 Found 211 hits with Last Name = 'fang' and Initial = 'k'
Found 211 hits with Last Name = 'fang' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372
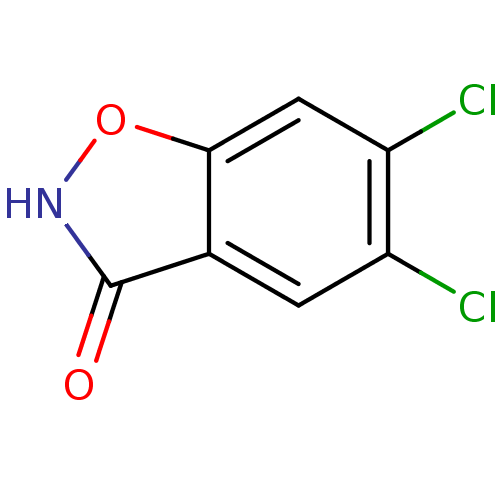
(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725
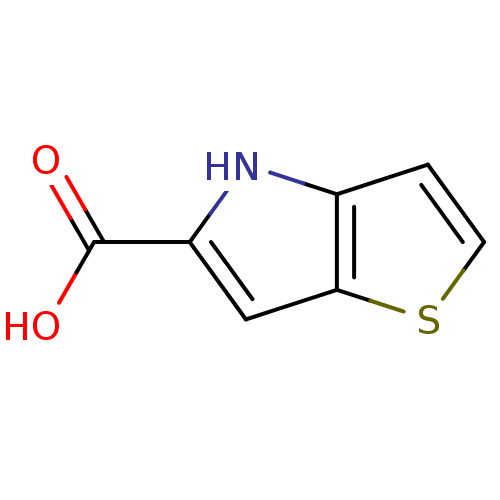
(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260722
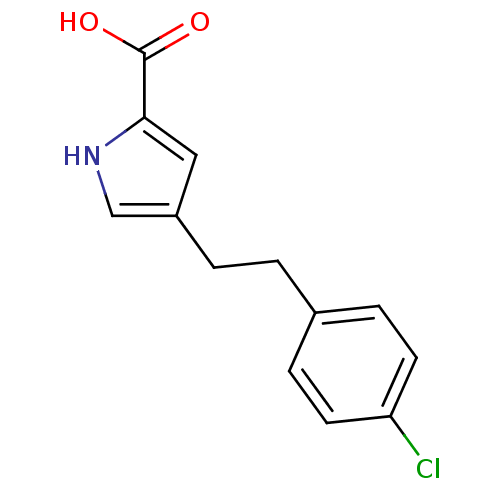
(4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...)Show InChI InChI=1S/C13H12ClNO2/c14-11-5-3-9(4-6-11)1-2-10-7-12(13(16)17)15-8-10/h3-8,15H,1-2H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50206007
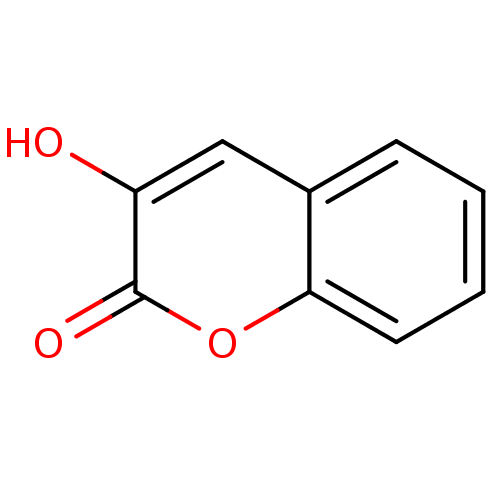
(3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...)Show InChI InChI=1S/C9H6O3/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449913
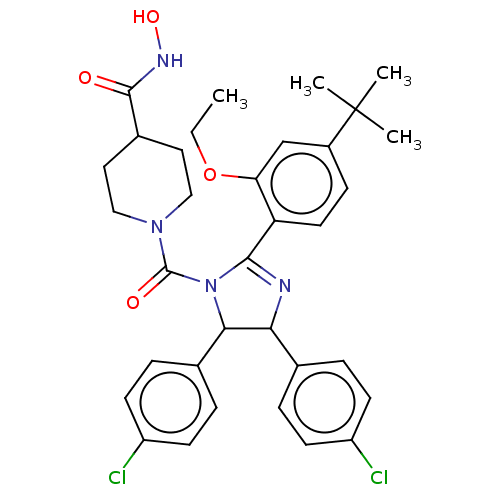
(CHEMBL4170056)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCC(CC1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C34H38Cl2N4O4/c1-5-44-28-20-24(34(2,3)4)10-15-27(28)31-37-29(21-6-11-25(35)12-7-21)30(22-8-13-26(36)14-9-22)40(31)33(42)39-18-16-23(17-19-39)32(41)38-43/h6-15,20,23,29-30,43H,5,16-19H2,1-4H3,(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449907
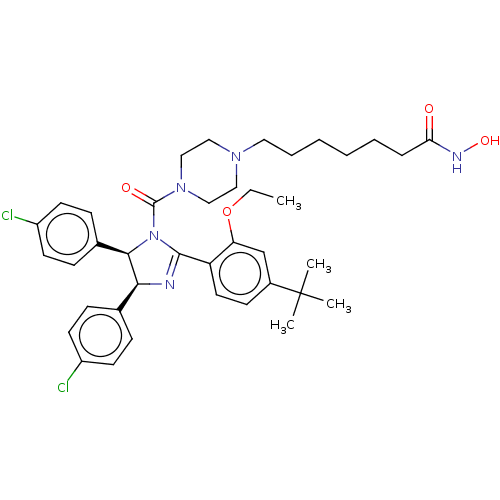
(CHEMBL4163675)Show SMILES CCOc1cc(ccc1C1=N[C@H]([C@H](N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47)/t35-,36+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449901
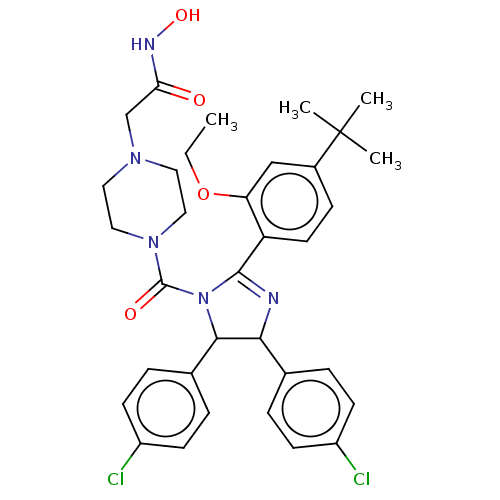
(CHEMBL4160754)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C34H39Cl2N5O4/c1-5-45-28-20-24(34(2,3)4)10-15-27(28)32-37-30(22-6-11-25(35)12-7-22)31(23-8-13-26(36)14-9-23)41(32)33(43)40-18-16-39(17-19-40)21-29(42)38-44/h6-15,20,30-31,44H,5,16-19,21H2,1-4H3,(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449900
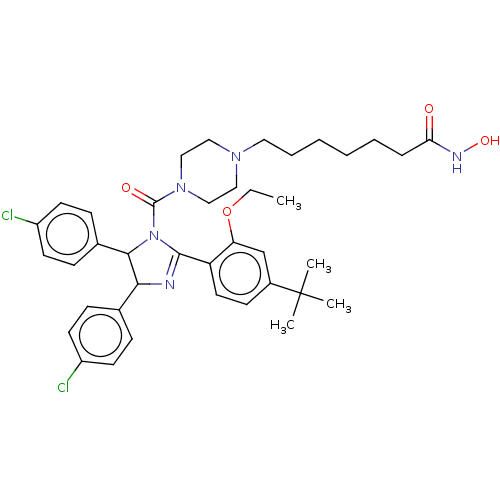
(CHEMBL4174805)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50229787
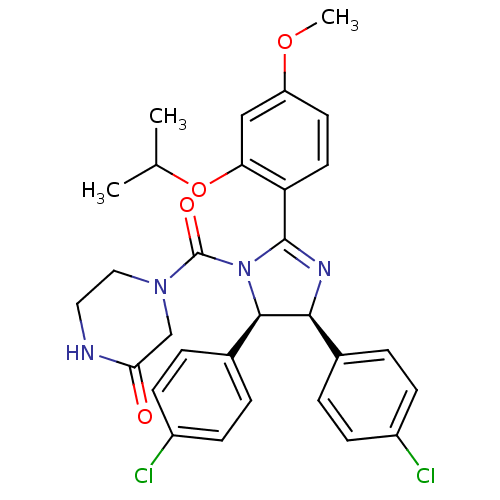
((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...)Show SMILES COc1ccc(C2=N[C@H]([C@H](N2C(=O)N2CCNC(=O)C2)c2ccc(Cl)cc2)c2ccc(Cl)cc2)c(OC(C)C)c1 |t:6| Show InChI InChI=1S/C30H30Cl2N4O4/c1-18(2)40-25-16-23(39-3)12-13-24(25)29-34-27(19-4-8-21(31)9-5-19)28(20-6-10-22(32)11-7-20)36(29)30(38)35-15-14-33-26(37)17-35/h4-13,16,18,27-28H,14-15,17H2,1-3H3,(H,33,37)/t27-,28+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449924
