(16)18/h3-6,8,11,13-14,19-20,23H,1,7,9-10,12H2,2H3/t13-,14-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Binding affinity for cytochrome P450 2D6 |
J Med Chem 47: 5340-6 (2004)
Article DOI: 10.1021/jm049934e
BindingDB Entry DOI: 10.7270/Q2R49RJH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Binding affinity for cytochrome P450 2D6 |
J Med Chem 47: 5340-6 (2004)
Article DOI: 10.1021/jm049934e
BindingDB Entry DOI: 10.7270/Q2R49RJH |
More data for this
Ligand-Target Pair | |
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
(Homo sapiens (Human)) | BDBM61867
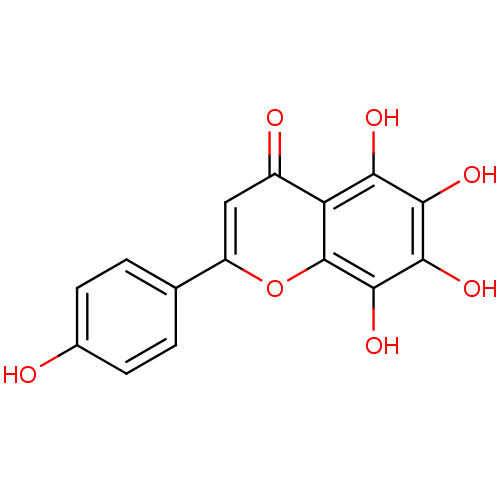
(2-(4-hydroxyphenyl)-5,6,7,8-tetrakis(oxidanyl)chro...)Show InChI InChI=1S/C15H10O7/c16-7-3-1-6(2-4-7)9-5-8(17)10-11(18)12(19)13(20)14(21)15(10)22-9/h1-5,16,18-21H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Competitive inhibition of PFKFB3 (unknown origin) using fructose 6-phosphate as substrate |
Bioorg Med Chem 22: 1029-39 (2014)
Article DOI: 10.1016/j.bmc.2013.12.041
BindingDB Entry DOI: 10.7270/Q2TB18C4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM87351
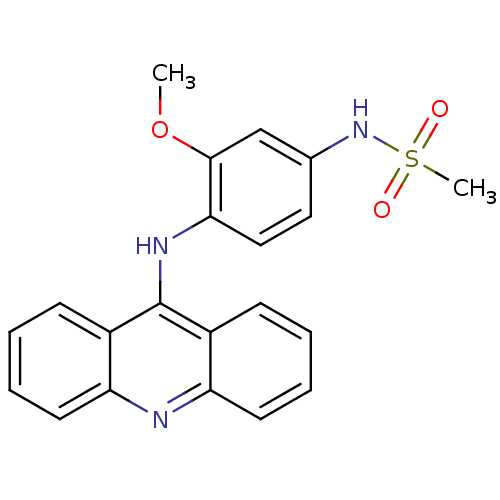
(Amsacrine hydrochloride | CHEMBL43 | MLS002153376 ...)Show SMILES COc1cc(NS(C)(=O)=O)ccc1Nc1c2ccccc2nc2ccccc12 Show InChI InChI=1S/C21H19N3O3S/c1-27-20-13-14(24-28(2,25)26)11-12-19(20)23-21-15-7-3-5-9-17(15)22-18-10-6-4-8-16(18)21/h3-13,24H,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Binding affinity for cytochrome P450 2D6 |
J Med Chem 47: 5340-6 (2004)
Article DOI: 10.1021/jm049934e
BindingDB Entry DOI: 10.7270/Q2R49RJH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C2
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C2 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50370411

(CINCHORINE | GNF-PF-3189)Show SMILES O[C@H]([C@H]1C[C@@H]2CC[N@]1C[C@@H]2C=C)c1ccnc2ccccc12 |r| Show InChI InChI=1S/C19H22N2O/c1-2-13-12-21-10-8-14(13)11-18(21)19(22)16-7-9-20-17-6-4-3-5-15(16)17/h2-7,9,13-14,18-19,22H,1,8,10-12H2/t13-,14-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leicester
Curated by ChEMBL
| Assay Description
Binding affinity for cytochrome P450 2D6 |
J Med Chem 47: 5340-6 (2004)
Article DOI: 10.1021/jm049934e
BindingDB Entry DOI: 10.7270/Q2R49RJH |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C4
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C4 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C1
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C1 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50396691

