

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255582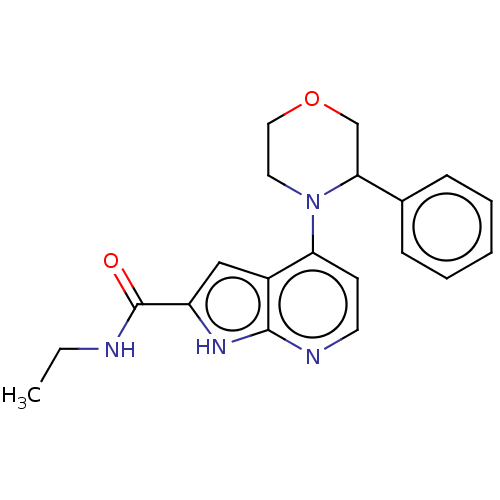 (CHEMBL4078783) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255603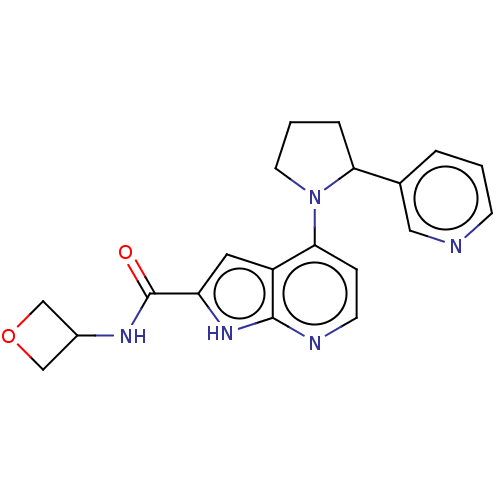 (CHEMBL4083992) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255566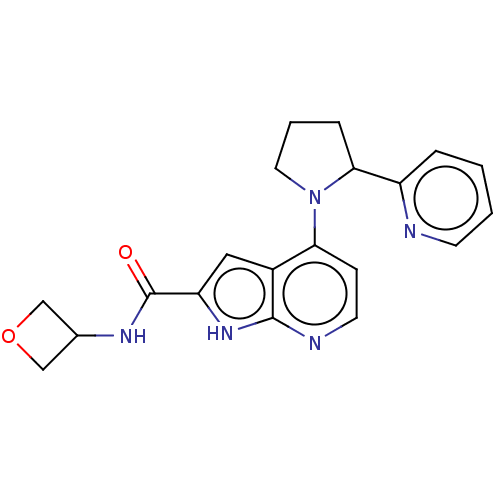 (CHEMBL4094252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255598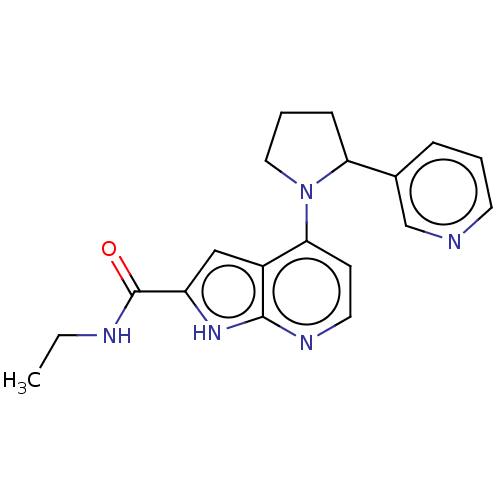 (CHEMBL4064004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255568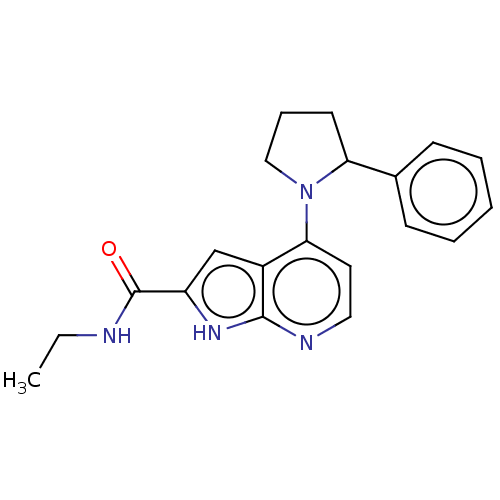 (CHEMBL4091768) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255621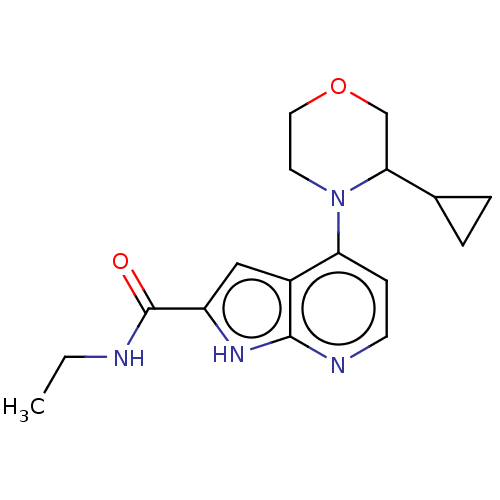 (CHEMBL4070624) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255622 (CHEMBL4067896) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255624 (CHEMBL4101983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255581 (CHEMBL4073623) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Inhibition