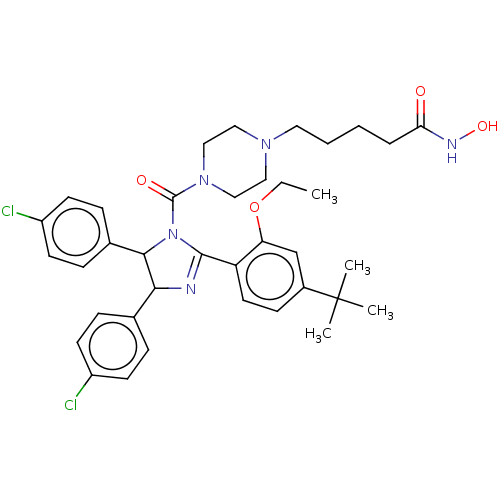
(CHEMBL4171353)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C37H45Cl2N5O4/c1-5-48-31-24-27(37(2,3)4)13-18-30(31)35-40-33(25-9-14-28(38)15-10-25)34(26-11-16-29(39)17-12-26)44(35)36(46)43-22-20-42(21-23-43)19-7-6-8-32(45)41-47/h9-18,24,33-34,47H,5-8,19-23H2,1-4H3,(H,41,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449896
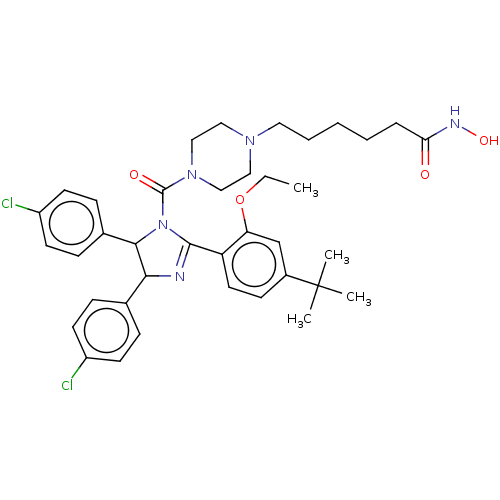
(CHEMBL4164148)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C38H47Cl2N5O4/c1-5-49-32-25-28(38(2,3)4)14-19-31(32)36-41-34(26-10-15-29(39)16-11-26)35(27-12-17-30(40)18-13-27)45(36)37(47)44-23-21-43(22-24-44)20-8-6-7-9-33(46)42-48/h10-19,25,34-35,48H,5-9,20-24H2,1-4H3,(H,42,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433371
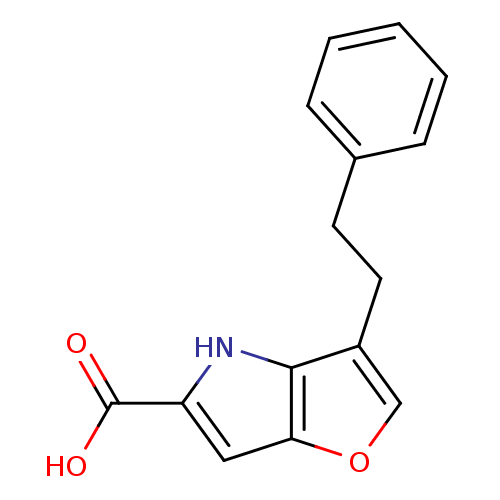
(CHEMBL2375519)Show InChI InChI=1S/C15H13NO3/c17-15(18)12-8-13-14(16-12)11(9-19-13)7-6-10-4-2-1-3-5-10/h1-5,8-9,16H,6-7H2,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449923
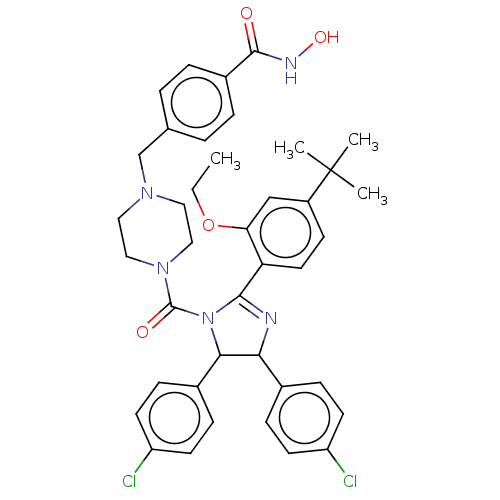
(CHEMBL4163017)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(Cc2ccc(cc2)C(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C40H43Cl2N5O4/c1-5-51-34-24-30(40(2,3)4)14-19-33(34)37-43-35(27-10-15-31(41)16-11-27)36(28-12-17-32(42)18-13-28)47(37)39(49)46-22-20-45(21-23-46)25-26-6-8-29(9-7-26)38(48)44-50/h6-19,24,35-36,50H,5,20-23,25H2,1-4H3,(H,44,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449903
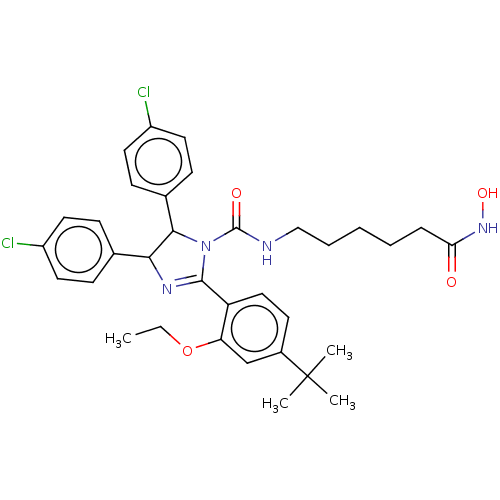
(CHEMBL4170573)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C34H40Cl2N4O4/c1-5-44-28-21-24(34(2,3)4)14-19-27(28)32-38-30(22-10-15-25(35)16-11-22)31(23-12-17-26(36)18-13-23)40(32)33(42)37-20-8-6-7-9-29(41)39-43/h10-19,21,30-31,43H,5-9,20H2,1-4H3,(H,37,42)(H,39,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449895
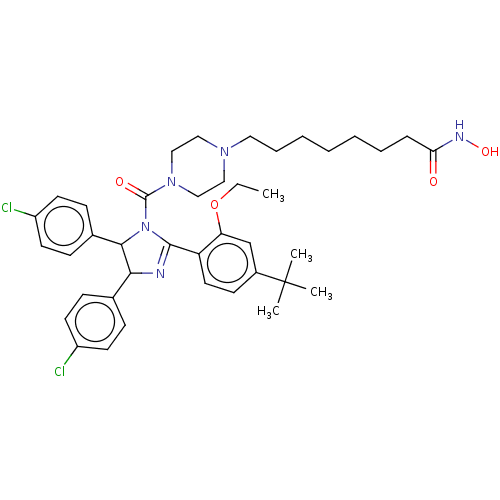
(CHEMBL4173404)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C40H51Cl2N5O4/c1-5-51-34-27-30(40(2,3)4)16-21-33(34)38-43-36(28-12-17-31(41)18-13-28)37(29-14-19-32(42)20-15-29)47(38)39(49)46-25-23-45(24-26-46)22-10-8-6-7-9-11-35(48)44-50/h12-21,27,36-37,50H,5-11,22-26H2,1-4H3,(H,44,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449925
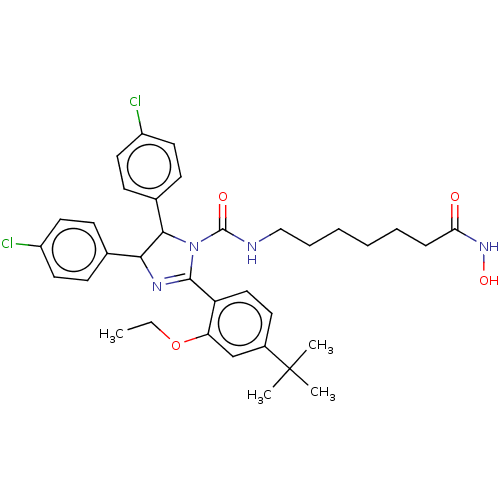