(CHEMBL449572 | US9271961, Jasmonic acid)Show InChI InChI=1S/C12H18O3/c1-2-3-4-5-10-9(8-12(14)15)6-7-11(10)13/h3-4,9-10H,2,5-8H2,1H3,(H,14,15)/b4-3-/t9-,10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.62E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant AKR1C3 expressed in Escherichia coli BL21(DE3) using phenanthrenequinone as substrate by spectrophotometric analysis |
J Med Chem 55: 7746-58 (2012)
Article DOI: 10.1021/jm3007867
BindingDB Entry DOI: 10.7270/Q28K7B6F |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50355682
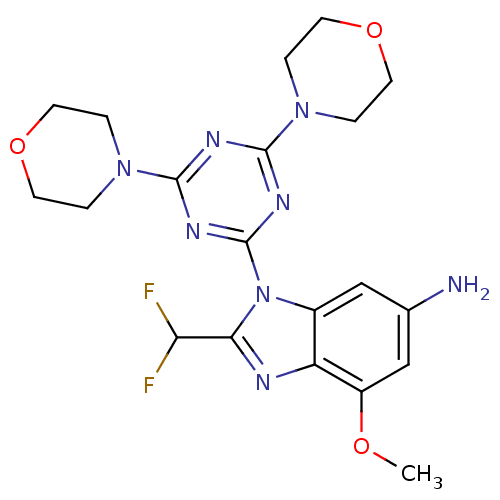
(CHEMBL1911117)Show SMILES COc1cc(N)cc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C20H24F2N8O3/c1-31-14-11-12(23)10-13-15(14)24-17(16(21)22)30(13)20-26-18(28-2-6-32-7-3-28)25-19(27-20)29-4-8-33-9-5-29/h10-11,16H,2-9,23H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PI3K p110alpha expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360084

(CHEMBL1928725)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1Cl)[N+]([O-])=O Show InChI InChI=1S/C16H11ClN6O4S/c1-21(28(26,27)16-7-13(23(24)25)2-3-14(16)17)19-9-12-10-20-22-5-4-11(8-18)6-15(12)22/h2-7,9-10H,1H3/b19-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360090
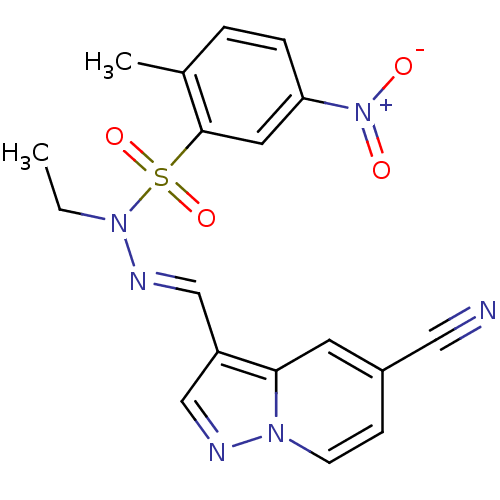
(CHEMBL1928555)Show SMILES CCN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C18H16N6O4S/c1-3-23(29(27,28)18-9-16(24(25)26)5-4-13(18)2)21-12-15-11-20-22-7-6-14(10-19)8-17(15)22/h4-9,11-12H,3H2,1-2H3/b21-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50355691
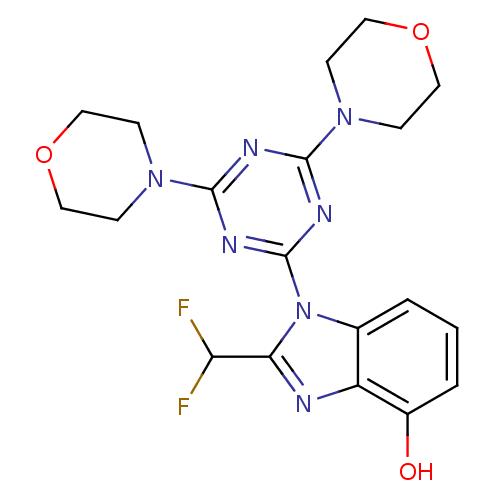
(CHEMBL1910998)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O3/c20-15(21)16-22-14-12(2-1-3-13(14)29)28(16)19-24-17(26-4-8-30-9-5-26)23-18(25-19)27-6-10-31-11-7-27/h1-3,15,29H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PI3K p110alpha after 2 hrs by HTRF assay |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50355691

(CHEMBL1910998)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O3/c20-15(21)16-22-14-12(2-1-3-13(14)29)28(16)19-24-17(26-4-8-30-9-5-26)23-18(25-19)27-6-10-31-11-7-27/h1-3,15,29H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PI3K p110delta after 2 hrs by HTRF assay |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50355691

(CHEMBL1910998)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O3/c20-15(21)16-22-14-12(2-1-3-13(14)29)28(16)19-24-17(26-4-8-30-9-5-26)23-18(25-19)27-6-10-31-11-7-27/h1-3,15,29H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K p110beta expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360085