of human full length MTH1 using 8-Oxo-2-dGTP as substrate preincubated for 15 mins followed substrate addition measured after 1 hr by PPil... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255583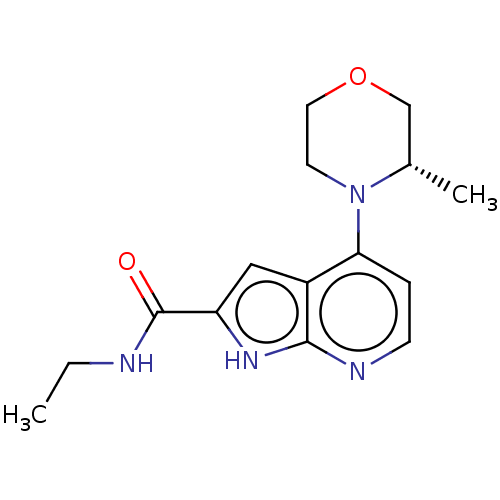 (CHEMBL4096813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255623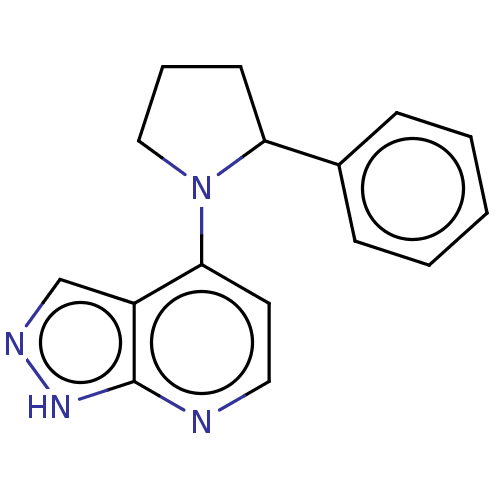 (CHEMBL4072758) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255585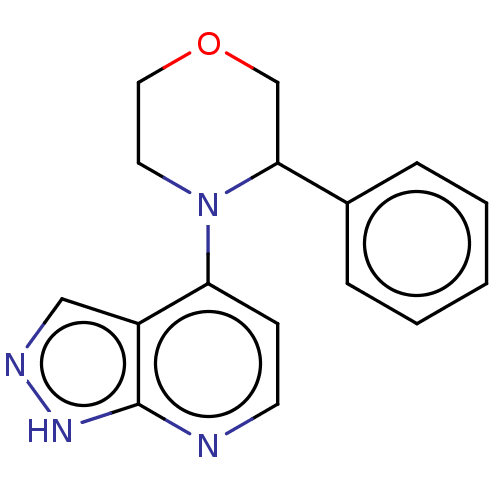 (CHEMBL4094381) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255615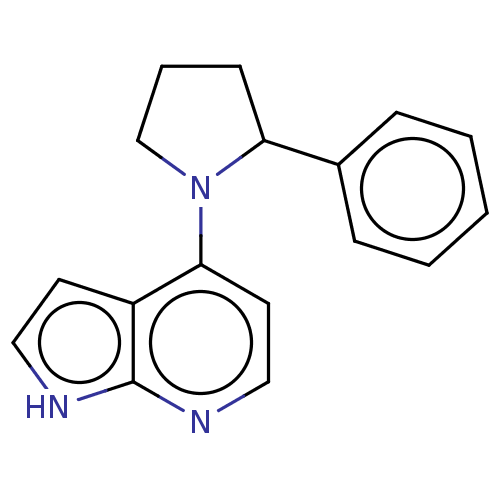 (CHEMBL4061204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255584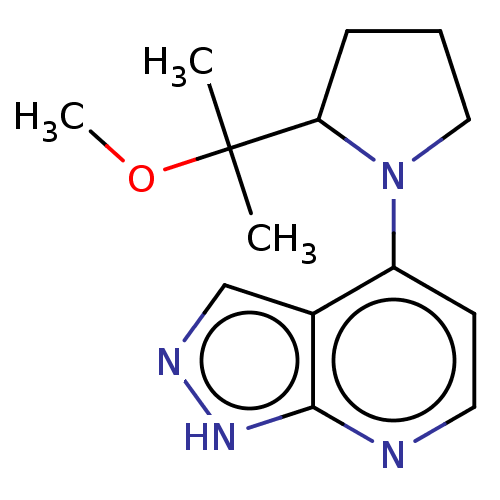 (CHEMBL4089106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50262998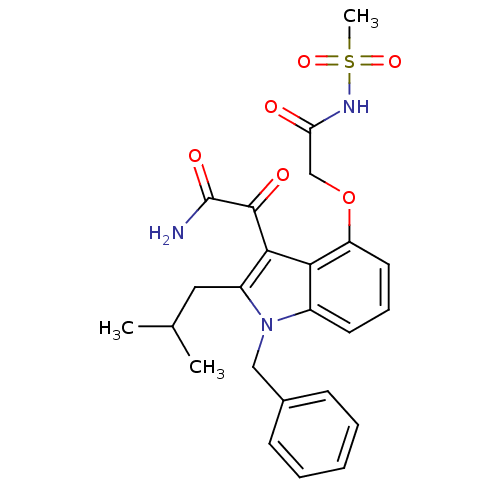 (CHEMBL477548 | mesyl-2-(3-(2-amino-2-oxoacetyl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to sPLA2X (unknown