(CHEMBL4159921)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C35H42Cl2N4O4/c1-5-45-29-22-25(35(2,3)4)15-20-28(29)33-39-31(23-11-16-26(36)17-12-23)32(24-13-18-27(37)19-14-24)41(33)34(43)38-21-9-7-6-8-10-30(42)40-44/h11-20,22,31-32,44H,5-10,21H2,1-4H3,(H,38,43)(H,40,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449898
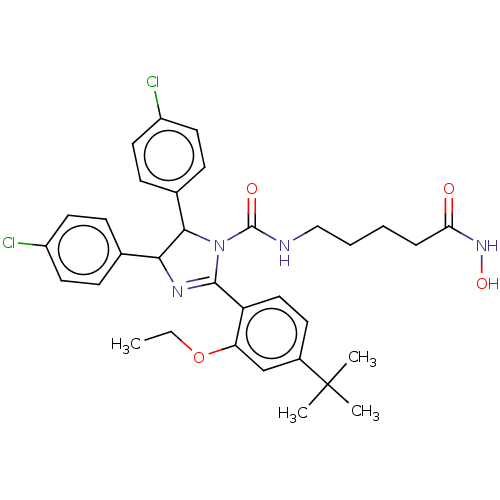
(CHEMBL4166471)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C33H38Cl2N4O4/c1-5-43-27-20-23(33(2,3)4)13-18-26(27)31-37-29(21-9-14-24(34)15-10-21)30(22-11-16-25(35)17-12-22)39(31)32(41)36-19-7-6-8-28(40)38-42/h9-18,20,29-30,42H,5-8,19H2,1-4H3,(H,36,41)(H,38,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449897
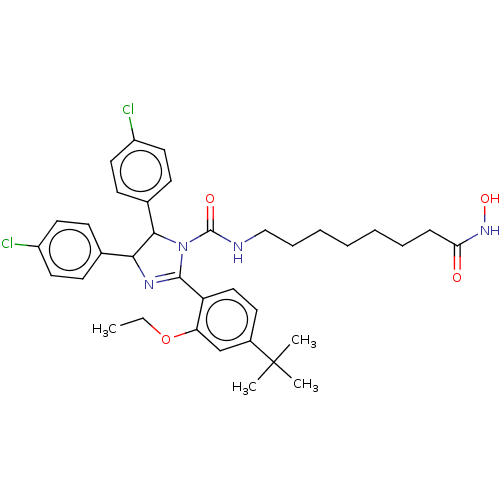
(CHEMBL4163120)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCCC(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C36H44Cl2N4O4/c1-5-46-30-23-26(36(2,3)4)16-21-29(30)34-40-32(24-12-17-27(37)18-13-24)33(25-14-19-28(38)20-15-25)42(34)35(44)39-22-10-8-6-7-9-11-31(43)41-45/h12-21,23,32-33,45H,5-11,22H2,1-4H3,(H,39,44)(H,41,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449894
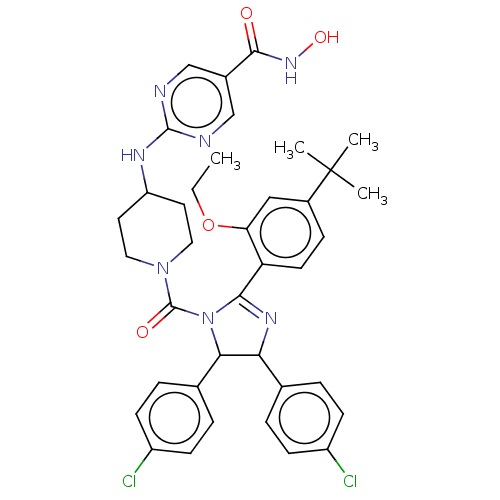
(CHEMBL4163059)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCC(CC1)Nc1ncc(cn1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C38H41Cl2N7O4/c1-5-51-31-20-26(38(2,3)4)10-15-30(31)34-44-32(23-6-11-27(39)12-7-23)33(24-8-13-28(40)14-9-24)47(34)37(49)46-18-16-29(17-19-46)43-36-41-21-25(22-42-36)35(48)45-50/h6-15,20-22,29,32-33,50H,5,16-19H2,1-4H3,(H,45,48)(H,41,42,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449902
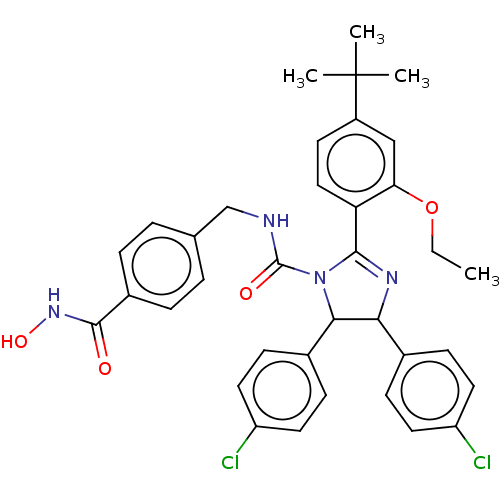
(CHEMBL4173507)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCc1ccc(cc1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C36H36Cl2N4O4/c1-5-46-30-20-26(36(2,3)4)14-19-29(30)33-40-31(23-10-15-27(37)16-11-23)32(24-12-17-28(38)18-13-24)42(33)35(44)39-21-22-6-8-25(9-7-22)34(43)41-45/h6-20,31-32,45H,5,21H2,1-4H3,(H,39,44)(H,41,43) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449905
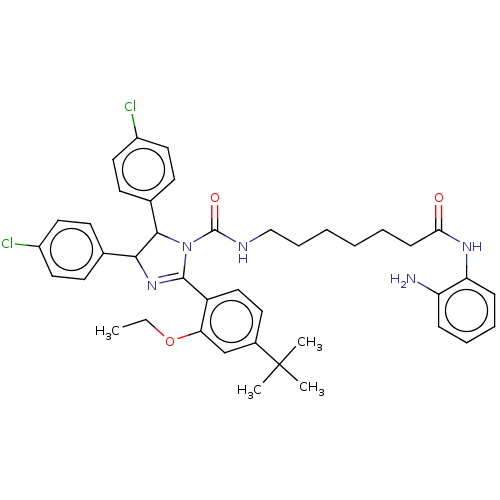
(CHEMBL4171605)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCC(=O)Nc1ccccc1N)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C41H47Cl2N5O3/c1-5-51-35-26-29(41(2,3)4)19-24-32(35)39-47-37(27-15-20-30(42)21-16-27)38(28-17-22-31(43)23-18-28)48(39)40(50)45-25-11-7-6-8-14-36(49)46-34-13-10-9-12-33(34)44/h9-10,12-13,15-24,26,37-38H,5-8,11,14,25,44H2,1-4H3,(H,45,50)(H,46,49) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449899