(CHEMBL1928726)Show SMILES COc1ccc(cc1S(=O)(=O)N(C)\N=C\c1cnn2ccc(cc12)C#N)[N+]([O-])=O Show InChI InChI=1S/C17H14N6O5S/c1-21(19-10-13-11-20-22-6-5-12(9-18)7-15(13)22)29(26,27)17-8-14(23(24)25)3-4-16(17)28-2/h3-8,10-11H,1-2H3/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50355687
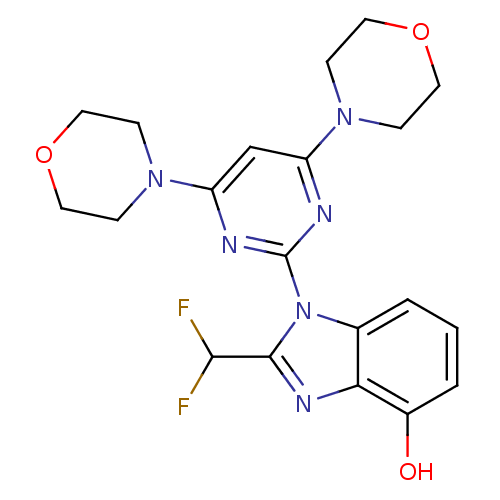
(CHEMBL1911122)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(cc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C20H22F2N6O3/c21-18(22)19-25-17-13(2-1-3-14(17)29)28(19)20-23-15(26-4-8-30-9-5-26)12-16(24-20)27-6-10-31-11-7-27/h1-3,12,18,29H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K p110beta expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50355691

(CHEMBL1910998)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O3/c20-15(21)16-22-14-12(2-1-3-13(14)29)28(16)19-24-17(26-4-8-30-9-5-26)23-18(25-19)27-6-10-31-11-7-27/h1-3,15,29H,4-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PI3K p110alpha expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360024

(CHEMBL1928541)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C17H14N6O4S/c1-12-3-4-15(23(24)25)8-17(12)28(26,27)21(2)19-10-14-11-20-22-6-5-13(9-18)7-16(14)22/h3-8,10-11H,1-2H3/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360024

(CHEMBL1928541)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C17H14N6O4S/c1-12-3-4-15(23(24)25)8-17(12)28(26,27)21(2)19-10-14-11-20-22-6-5-13(9-18)7-16(14)22/h3-8,10-11H,1-2H3/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50355683
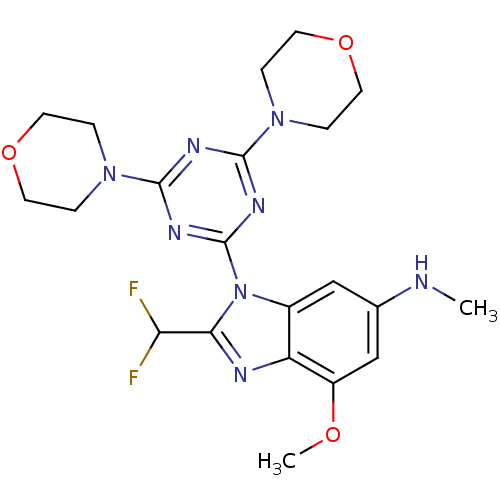
(CHEMBL1911118)Show SMILES CNc1cc(OC)c2nc(C(F)F)n(-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)c2c1 Show InChI InChI=1S/C21H26F2N8O3/c1-24-13-11-14-16(15(12-13)32-2)25-18(17(22)23)31(14)21-27-19(29-3-7-33-8-4-29)26-20(28-21)30-5-9-34-10-6-30/h11-12,17,24H,3-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K p110beta expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360063
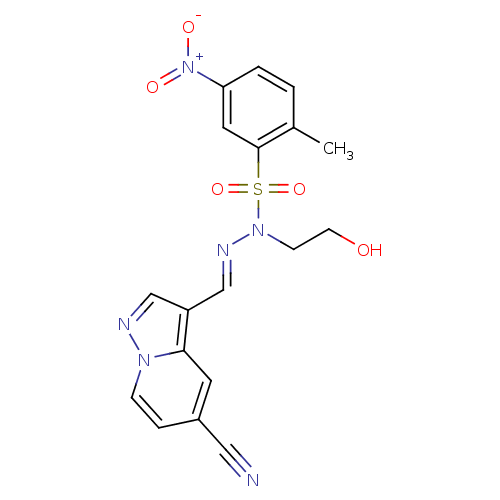
(CHEMBL1928561)Show SMILES Cc1ccc(cc1S(=O)(=O)N(CCO)\N=C\c1cnn2ccc(cc12)C#N)[N+]([O-])=O Show InChI InChI=1S/C18H16N6O5S/c1-13-2-3-16(24(26)27)9-18(13)30(28,29)23(6-7-25)21-12-15-11-20-22-5-4-14(10-19)8-17(15)22/h2-5,8-9,11-12,25H,6-7H2,1H3/b21-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50355691

