

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085666 ((2S,3S,4R,5R)-5-{6-(2,2-Diphenyl-ethylamino)-2-[2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085674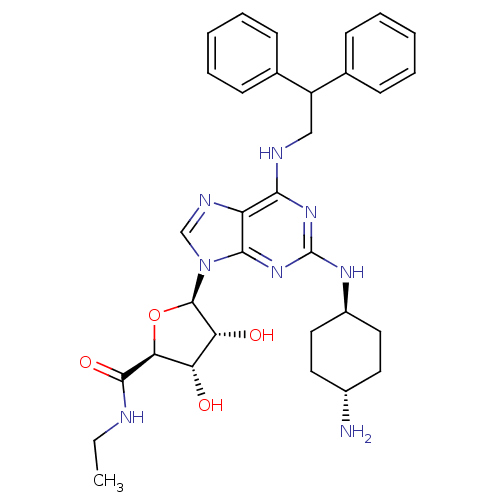 ((2S,3S,4R,5R)-5-[2-(4-Amino-cyclohexylamino)-6-(2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085671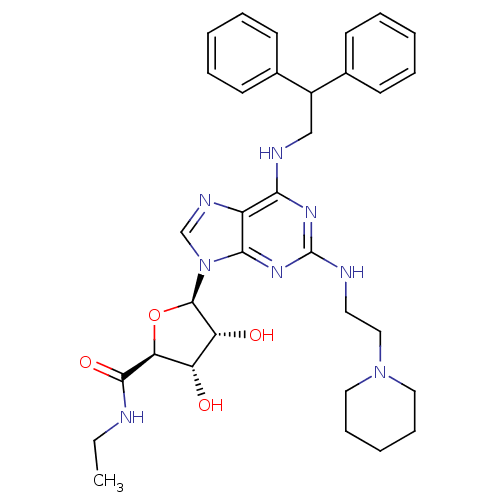 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-(2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085668 ((2S,3S,4R,5R)-5-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085662 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-((1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085661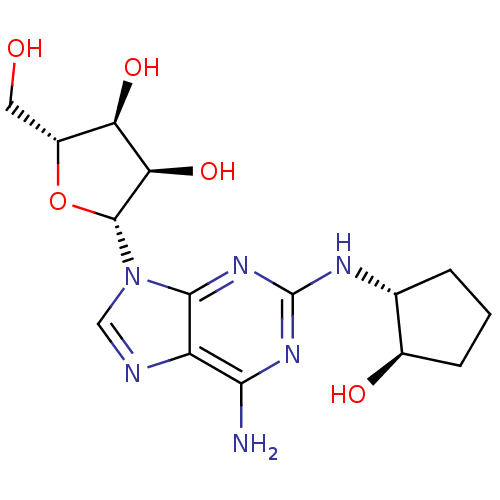 ((2R,3R,4S,5R)-2-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM21220 ((2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-N-ethyl-3,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085664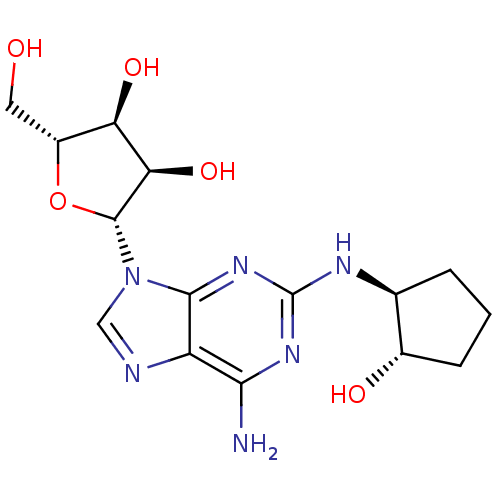 ((2R,3R,4S,5R)-2-[6-Amino-2-((1S,2S)-2-hydroxy-cycl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085663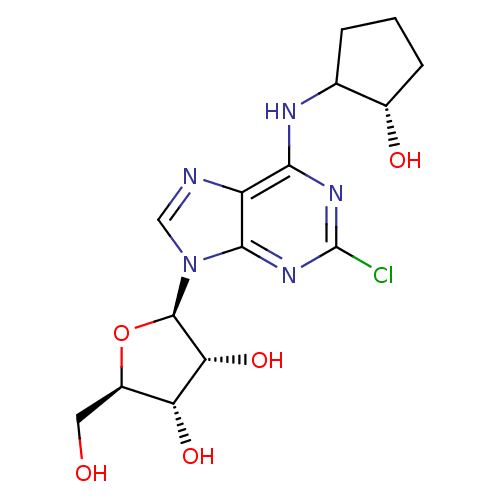 ((2R,3R,4S,5R)-2-[2-Chloro-6-((R)-(S)-2-hydroxy-cyc...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085670 ((2S,3S,4R,5R)-5-(6-Amino-2-cyclopentylamino-purin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085672 ((2R,3R,4S,5R)-2-(6-Amino-2-cyclopentylamino-purin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085665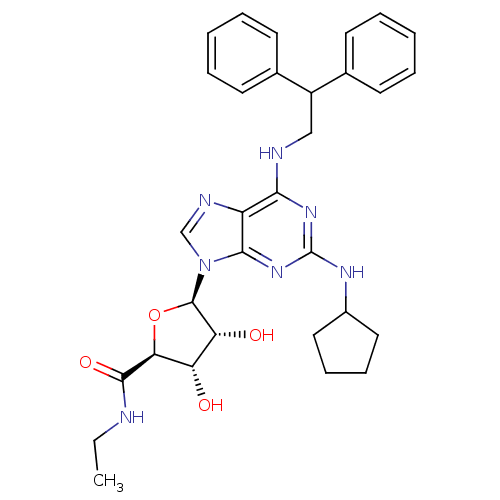 ((2S,3S,4R,5R)-5-[2-Cyclopentylamino-6-(2,2-dipheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50085667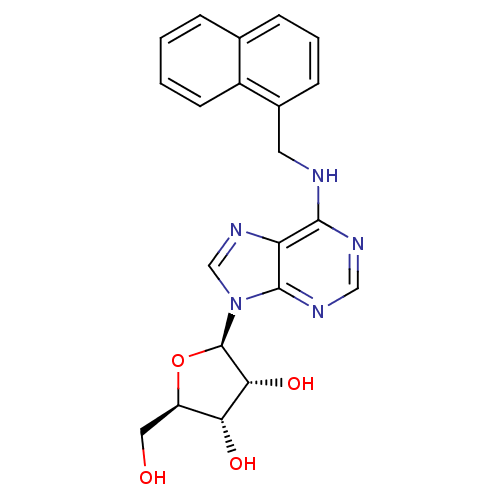 ((2R,3S,4R,5R)-2-Hydroxymethyl-5-{6-[(naphthalen-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Agonistic activity against Adenosine A2A receptor on rat striatal membranes | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085658 ((2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50085667 ((2R,3S,4R,5R)-2-Hydroxymethyl-5-{6-[(naphthalen-1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Agonistic activity against Adenosine A1 receptor on rat whole-brain membranes | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085669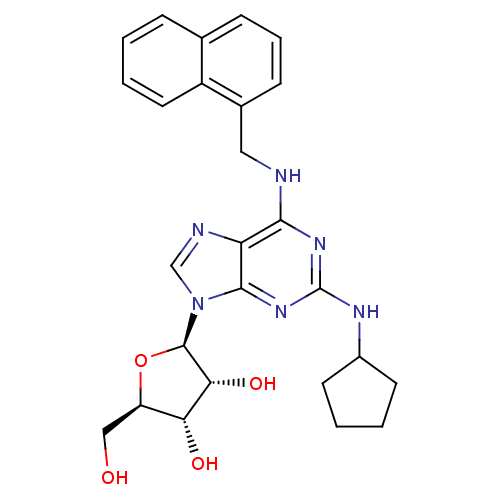 ((2R,3R,4S,5R)-2-{2-Cyclopentylamino-6-[(naphthalen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085673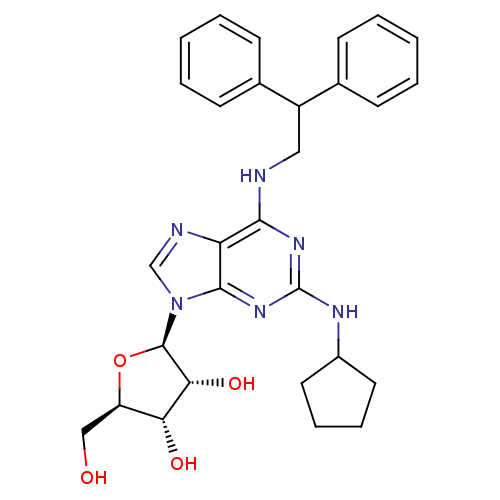 ((2R,3R,4S,5R)-2-[2-Cyclopentylamino-6-(2,2-dipheny...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085660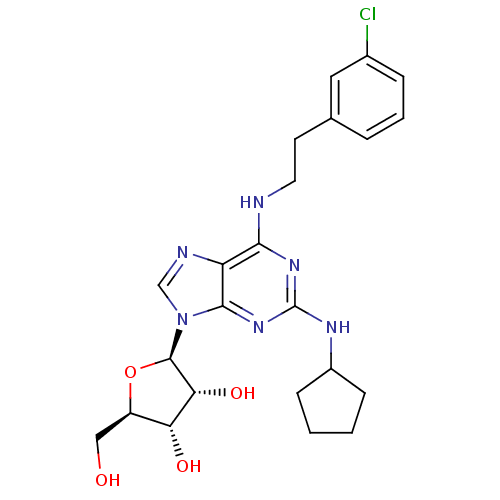 ((2R,3R,4S,5R)-2-{6-[2-(3-Chloro-phenyl)-ethylamino...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085670 ((2S,3S,4R,5R)-5-(6-Amino-2-cyclopentylamino-purin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085664 ((2R,3R,4S,5R)-2-[6-Amino-2-((1S,2S)-2-hydroxy-cycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085675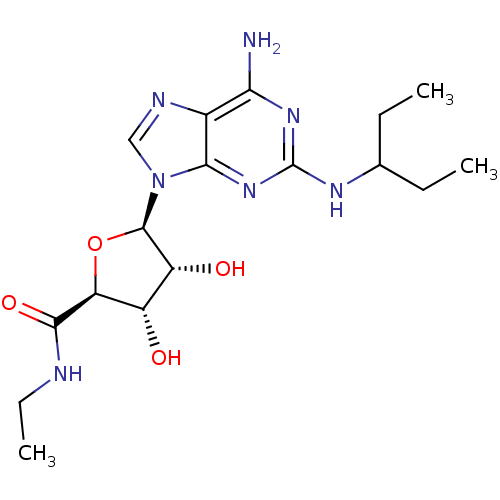 ((2S,3S,4R,5R)-5-[6-Amino-2-(1-ethyl-propylamino)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50085663 ((2R,3R,4S,5R)-2-[2-Chloro-6-((R)-(S)-2-hydroxy-cyc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of human neutrophil activation via Adenosine A2A receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085668 ((2S,3S,4R,5R)-5-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085671 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-(2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 263 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085674 ((2S,3S,4R,5R)-5-[2-(4-Amino-cyclohexylamino)-6-(2,...