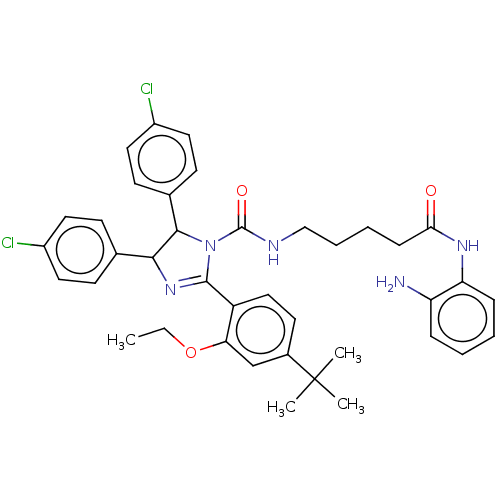
(CHEMBL4175049)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCC(=O)Nc1ccccc1N)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H43Cl2N5O3/c1-5-49-33-24-27(39(2,3)4)17-22-30(33)37-45-35(25-13-18-28(40)19-14-25)36(26-15-20-29(41)21-16-26)46(37)38(48)43-23-9-8-12-34(47)44-32-11-7-6-10-31(32)42/h6-7,10-11,13-22,24,35-36H,5,8-9,12,23,42H2,1-4H3,(H,43,48)(H,44,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449904
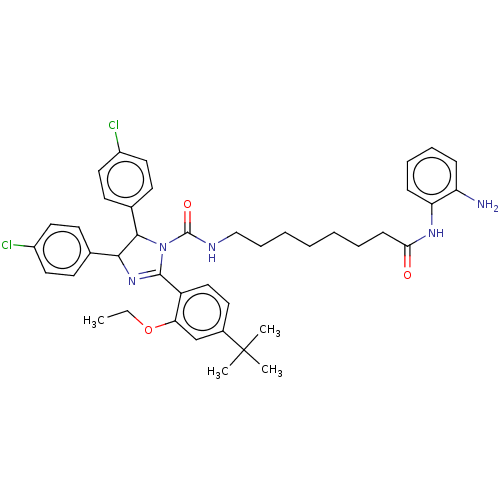
(CHEMBL4174687)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCCCC(=O)Nc1ccccc1N)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C42H49Cl2N5O3/c1-5-52-36-27-30(42(2,3)4)20-25-33(36)40-48-38(28-16-21-31(43)22-17-28)39(29-18-23-32(44)24-19-29)49(40)41(51)46-26-12-8-6-7-9-15-37(50)47-35-14-11-10-13-34(35)45/h10-11,13-14,16-25,27,38-39H,5-9,12,15,26,45H2,1-4H3,(H,46,51)(H,47,50) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449922
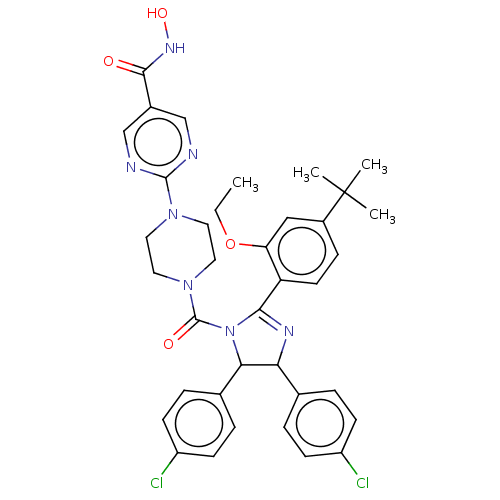
(CHEMBL4170975)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CC1)c1ncc(cn1)C(=O)NO)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C37H39Cl2N7O4/c1-5-50-30-20-26(37(2,3)4)10-15-29(30)33-42-31(23-6-11-27(38)12-7-23)32(24-8-13-28(39)14-9-24)46(33)36(48)45-18-16-44(17-19-45)35-40-21-25(22-41-35)34(47)43-49/h6-15,20-22,31-32,49H,5,16-19H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449906
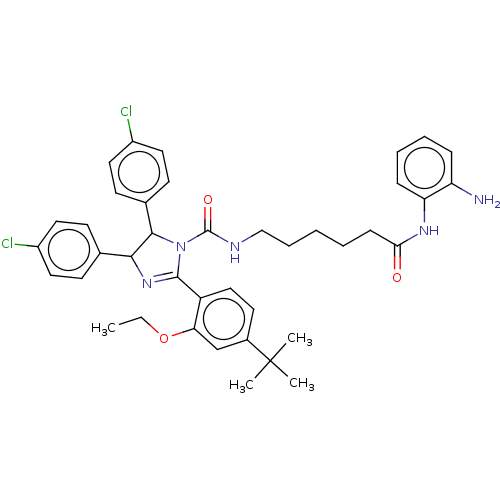
(CHEMBL4163726)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)NCCCCCC(=O)Nc1ccccc1N)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C40H45Cl2N5O3/c1-5-50-34-25-28(40(2,3)4)18-23-31(34)38-46-36(26-14-19-29(41)20-15-26)37(27-16-21-30(42)22-17-27)47(38)39(49)44-24-10-6-7-13-35(48)45-33-12-9-8-11-32(33)43/h8-9,11-12,14-23,25,36-37H,5-7,10,13,24,43H2,1-4H3,(H,44,49)(H,45,48) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM36181
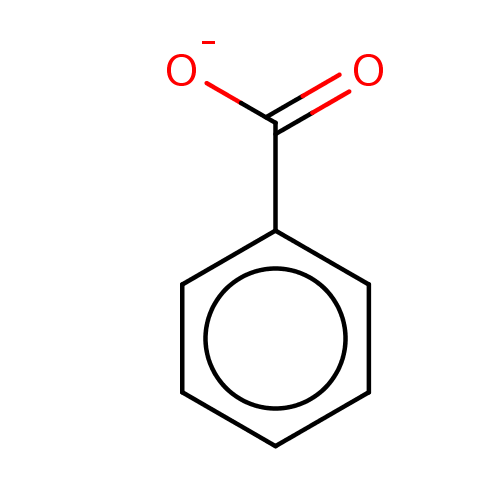
(SODIUM BENZOATE | benzoate | benzoic acid | benzoi...)Show InChI InChI=1S/C7H6O2/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H,8,9)/p-1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO by Michaelis-Menten plot analysis in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449921
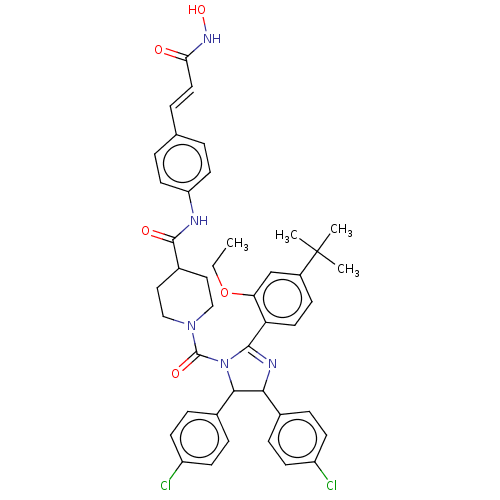