(CHEMBL1910998)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O3/c20-15(21)16-22-14-12(2-1-3-13(14)29)28(16)19-24-17(26-4-8-30-9-5-26)23-18(25-19)27-6-10-31-11-7-27/h1-3,15,29H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of PIK3CA-mediated cell signaling in PTEN-deficient human U87MG cells assessed as inhibition of insulin-induced pAkt/PKB phosphorylation a... |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50355682

(CHEMBL1911117)Show SMILES COc1cc(N)cc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C20H24F2N8O3/c1-31-14-11-12(23)10-13-15(14)24-17(16(21)22)30(13)20-26-18(28-2-6-32-7-3-28)25-19(27-20)29-4-8-33-9-5-29/h10-11,16H,2-9,23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant PI3K p110beta expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360081
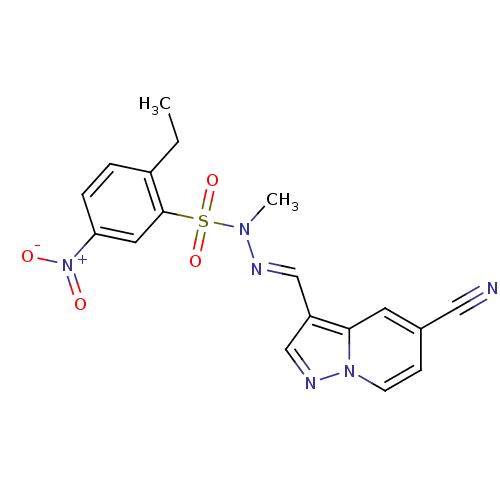
(CHEMBL1928722)Show SMILES CCc1ccc(cc1S(=O)(=O)N(C)\N=C\c1cnn2ccc(cc12)C#N)[N+]([O-])=O Show InChI InChI=1S/C18H16N6O4S/c1-3-14-4-5-16(24(25)26)9-18(14)29(27,28)22(2)20-11-15-12-21-23-7-6-13(10-19)8-17(15)23/h4-9,11-12H,3H2,1-2H3/b20-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50355687

(CHEMBL1911122)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(cc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C20H22F2N6O3/c21-18(22)19-25-17-13(2-1-3-14(17)29)28(19)20-23-15(26-4-8-30-9-5-26)12-16(24-20)27-6-10-31-11-7-27/h1-3,12,18,29H,4-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PI3K p110alpha expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50355682

(CHEMBL1911117)Show SMILES COc1cc(N)cc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C20H24F2N8O3/c1-31-14-11-12(23)10-13-15(14)24-17(16(21)22)30(13)20-26-18(28-2-6-32-7-3-28)25-19(27-20)29-4-8-33-9-5-29/h10-11,16H,2-9,23H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PI3K p110delta after 2 hrs by HTRF assay |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360082

(CHEMBL1928723)Show SMILES CC(C)c1ccc(cc1S(=O)(=O)N(C)\N=C\c1cnn2ccc(cc12)C#N)[N+]([O-])=O Show InChI InChI=1S/C19H18N6O4S/c1-13(2)17-5-4-16(25(26)27)9-19(17)30(28,29)23(3)21-11-15-12-22-24-7-6-14(10-20)8-18(15)24/h4-9,11-13H,1-3H3/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50355683