origin) | Bioorg Med Chem Lett 24: 5251-5 (2014) Article DOI: 10.1016/j.bmcl.2014.09.058 BindingDB Entry DOI: 10.7270/Q2668FS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255617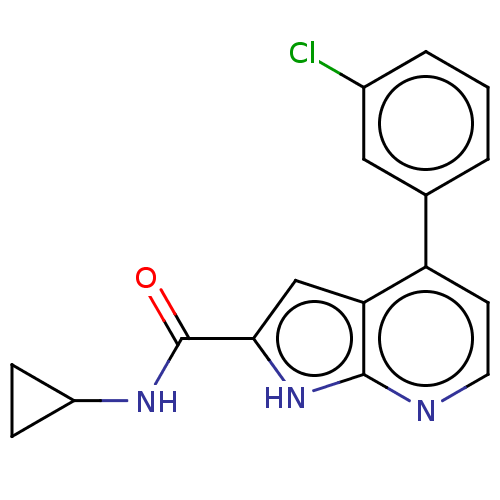 (CHEMBL4095468) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255610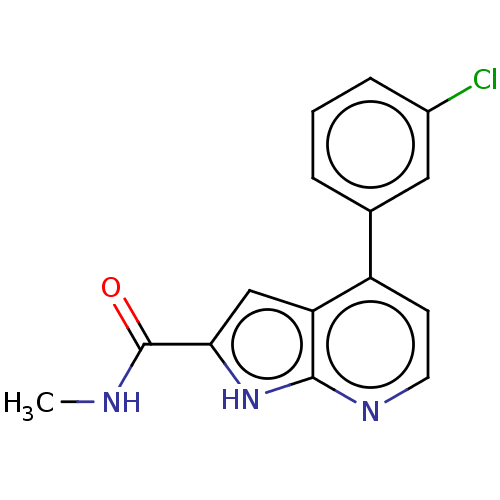 (CHEMBL4079884) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255618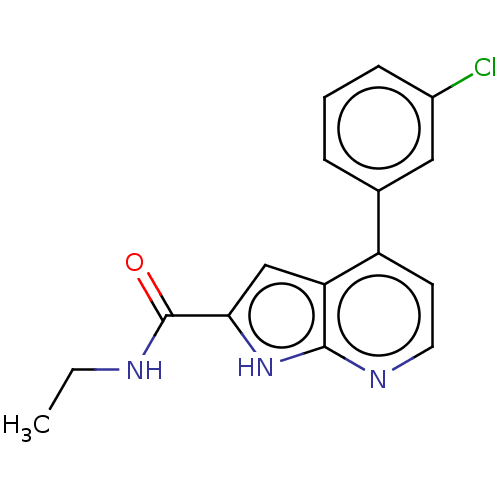 (CHEMBL4104130) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366784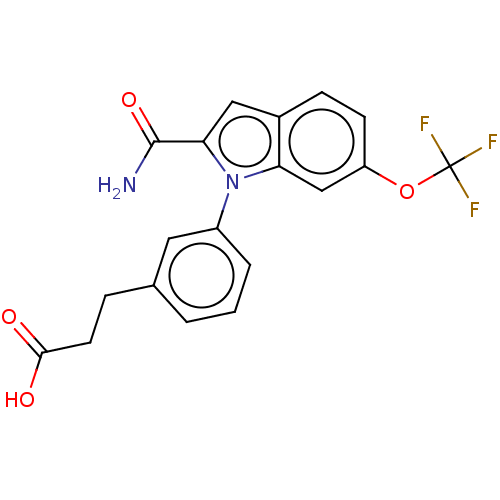 (CHEMBL4171084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255567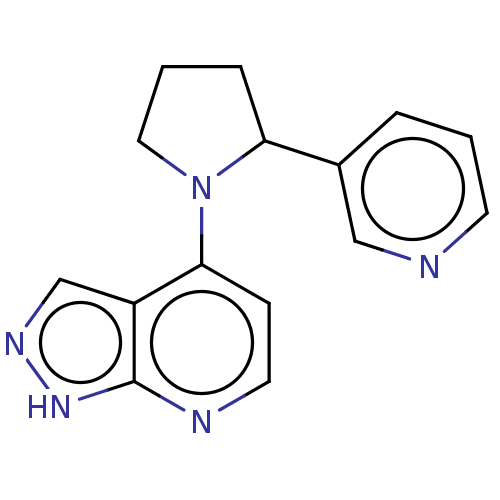 (CHEMBL4066892) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255604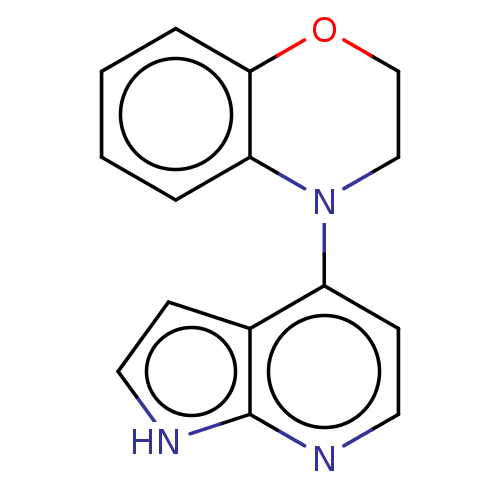 (CHEMBL4093037) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366811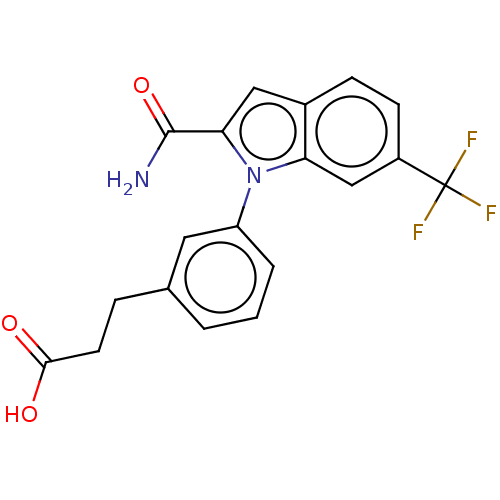 (CHEMBL4176544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50031106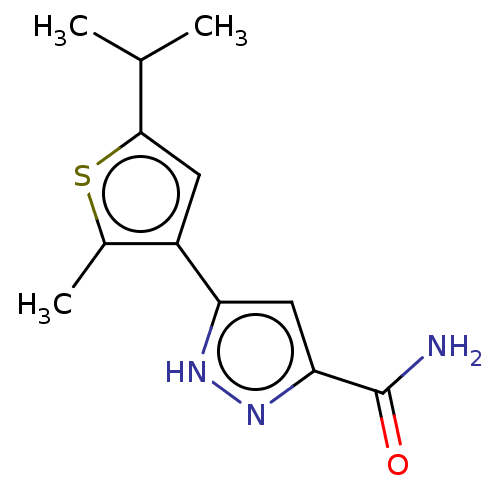 (CHEMBL3337975) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins | Bioorg Med Chem Lett 24: 5251-5 (2014) Article DOI: 10.1016/j.bmcl.2014.09.058 BindingDB Entry DOI: 10.7270/Q2668FS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350728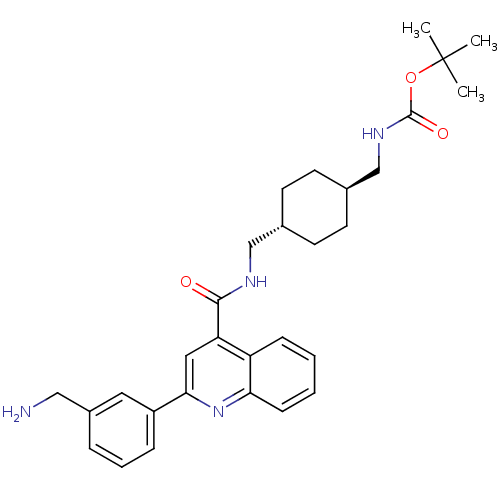 (CHEMBL1818291) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366983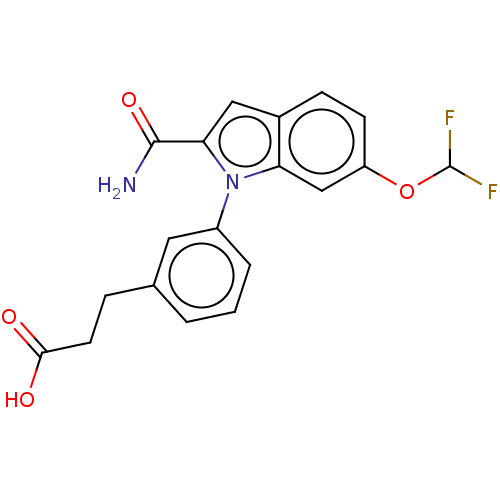 (CHEMBL4160483) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350748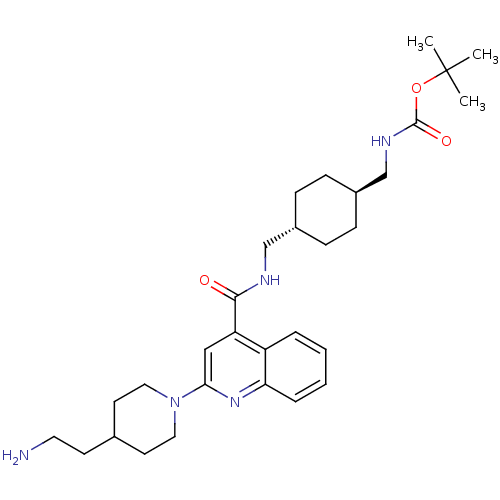 (CHEMBL1818297) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366807 (CHEMBL4172222) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366839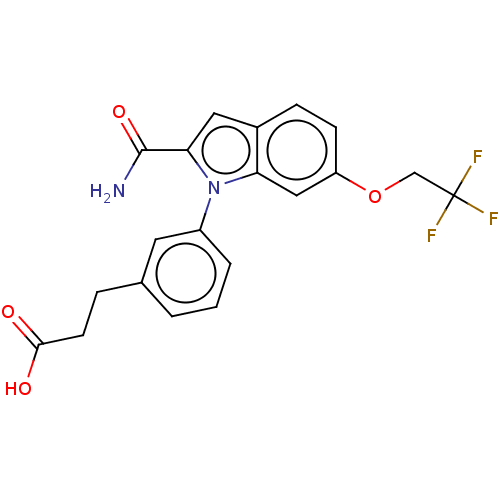 (CHEMBL4175583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Mus musculus) | BDBM50366784 (CHEMBL4171084) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of mouse sPLA2-10 using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substrate additio... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350750 (CHEMBL1818300) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins | Bioorg Med Chem Lett 24: 5251-5 (2014) Article DOI: 10.1016/j.bmcl.2014.09.058 BindingDB Entry DOI: 10.7270/Q2668FS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350747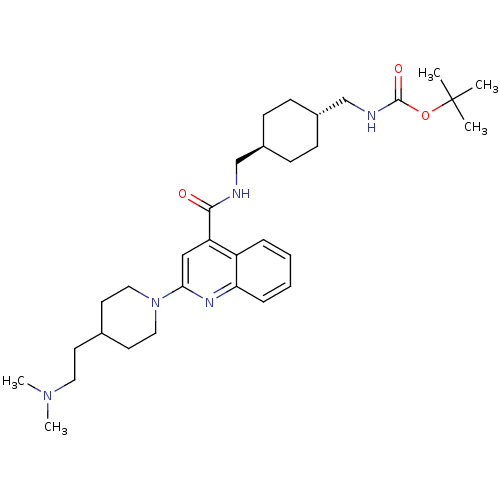 (CHEMBL1818296) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366840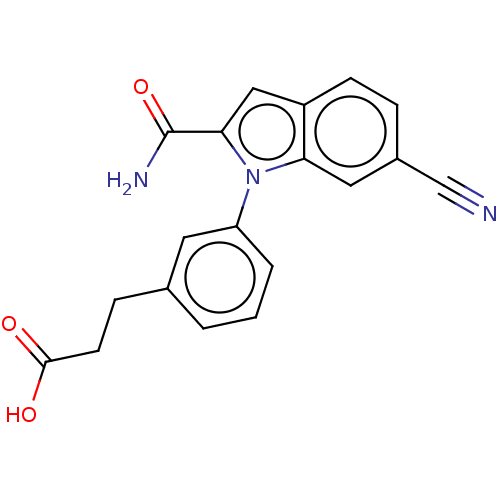 (CHEMBL4174522) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2 group V (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-5 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate p... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350753 (CHEMBL1818303) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using HDL as substrate pretreated for 20 mins followed by substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM50350750 (CHEMBL1818300) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of ACC2 in obese Zucker rat assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malac... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366905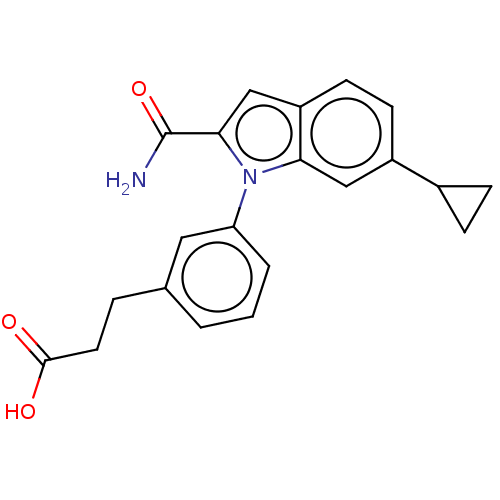 (CHEMBL4173359) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366889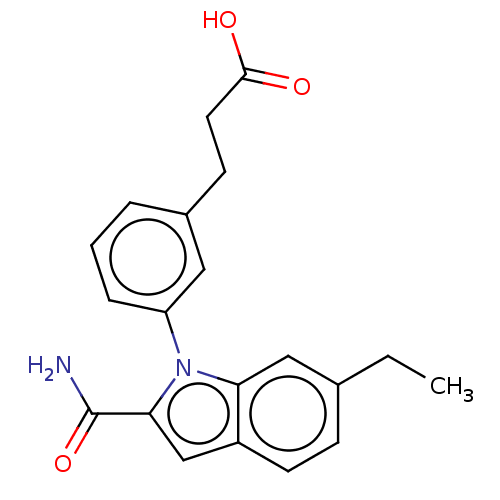 (CHEMBL4175643) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM50189617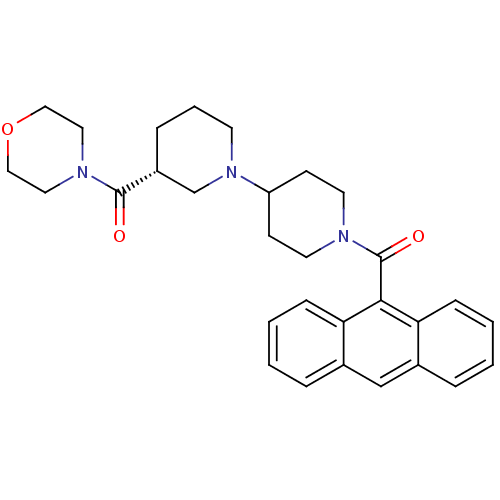 ((3R)-1'-(9-anthrylcarbonyl)-3-(morpholin-4-ylcarbo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of ACC2 in obese Zucker rat assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malac... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50031105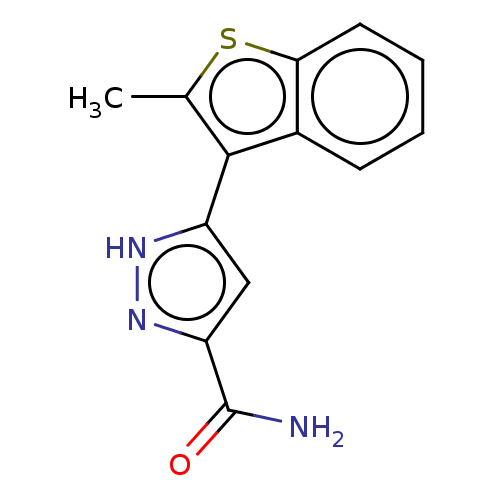 (CHEMBL3337976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human sPLA2X using 1,2-bis(heptanoylthio) glycerophosphocholine