(CHEMBL4161826)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCC(CC1)C(=O)Nc1ccc(\C=C\C(=O)NO)cc1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C43H45Cl2N5O5/c1-5-55-36-26-31(43(2,3)4)13-20-35(36)40-47-38(28-9-14-32(44)15-10-28)39(29-11-16-33(45)17-12-29)50(40)42(53)49-24-22-30(23-25-49)41(52)46-34-18-6-27(7-19-34)8-21-37(51)48-54/h6-21,26,30,38-39,54H,5,22-25H2,1-4H3,(H,46,52)(H,48,51)/b21-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Sus scrofa (pig)) | BDBM50004955
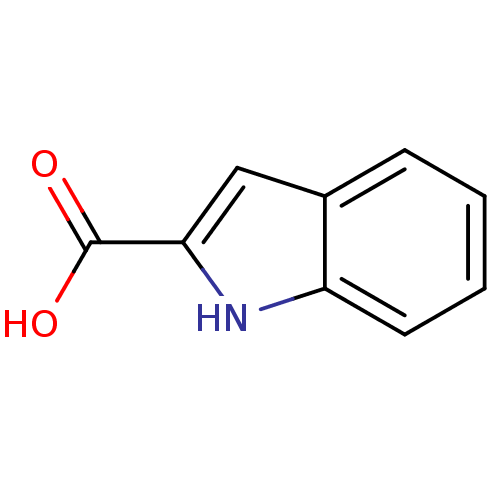
(1H-Indole-2-carboxylic acid | CHEMBL278390 | Indol...)Show InChI InChI=1S/C9H7NO2/c11-9(12)8-5-6-3-1-2-4-7(6)10-8/h1-5,10H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of pig kidney DAAO in presence of D-alanine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM50449908
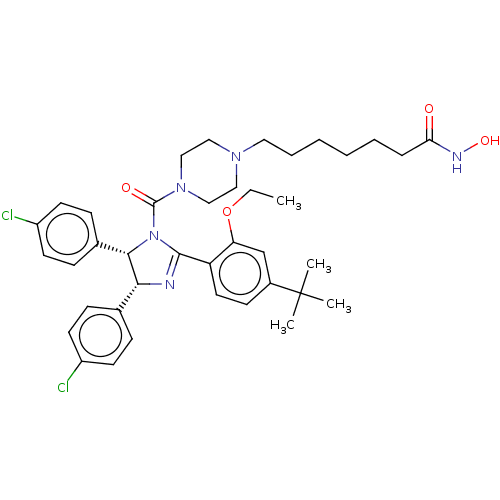
(CHEMBL4168217)Show SMILES CCOc1cc(ccc1C1=N[C@@H]([C@@H](N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |r,t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47)/t35-,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
E3 ubiquitin-protein ligase Mdm2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of MDM2 (unknown origin) preincubated for 30 mins by fluorescence polarization assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50433372

(CHEMBL2375520)Show InChI InChI=1S/C7H3Cl2NO2/c8-4-1-3-6(2-5(4)9)12-10-7(3)11/h1-2H,(H,10,11) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725

(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260725

(4H-thieno[3,2-b]pyrrole-5-carboxylic acid | CHEMBL...)Show InChI InChI=1S/C7H5NO2S/c9-7(10)5-3-6-4(8-5)1-2-11-6/h1-3,8H,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DAAO (unknown origin) |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50465408
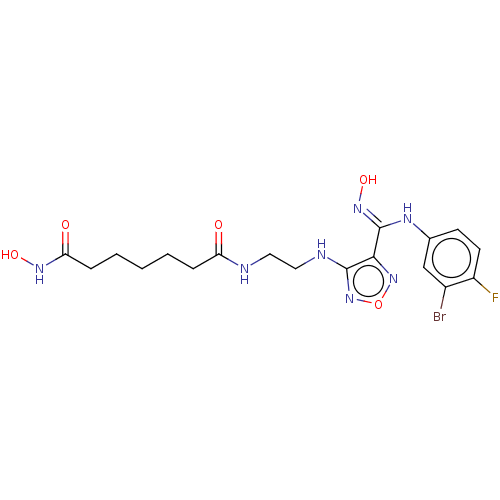
(CHEMBL4288076)Show SMILES ONC(=O)CCCCCC(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C18H23BrFN7O5/c19-12-10-11(6-7-13(12)20)23-18(25-31)16-17(27-32-26-16)22-9-8-21-14(28)4-2-1-3-5-15(29)24-30/h6-7,10,30-31H,1-5,8-9H2,(H,21,28)(H,22,27)(H,23,25)(H,24,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
ACS Med Chem Lett 9: 312-317 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00487
BindingDB Entry DOI: 10.7270/Q2HM5C55 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50260722