(CHEMBL1911118)Show SMILES CNc1cc(OC)c2nc(C(F)F)n(-c3nc(nc(n3)N3CCOCC3)N3CCOCC3)c2c1 Show InChI InChI=1S/C21H26F2N8O3/c1-24-13-11-14-16(15(12-13)32-2)25-18(17(22)23)31(14)21-27-19(29-3-7-33-8-4-29)26-20(28-21)30-5-9-34-10-6-30/h11-12,17,24H,3-10H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PI3K p110alpha expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50355679
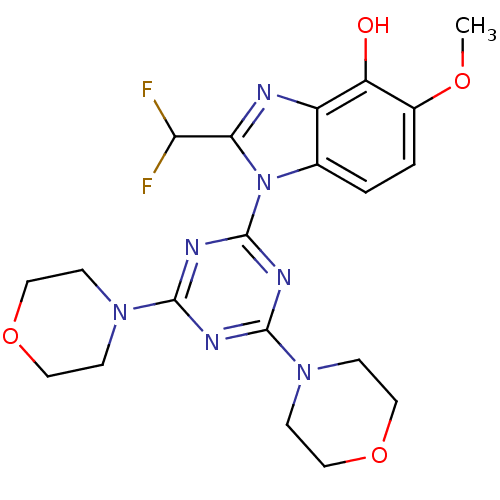
(CHEMBL1911114)Show SMILES COc1ccc2n(c(nc2c1O)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C20H23F2N7O4/c1-31-13-3-2-12-14(15(13)30)23-17(16(21)22)29(12)20-25-18(27-4-8-32-9-5-27)24-19(26-20)28-6-10-33-11-7-28/h2-3,16,30H,4-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PI3K p110delta after 2 hrs by HTRF assay |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360086
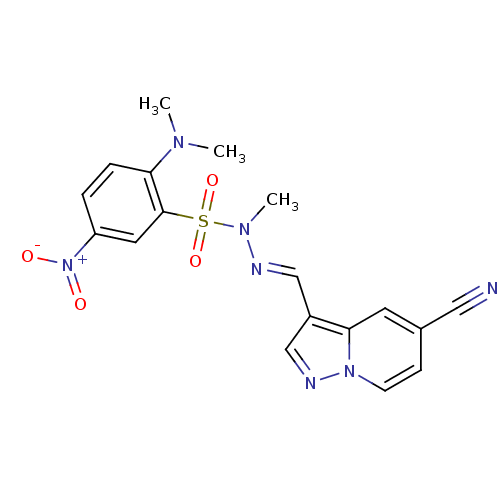
(CHEMBL1928727)Show SMILES CN(C)c1ccc(cc1S(=O)(=O)N(C)\N=C\c1cnn2ccc(cc12)C#N)[N+]([O-])=O Show InChI InChI=1S/C18H17N7O4S/c1-22(2)16-5-4-15(25(26)27)9-18(16)30(28,29)23(3)20-11-14-12-21-24-7-6-13(10-19)8-17(14)24/h4-9,11-12H,1-3H3/b20-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50451184
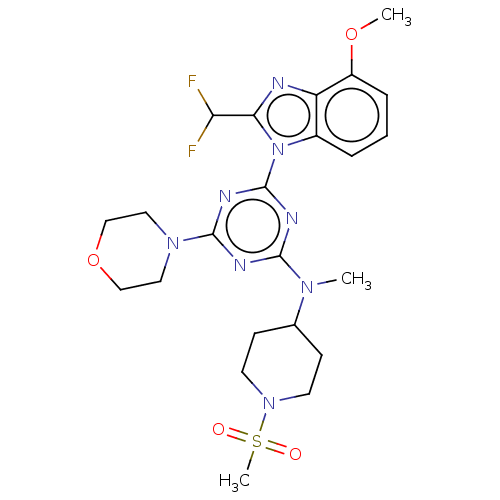
(CHEMBL4213621)Show SMILES COc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N(C)C1CCN(CC1)S(C)(=O)=O Show InChI InChI=1S/C23H30F2N8O4S/c1-30(15-7-9-32(10-8-15)38(3,34)35)21-27-22(31-11-13-37-14-12-31)29-23(28-21)33-16-5-4-6-17(36-2)18(16)26-20(33)19(24)25/h4-6,15,19H,7-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in Sf9 insect cells by HTRF assay |
Bioorg Med Chem 25: 5859-5874 (2017)
Article DOI: 10.1016/j.bmc.2017.09.025
BindingDB Entry DOI: 10.7270/Q2G73HB6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360073

(CHEMBL1928571)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#N)S(=O)(=O)c1cc(ccc1C)C#N Show InChI InChI=1S/C18H14N6O2S/c1-13-3-4-14(9-19)8-18(13)27(25,26)23(2)21-11-16-12-22-24-6-5-15(10-20)7-17(16)24/h3-8,11-12H,1-2H3/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 58-68 (2011)
Article DOI: 10.1016/j.bmc.2011.11.031
BindingDB Entry DOI: 10.7270/Q21C1X99 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360040

(CHEMBL1928536)Show SMILES CN(\N=C\c1cnn2ccc(cc12)C#C)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C18H15N5O4S/c1-4-14-7-8-22-17(9-14)15(12-20-22)11-19-21(3)28(26,27)18-10-16(23(24)25)6-5-13(18)2/h1,5-12H,2-3H3/b19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50451223