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085662 ((2S,3S,4R,5R)-5-[6-(2,2-Diphenyl-ethylamino)-2-((1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085672 ((2R,3R,4S,5R)-2-(6-Amino-2-cyclopentylamino-purin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 549 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085673 ((2R,3R,4S,5R)-2-[2-Cyclopentylamino-6-(2,2-dipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >612 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085666 ((2S,3S,4R,5R)-5-{6-(2,2-Diphenyl-ethylamino)-2-[2-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 672 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085660 ((2R,3R,4S,5R)-2-{6-[2-(3-Chloro-phenyl)-ethylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085661 ((2R,3R,4S,5R)-2-[6-Amino-2-((1R,2R)-2-hydroxy-cycl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50085665 ((2S,3S,4R,5R)-5-[2-Cyclopentylamino-6-(2,2-dipheny...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Ex vivo inhibition of guinea pig ileum twitch via Adenosine A1 receptor. | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50085659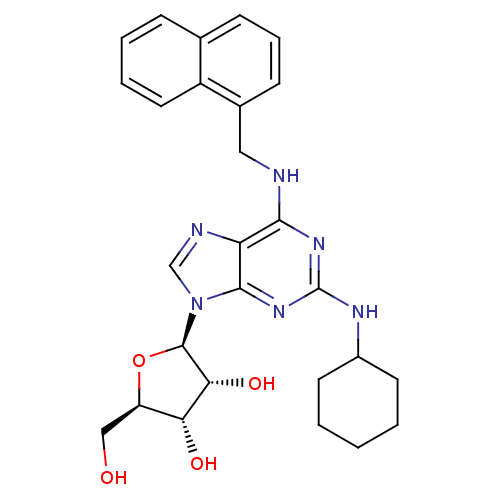 ((2R,3R,4S,5R)-2-{2-Cyclohexylamino-6-[(naphthalen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Agonistic activity against Adenosine A2A receptor on rat striatal membranes | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50085659 ((2R,3R,4S,5R)-2-{2-Cyclohexylamino-6-[(naphthalen-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Medicines Research Centre Curated by ChEMBL | Assay Description Agonistic activity against Adenosine A1 receptor on rat whole-brain membranes | Bioorg Med Chem Lett 10: 403-6 (2000) BindingDB Entry DOI: 10.7270/Q2XK8G2K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50391318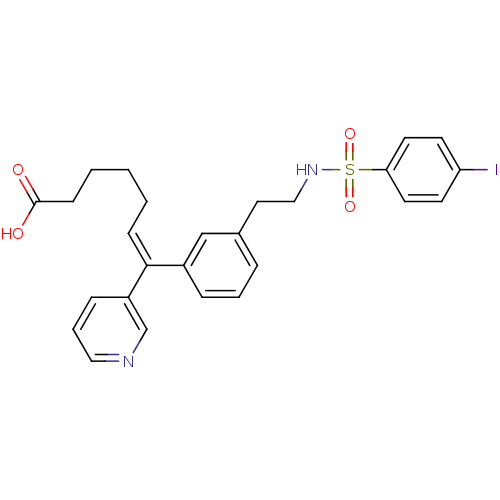 (CHEMBL161422) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of collagen-induced thromboxane A2 production in human whole blood | Bioorg Med Chem Lett 1: 699-704 (1991) Article DOI: 10.1016/S0960-894X(01)81051-9 BindingDB Entry DOI: 10.7270/Q2WD41RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 0 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50391322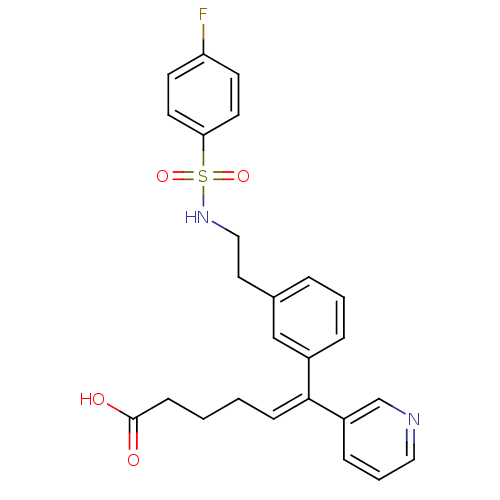 (CHEMBL351657) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of collagen-induced thromboxane A2 production in human whole blood | Bioorg Med Chem Lett 1: 699-704 (1991) Article DOI: 10.1016/S0960-894X(01)81051-9 BindingDB Entry DOI: 10.7270/Q2WD41RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50003795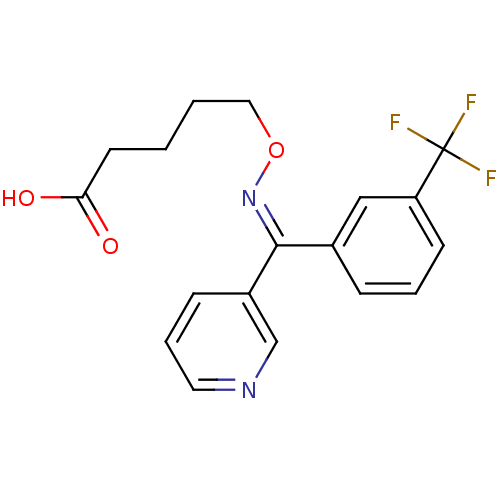 (5-[1-Pyridin-3-yl-1-(3-trifluoromethyl-phenyl)-met...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro TXA2 synthase antagonism through inhibition of collagen induced thromboxane-A2 production in human citrated whole blood | Bioorg Med Chem Lett 1: 695-698 (1991) Checked by Author Article DOI: 10.1016/S0960-894X(01)81050-7 BindingDB Entry DOI: 10.7270/Q2125TT6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50391324 (CHEMBL348790 | GR-85305) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of collagen-induced thromboxane A2 production in human whole blood | Bioorg Med Chem Lett 1: 699-704 (1991) Article DOI: 10.1016/S0960-894X(01)81051-9 BindingDB Entry DOI: 10.7270/Q2WD41RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50391317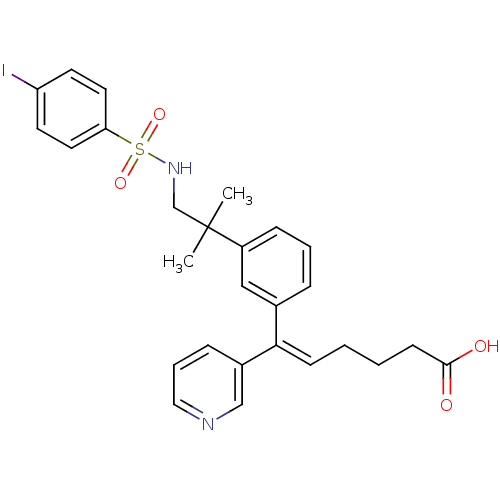 (CHEMBL162641 | GR-108774) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of collagen-induced thromboxane A2 production in human whole blood | Bioorg Med Chem Lett 1: 699-704 (1991) Article DOI: 10.1016/S0960-894X(01)81051-9 BindingDB Entry DOI: 10.7270/Q2WD41RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50003795 (5-[1-Pyridin-3-yl-1-(3-trifluoromethyl-phenyl)-met...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | DrugBank Article | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of collagen-induced thromboxane A2 production in human whole blood | Bioorg Med Chem Lett 1: 699-704 (1991) Article DOI: 10.1016/S0960-894X(01)81051-9 BindingDB Entry DOI: 10.7270/Q2WD41RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066999 ((S)-3,3-Diethyl-2-[4-(4-methyl-piperazine-1-carbon...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50391321 (CHEMBL162879) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of collagen-induced thromboxane A2 production in human whole blood | Bioorg Med Chem Lett 1: 699-704 (1991) Article DOI: 10.1016/S0960-894X(01)81051-9 BindingDB Entry DOI: 10.7270/Q2WD41RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50067002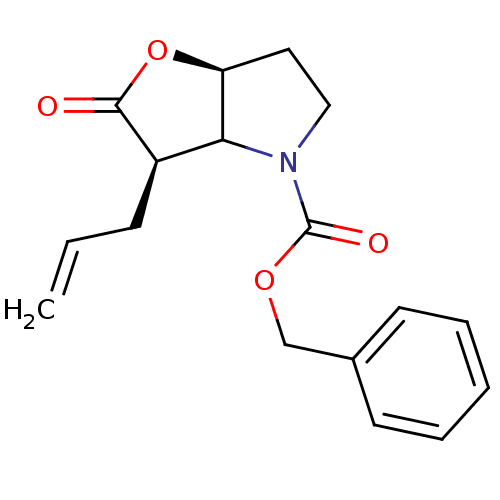 ((3R,6aS)-3-Allyl-2-oxo-hexahydro-furo[3,2-b]pyrrol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50391319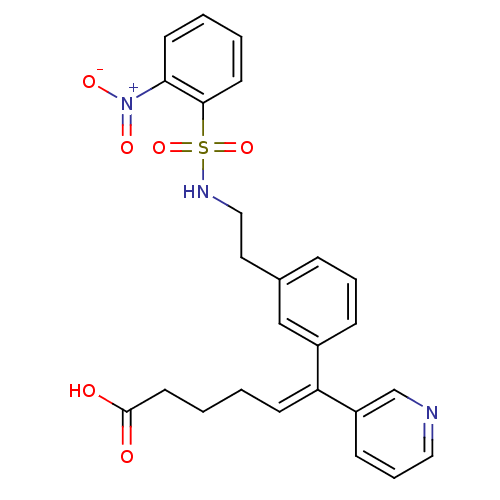 (CHEMBL164109) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of collagen-induced thromboxane A2 production in human whole blood | Bioorg Med Chem Lett 1: 699-704 (1991) Article DOI: 10.1016/S0960-894X(01)81051-9 BindingDB Entry DOI: 10.7270/Q2WD41RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50391316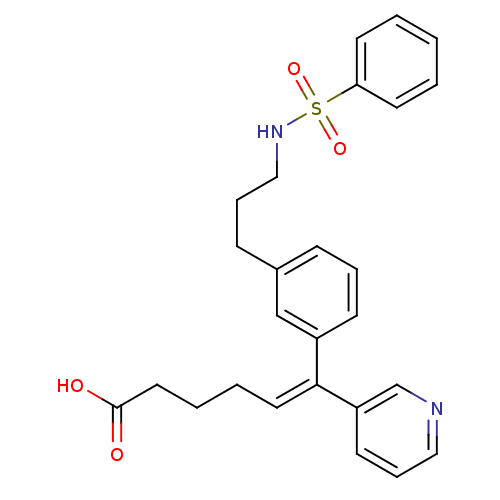 (CHEMBL422017) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of collagen-induced thromboxane A2 production in human whole blood | Bioorg Med Chem Lett 1: 699-704 (1991) Article DOI: 10.1016/S0960-894X(01)81051-9 BindingDB Entry DOI: 10.7270/Q2WD41RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane-A synthase (Homo sapiens (Human)) | BDBM50391325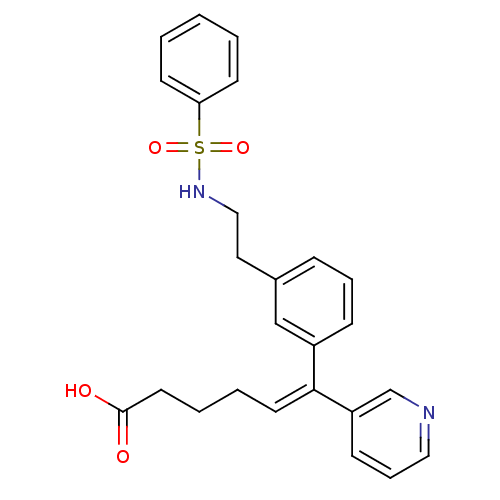 (CHEMBL159447) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition of collagen-induced thromboxane A2 production in human whole blood | Bioorg Med Chem Lett 1: 699-704 (1991) Article DOI: 10.1016/S0960-894X(01)81051-9 BindingDB Entry DOI: 10.7270/Q2WD41RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50066997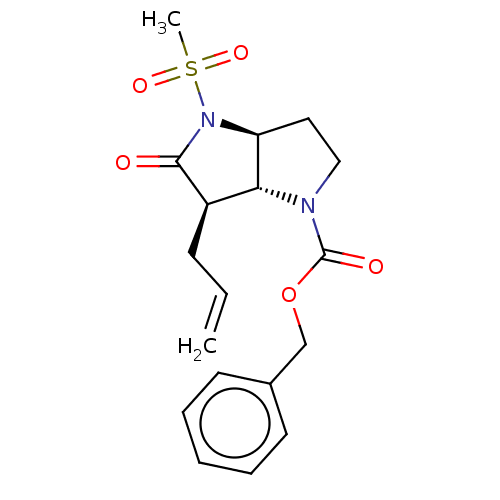 ((3aS,6R)-6-Allyl-4-methanesulfonyl-5-oxo-hexahydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoWellcome Medicines Research Centre Curated by ChEMBL | Assay Description The compound was tested for inhibition of human neutrophil elastase enzyme with a preincubation time of 40 min. | J Med Chem 41: 3919-22 (1998) Article DOI: 10.1021/jm981026s BindingDB Entry DOI: 10.7270/Q2X34WMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 277 total ) | Next | Last >> |