(4-(4-chlorophenethyl)-1H-pyrrole-2-carboxylic acid...)Show InChI InChI=1S/C13H12ClNO2/c14-11-5-3-9(4-6-11)1-2-10-7-12(13(16)17)15-8-10/h3-8,15H,1-2H2,(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
ACS Med Chem Lett 9: 312-317 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00487
BindingDB Entry DOI: 10.7270/Q2HM5C55 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50449900

(CHEMBL4174805)Show SMILES CCOc1cc(ccc1C1=NC(C(N1C(=O)N1CCN(CCCCCCC(=O)NO)CC1)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(C)(C)C |t:10| Show InChI InChI=1S/C39H49Cl2N5O4/c1-5-50-33-26-29(39(2,3)4)15-20-32(33)37-42-35(27-11-16-30(40)17-12-27)36(28-13-18-31(41)19-14-28)46(37)38(48)45-24-22-44(23-25-45)21-9-7-6-8-10-34(47)43-49/h11-20,26,35-36,49H,5-10,21-25H2,1-4H3,(H,43,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50465404
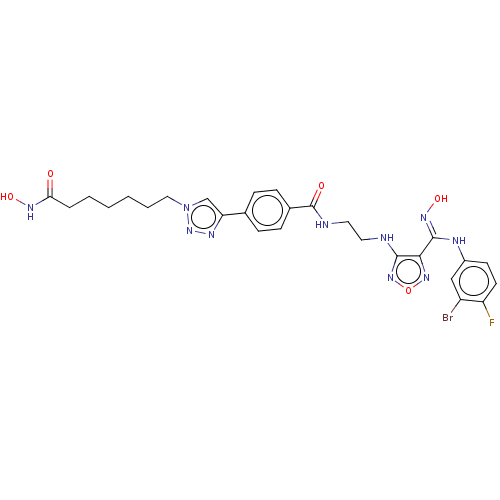
(CHEMBL4279657)Show SMILES ONC(=O)CCCCCCn1cc(nn1)-c1ccc(cc1)C(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C27H30BrFN10O5/c28-20-15-19(10-11-21(20)29)32-26(35-43)24-25(37-44-36-24)30-12-13-31-27(41)18-8-6-17(7-9-18)22-16-39(38-33-22)14-4-2-1-3-5-23(40)34-42/h6-11,15-16,42-43H,1-5,12-14H2,(H,30,37)(H,31,41)(H,32,35)(H,34,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
ACS Med Chem Lett 9: 312-317 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00487
BindingDB Entry DOI: 10.7270/Q2HM5C55 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50371825
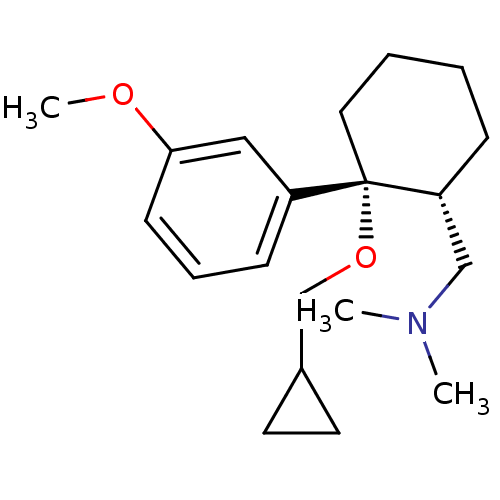
(CHEMBL404197)Show SMILES COc1cccc(c1)[C@]1(CCCC[C@@H]1CN(C)C)OCC1CC1 Show InChI InChI=1S/C20H31NO2/c1-21(2)14-18-7-4-5-12-20(18,23-15-16-10-11-16)17-8-6-9-19(13-17)22-3/h6,8-9,13,16,18H,4-5,7,10-12,14-15H2,1-3H3/t18-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT mediated 5-hydroxytryptamine uptake |
Bioorg Med Chem Lett 18: 1674-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.051
BindingDB Entry DOI: 10.7270/Q2VQ33HQ |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Homo sapiens (Human)) | BDBM50371827
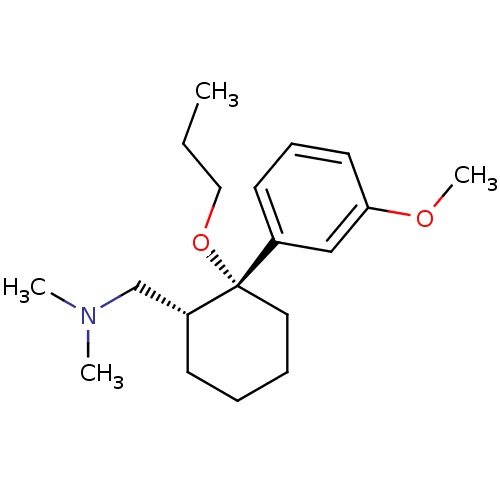
(CHEMBL256227)Show InChI InChI=1S/C19H31NO2/c1-5-13-22-19(16-10-8-11-18(14-16)21-4)12-7-6-9-17(19)15-20(2)3/h8,10-11,14,17H,5-7,9,12-13,15H2,1-4H3/t17-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Inhibition of SERT mediated 5-hydroxytryptamine uptake |
Bioorg Med Chem Lett 18: 1674-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.051