(CHEMBL4214500)Show SMILES COc1cccc2n(c(nc12)C(F)F)-c1nc(N2CCOCC2)c2cnn(C3CCN(CC3)S(C)(=O)=O)c2n1 Show InChI InChI=1S/C24H28F2N8O4S/c1-37-18-5-3-4-17-19(18)28-23(20(25)26)33(17)24-29-21(31-10-12-38-13-11-31)16-14-27-34(22(16)30-24)15-6-8-32(9-7-15)39(2,35)36/h3-5,14-15,20H,6-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in Sf9 insect cells by HTRF assay |
Bioorg Med Chem 25: 5859-5874 (2017)
Article DOI: 10.1016/j.bmc.2017.09.025
BindingDB Entry DOI: 10.7270/Q2G73HB6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50435583
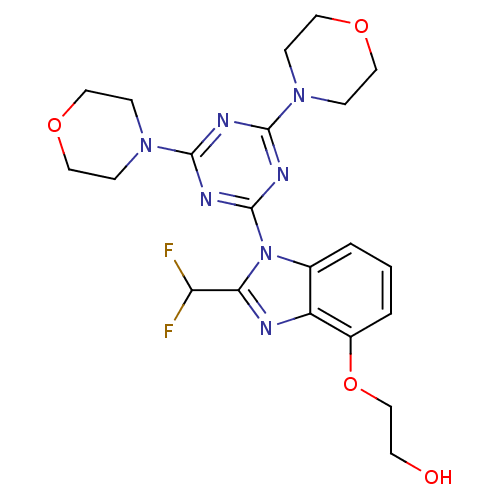
(CHEMBL2393147)Show SMILES OCCOc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C21H25F2N7O4/c22-17(23)18-24-16-14(2-1-3-15(16)34-13-8-31)30(18)21-26-19(28-4-9-32-10-5-28)25-20(27-21)29-6-11-33-12-7-29/h1-3,17,31H,4-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110delta/p85alpha (unknown origin) using phosphotidylinositol as susbstrate after 1 hr by thin layer paper chromatogr... |
Eur J Med Chem 64: 137-47 (2013)
Article DOI: 10.1016/j.ejmech.2013.03.038
BindingDB Entry DOI: 10.7270/Q2377B33 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50355694
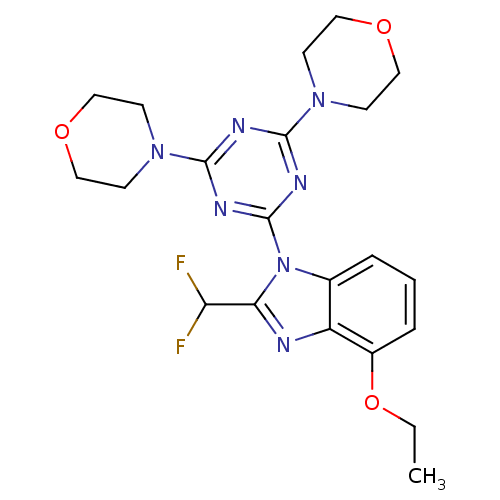
(CHEMBL1911000)Show SMILES CCOc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C21H25F2N7O3/c1-2-33-15-5-3-4-14-16(15)24-18(17(22)23)30(14)21-26-19(28-6-10-31-11-7-28)25-20(27-21)29-8-12-32-13-9-29/h3-5,17H,2,6-13H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PI3K p110alpha expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50355693
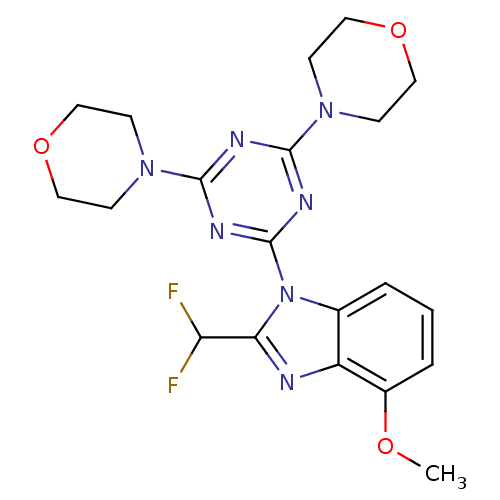
(CHEMBL1910999)Show SMILES COc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C20H23F2N7O3/c1-30-14-4-2-3-13-15(14)23-17(16(21)22)29(13)20-25-18(27-5-9-31-10-6-27)24-19(26-20)28-7-11-32-12-8-28/h2-4,16H,5-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PI3K p110alpha expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360037

(CHEMBL1928533)Show SMILES CN(\N=C\c1cnn2ccc(Cl)cc12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14ClN5O4S/c1-11-3-4-14(22(23)24)8-16(11)27(25,26)20(2)18-9-12-10-19-21-6-5-13(17)7-15(12)21/h3-10H,1-2H3/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50451217
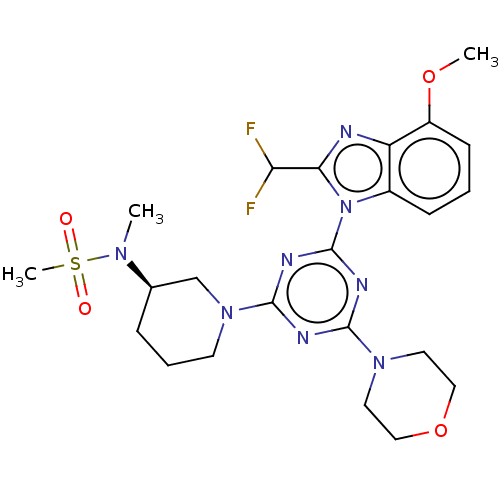
(CHEMBL4214284)Show SMILES COc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCC[C@H](C1)N(C)S(C)(=O)=O)N1CCOCC1 |r| Show InChI InChI=1S/C23H30F2N8O4S/c1-30(38(3,34)35)15-6-5-9-32(14-15)22-27-21(31-10-12-37-13-11-31)28-23(29-22)33-16-7-4-8-17(36-2)18(16)26-20(33)19(24)25/h4,7-8,15,19H,5-6,9-14H2,1-3H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in Sf9 insect cells by HTRF assay |
Bioorg Med Chem 25: 5859-5874 (2017)
Article DOI: 10.1016/j.bmc.2017.09.025
BindingDB Entry DOI: 10.7270/Q2G73HB6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50355691