substrate incubated for 30 mins | Bioorg Med Chem Lett 24: 5251-5 (2014) Article DOI: 10.1016/j.bmcl.2014.09.058 BindingDB Entry DOI: 10.7270/Q2668FS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase (Rattus norvegicus (Rat)) | BDBM50350747 (CHEMBL1818296) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of ACC2 in obese Zucker rat assessed as reduction in hepatic malonyl-coA level preincubated for 15 mins measured after 1.5 hrs using Malac... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255620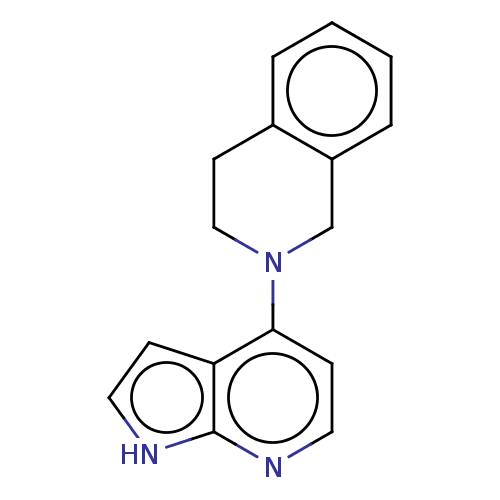 (CHEMBL4083073) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255611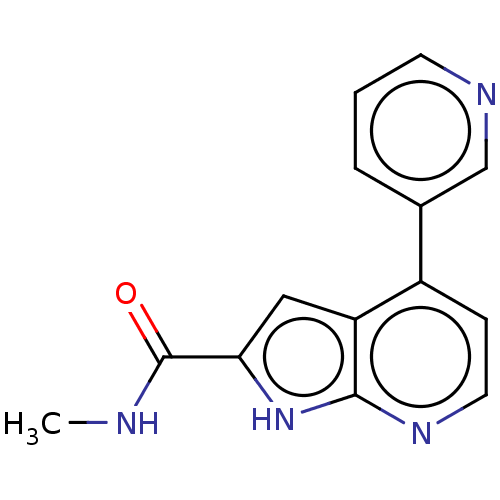 (CHEMBL4091375) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sprint Bioscience AB, Novum , 14157 Huddinge , Sweden. Curated by ChEMBL | Assay Description Binding affinity to recombinant human full length N-terminal His tagged-MTH1 expressed in Escherichia coli