BindingDB Entry DOI: 10.7270/Q2VQ33HQ |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50465406
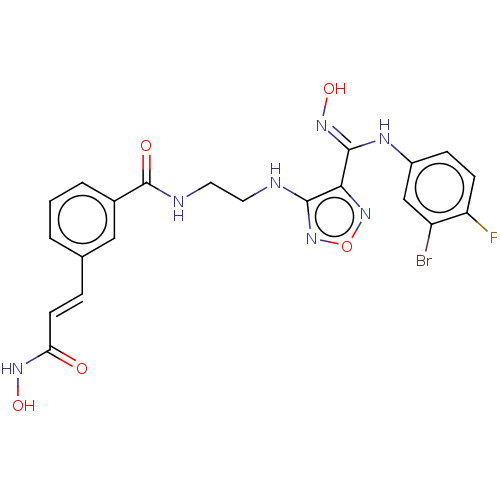
(CHEMBL4281734)Show SMILES ONC(=O)\C=C\c1cccc(c1)C(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C21H19BrFN7O5/c22-15-11-14(5-6-16(15)23)26-20(28-34)18-19(30-35-29-18)24-8-9-25-21(32)13-3-1-2-12(10-13)4-7-17(31)27-33/h1-7,10-11,33-34H,8-9H2,(H,24,30)(H,25,32)(H,26,28)(H,27,31)/b7-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human GST-tagged IDO1 (1 to 404 residues) using D-Tryptophan as substrate |
ACS Med Chem Lett 9: 312-317 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00487
BindingDB Entry DOI: 10.7270/Q2HM5C55 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) after 30 mins by fluorescence assay |
J Med Chem 61: 7245-7260 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00664
BindingDB Entry DOI: 10.7270/Q2R213Z9 |
More data for this
Ligand-Target Pair | |
D-amino-acid oxidase
(Homo sapiens (Human)) | BDBM50206007

(3-Hydroxy-chromen-2-one | 3-hydroxy-2H-chromen-2-o...)Show InChI InChI=1S/C9H6O3/c10-7-5-6-3-1-2-4-8(6)12-9(7)11/h1-5,10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunovion Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant DAAO after 1 hr by coupled enzyme assay in presence of D-serine |
J Med Chem 56: 3710-24 (2013)
Article DOI: 10.1021/jm4002583
BindingDB Entry DOI: 10.7270/Q2X92CPC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50465399
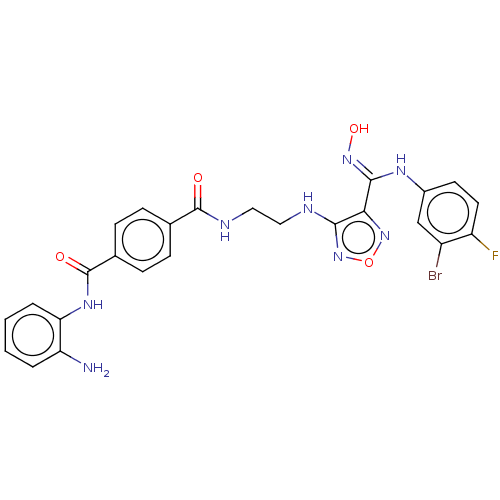
(CHEMBL4291940)Show SMILES Nc1ccccc1NC(=O)c1ccc(cc1)C(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C25H22BrFN8O4/c26-17-13-16(9-10-18(17)27)31-23(33-38)21-22(35-39-34-21)29-11-12-30-24(36)14-5-7-15(8-6-14)25(37)32-20-4-2-1-3-19(20)28/h1-10,13,38H,11-12,28H2,(H,29,35)(H,30,36)(H,31,33)(H,32,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) using substrate 3 after 30 mins by fluorescence assay |
ACS Med Chem Lett 9: 312-317 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00487
BindingDB Entry DOI: 10.7270/Q2HM5C55 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50465400
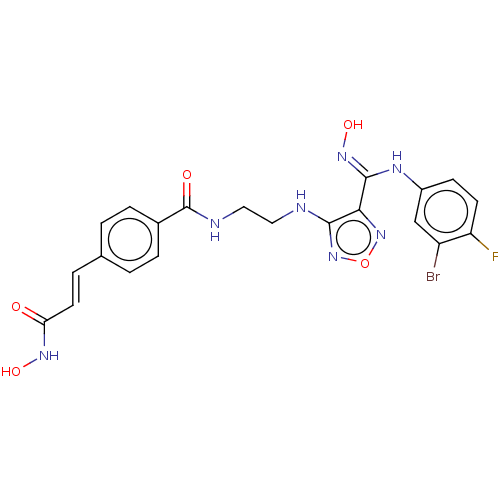
(CHEMBL4289614)Show SMILES ONC(=O)\C=C\c1ccc(cc1)C(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C21H19BrFN7O5/c22-15-11-14(6-7-16(15)23)26-20(28-34)18-19(30-35-29-18)24-9-10-25-21(32)13-4-1-12(2-5-13)3-8-17(31)27-33/h1-8,11,33-34H,9-10H2,(H,24,30)(H,25,32)(H,26,28)(H,27,31)/b8-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