(CHEMBL1910998)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O3/c20-15(21)16-22-14-12(2-1-3-13(14)29)28(16)19-24-17(26-4-8-30-9-5-26)23-18(25-19)27-6-10-31-11-7-27/h1-3,15,29H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of PIK3CA-mediated cell signaling in PTEN-deficient human U87MG cells assessed as inhibition of insulin-induced pAkt/PKB phosphorylation a... |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Aldo-keto reductase family 1 member C3
(Homo sapiens (Human)) | BDBM50446016

(CHEMBL3103330)Show InChI InChI=1S/C15H19Cl2N3O2/c16-12-1-2-14(13(17)11-12)18-3-5-19(6-4-18)15(21)20-7-9-22-10-8-20/h1-2,11H,3-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of AKR1C3 (unknown origin) expressed in human HCT116 cells assessed as formation of PR-104H from PR-104A preincubated for 2 hrs |
Bioorg Med Chem 22: 967-77 (2014)
Article DOI: 10.1016/j.bmc.2013.12.050
BindingDB Entry DOI: 10.7270/Q25H7HQM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50451240
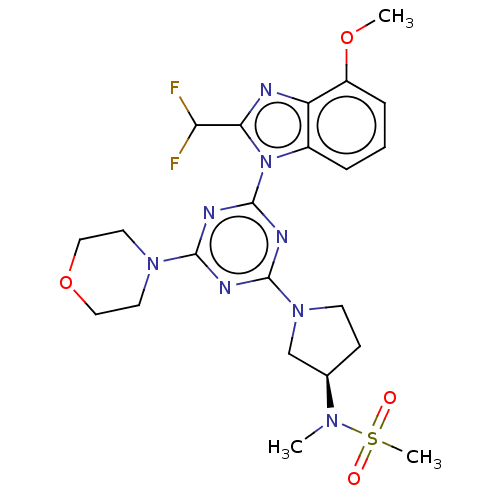
(CHEMBL4211457)Show SMILES COc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CC[C@H](C1)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28F2N8O4S/c1-29(37(3,33)34)14-7-8-31(13-14)21-26-20(30-9-11-36-12-10-30)27-22(28-21)32-15-5-4-6-16(35-2)17(15)25-19(32)18(23)24/h4-6,14,18H,7-13H2,1-3H3/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in Sf9 insect cells by HTRF assay |
Bioorg Med Chem 25: 5859-5874 (2017)
Article DOI: 10.1016/j.bmc.2017.09.025
BindingDB Entry DOI: 10.7270/Q2G73HB6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50451236

(CHEMBL4208958)Show SMILES COc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCC(CNS(C)(=O)=O)CC1)N1CCOCC1 Show InChI InChI=1S/C23H30F2N8O4S/c1-36-17-5-3-4-16-18(17)27-20(19(24)25)33(16)23-29-21(28-22(30-23)32-10-12-37-13-11-32)31-8-6-15(7-9-31)14-26-38(2,34)35/h3-5,15,19,26H,6-14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in Sf9 insect cells by HTRF assay |
Bioorg Med Chem 25: 5859-5874 (2017)
Article DOI: 10.1016/j.bmc.2017.09.025
BindingDB Entry DOI: 10.7270/Q2G73HB6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50355691

(CHEMBL1910998)Show SMILES Oc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O3/c20-15(21)16-22-14-12(2-1-3-13(14)29)28(16)19-24-17(26-4-8-30-9-5-26)23-18(25-19)27-6-10-31-11-7-27/h1-3,15,29H,4-11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of human His-tagged PI3K p110beta after 2 hrs by HTRF assay |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50451229

(CHEMBL4205087)Show SMILES COc1cccc2n(c(nc12)C(F)F)-c1nc(NCC2CCN(CC2)S(C)(=O)=O)nc(n1)N1CCOCC1 Show InChI InChI=1S/C23H30F2N8O4S/c1-36-17-5-3-4-16-18(17)27-20(19(24)25)33(16)23-29-21(28-22(30-23)31-10-12-37-13-11-31)26-14-15-6-8-32(9-7-15)38(2,34)35/h3-5,15,19H,6-14H2,1-2H3,(H,26,28,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in Sf9 insect cells by HTRF assay |
Bioorg Med Chem 25: 5859-5874 (2017)
Article DOI: 10.1016/j.bmc.2017.09.025
BindingDB Entry DOI: 10.7270/Q2G73HB6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50360028