at 3 mM by isothermal titration calorimetr... | J Med Chem 61: 2533-2551 (2018) Article DOI: 10.1021/acs.jmedchem.7b01884 BindingDB Entry DOI: 10.7270/Q25D8V9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350739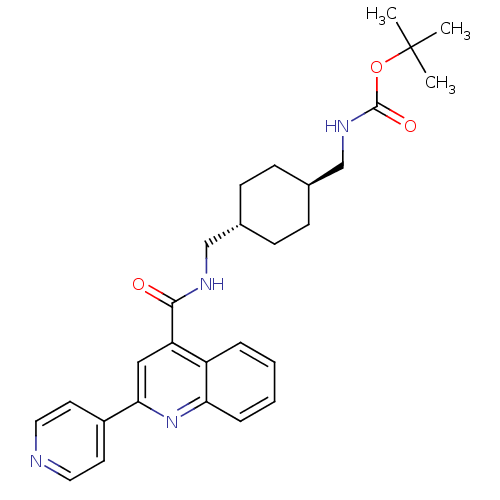 (CHEMBL1818194) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350732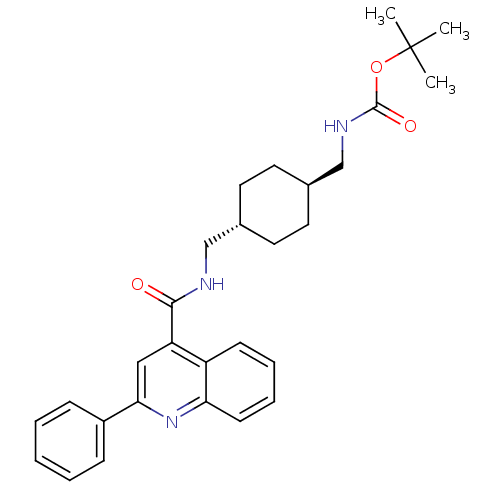 (CHEMBL1818187) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366876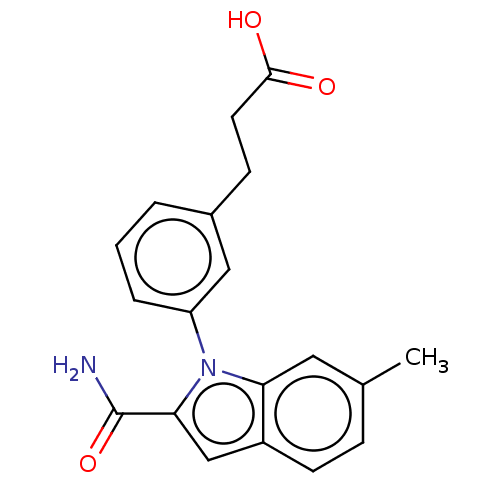 (CHEMBL4171797) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50350746 (CHEMBL1818295) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant his-tagged ACC2 expressed in baculovirus/Sf9 cell assessed as inorganic phosphate formation preincubated for 15 mins ... | Bioorg Med Chem 19: 3039-53 (2011) Article DOI: 10.1016/j.bmc.2011.04.014 BindingDB Entry DOI: 10.7270/Q2G44QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 169 total ) | Next | Last >> |