ACS Med Chem Lett 9: 312-317 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00487
BindingDB Entry DOI: 10.7270/Q2HM5C55 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50176263
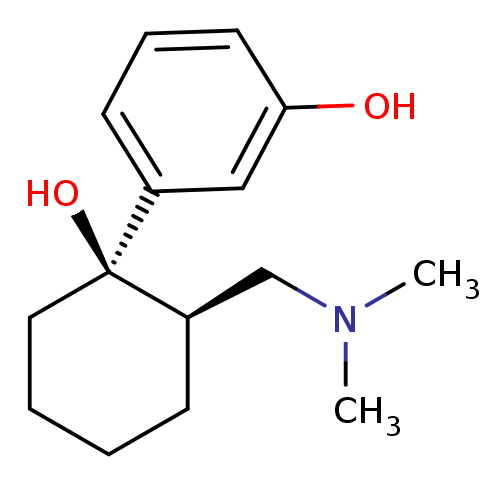
(3-((1R,2R)-2-((dimethylamino)methyl)-1-hydroxycycl...)Show InChI InChI=1S/C15H23NO2/c1-16(2)11-13-6-3-4-9-15(13,18)12-7-5-8-14(17)10-12/h5,7-8,10,13,17-18H,3-4,6,9,11H2,1-2H3/t13-,15+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor |
Bioorg Med Chem Lett 18: 1674-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.051
BindingDB Entry DOI: 10.7270/Q2VQ33HQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50371815
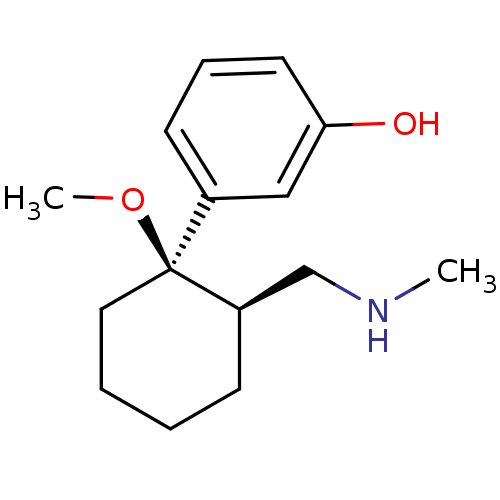
(CHEMBL402116)Show InChI InChI=1S/C15H23NO2/c1-16-11-13-6-3-4-9-15(13,18-2)12-7-5-8-14(17)10-12/h5,7-8,10,13,16-17H,3-4,6,9,11H2,1-2H3/t13-,15+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor |
Bioorg Med Chem Lett 18: 1674-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.051
BindingDB Entry DOI: 10.7270/Q2VQ33HQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50371815

(CHEMBL402116)Show InChI InChI=1S/C15H23NO2/c1-16-11-13-6-3-4-9-15(13,18-2)12-7-5-8-14(17)10-12/h5,7-8,10,13,16-17H,3-4,6,9,11H2,1-2H3/t13-,15+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Sepracor Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to mu opioid receptor |
Bioorg Med Chem Lett 18: 1674-80 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.051
BindingDB Entry DOI: 10.7270/Q2VQ33HQ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50465402
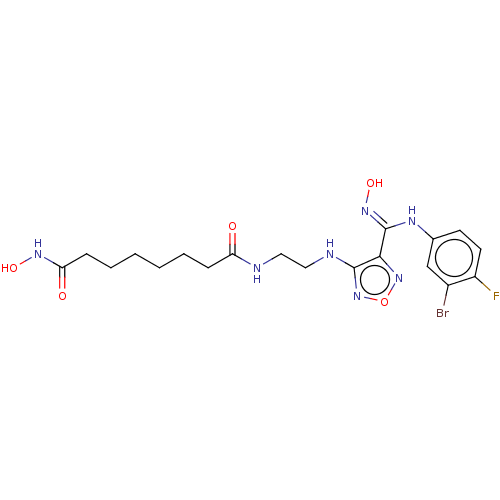
(CHEMBL4283574)Show SMILES ONC(=O)CCCCCCC(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C19H25BrFN7O5/c20-13-11-12(7-8-14(13)21)24-19(26-32)17-18(28-33-27-17)23-10-9-22-15(29)5-3-1-2-4-6-16(30)25-31/h7-8,11,31-32H,1-6,9-10H2,(H,22,29)(H,23,28)(H,24,26)(H,25,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
East China University of Science and Technology
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length HDAC1 using fluorogenic substrate 3 after 30 mins by fluorescence assay |
ACS Med Chem Lett 9: 312-317 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00487
BindingDB Entry DOI: 10.7270/Q2HM5C55 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data