(CHEMBL1928525)Show SMILES CN(\N=C\c1cnn2ccc(Br)cc12)S(=O)(=O)c1cc(ccc1C)[N+]([O-])=O Show InChI InChI=1S/C16H14BrN5O4S/c1-11-3-4-14(22(23)24)8-16(11)27(25,26)20(2)18-9-12-10-19-21-6-5-13(17)7-15(12)21/h3-10H,1-2H3/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3K p110alpha using L-alpha-phosphotidylinositol as substrate and [gamma33P]ATP after 60 mins by thin layer chromatographi... |
Bioorg Med Chem 20: 69-85 (2011)
Article DOI: 10.1016/j.bmc.2011.11.029
BindingDB Entry DOI: 10.7270/Q2513ZNR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50451187
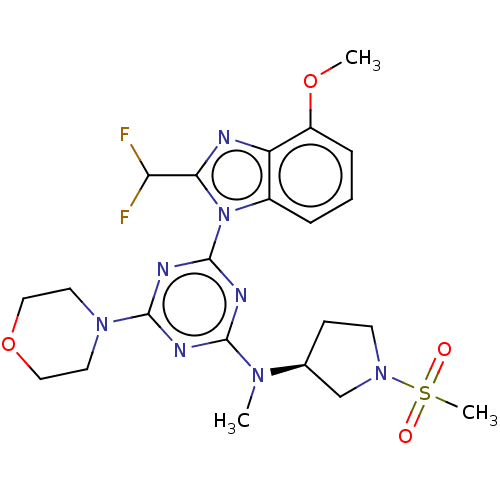
(CHEMBL4214405)Show SMILES COc1cccc2n(c(nc12)C(F)F)-c1nc(nc(n1)N1CCOCC1)N(C)[C@H]1CCN(C1)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28F2N8O4S/c1-29(14-7-8-31(13-14)37(3,33)34)20-26-21(30-9-11-36-12-10-30)28-22(27-20)32-15-5-4-6-16(35-2)17(15)25-19(32)18(23)24/h4-6,14,18H,7-13H2,1-3H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human PI3K p110alpha/p85alpha expressed in Sf9 insect cells by HTRF assay |
Bioorg Med Chem 25: 5859-5874 (2017)
Article DOI: 10.1016/j.bmc.2017.09.025
BindingDB Entry DOI: 10.7270/Q2G73HB6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50355690

(CHEMBL1911125)Show SMILES COc1cccc2n(c(nc12)C(F)F)-c1cc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C21H24F2N6O3/c1-30-15-4-2-3-14-18(15)26-20(19(22)23)29(14)17-13-16(27-5-9-31-10-6-27)24-21(25-17)28-7-11-32-12-8-28/h2-4,13,19H,5-12H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant PI3K p110alpha expressed in Sf21 insect cells using phosphatidylinositol as substrate after 1 hr by phosphoimaging |
J Med Chem 54: 7105-26 (2011)
Article DOI: 10.1021/jm200688y
BindingDB Entry DOI: 10.7270/Q20P10FQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50447094
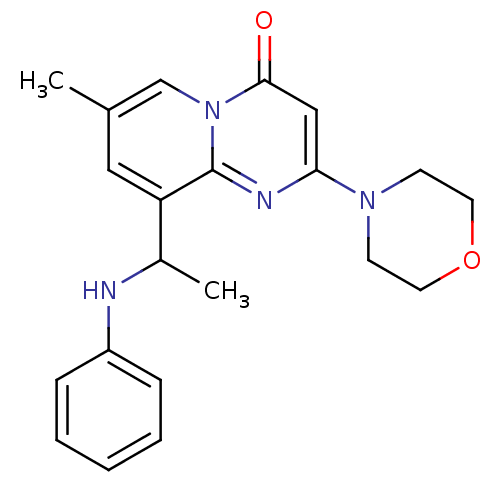
(CHEMBL1972466)Show InChI InChI=1S/C21H24N4O2/c1-15-12-18(16(2)22-17-6-4-3-5-7-17)21-23-19(13-20(26)25(21)14-15)24-8-10-27-11-9-24/h3-7,12-14,16,22H,8-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human p110beta expressed in Sf9 cells co-expressing p85alpha regulatory subunit using phosphatidylinositol substr... |
Bioorg Med Chem 23: 3796-808 (2015)
Article DOI: 10.1016/j.bmc.2015.03.073
BindingDB Entry DOI: 10.7270/Q29K4D0R |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data