 Found 467 hits with Last Name = 'ohata' and Initial = 'k'
Found 467 hits with Last Name = 'ohata' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mu-type opioid receptor
(GUINEA PIG) | BDBM50297169
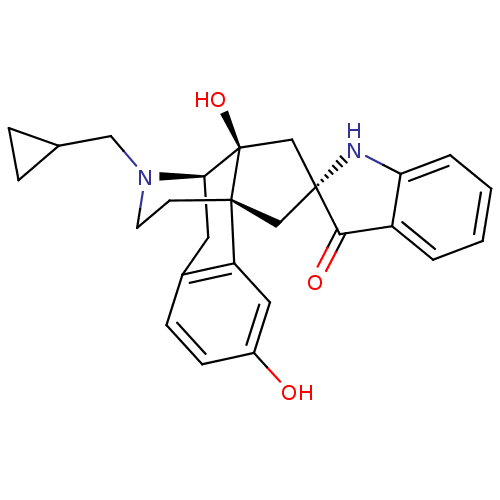
((6R)-16-(Cyclopropylmethyl)-3,13b-dihydroxy-Cnormo...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]4(C[C@]5(C[C@@]34O)Nc3ccccc3C5=O)c2c1 |r,THB:8:7:18:29.4.5| Show InChI InChI=1S/C26H28N2O3/c29-18-8-7-17-11-22-26(31)15-25(23(30)19-3-1-2-4-21(19)27-25)14-24(26,20(17)12-18)9-10-28(22)13-16-5-6-16/h1-4,7-8,12,16,22,27,29,31H,5-6,9-11,13-15H2/t22-,24-,25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Hartley guinea pig mu opioid receptor by liquid scintillation counter |
Bioorg Med Chem 17: 5983-8 (2009)
Article DOI: 10.1016/j.bmc.2009.06.067
BindingDB Entry DOI: 10.7270/Q2154H3Q |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50297170
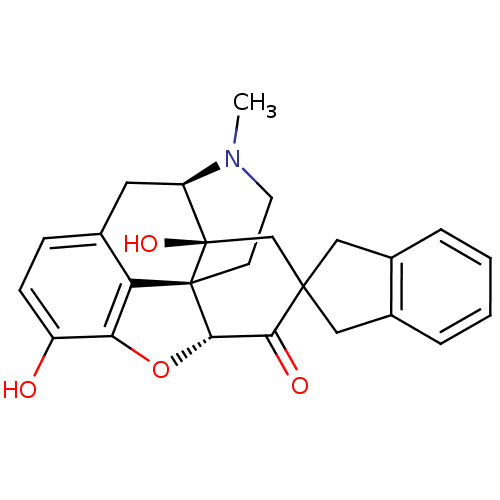
(7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)CC1(Cc2ccccc2C1)C4=O)ccc5O Show InChI InChI=1S/C25H25NO4/c1-26-9-8-24-19-14-6-7-17(27)20(19)30-22(24)21(28)23(13-25(24,29)18(26)10-14)11-15-4-2-3-5-16(15)12-23/h2-7,18,22,27,29H,8-13H2,1H3/t18-,22+,24+,25-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Hartley guinea pig mu opioid receptor by liquid scintillation counter |
Bioorg Med Chem 17: 5983-8 (2009)
Article DOI: 10.1016/j.bmc.2009.06.067
BindingDB Entry DOI: 10.7270/Q2154H3Q |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50297169

((6R)-16-(Cyclopropylmethyl)-3,13b-dihydroxy-Cnormo...)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]4(C[C@]5(C[C@@]34O)Nc3ccccc3C5=O)c2c1 |r,THB:8:7:18:29.4.5| Show InChI InChI=1S/C26H28N2O3/c29-18-8-7-17-11-22-26(31)15-25(23(30)19-3-1-2-4-21(19)27-25)14-24(26,20(17)12-18)9-10-28(22)13-16-5-6-16/h1-4,7-8,12,16,22,27,29,31H,5-6,9-11,13-15H2/t22-,24-,25-,26-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 13.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69493 from Hartley guinea pig kappa opioid receptor by liquid scintillation counter |
Bioorg Med Chem 17: 5983-8 (2009)
Article DOI: 10.1016/j.bmc.2009.06.067
BindingDB Entry DOI: 10.7270/Q2154H3Q |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(MOUSE) | BDBM50060080
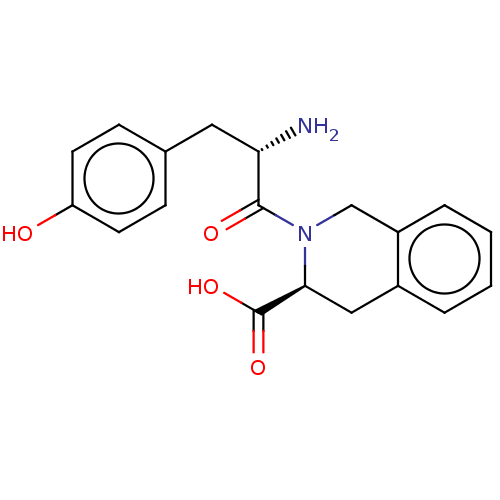
(2-({2-[(R)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C19H20N2O4/c20-16(9-12-5-7-15(22)8-6-12)18(23)21-11-14-4-2-1-3-13(14)10-17(21)19(24)25/h1-8,16-17,22H,9-11,20H2,(H,24,25)/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the binding affinity towards delta-opioid receptor by displacement of [3H]p-CI-DPDPE radioligand from mouse vas defere... |
Bioorg Med Chem Lett 7: 3049-3052 (1997)
Article DOI: 10.1016/S0960-894X(97)10145-7
BindingDB Entry DOI: 10.7270/Q2H13211 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Cavia porcellus (domestic guinea pig)) | BDBM50297170

(7-(2'-spiroindanyl)oxymorphone | 7-(Spiroindano)Ox...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@]3(O)CC1(Cc2ccccc2C1)C4=O)ccc5O Show InChI InChI=1S/C25H25NO4/c1-26-9-8-24-19-14-6-7-17(27)20(19)30-22(24)21(28)23(13-25(24,29)18(26)10-14)11-15-4-2-3-5-16(15)12-23/h2-7,18,22,27,29H,8-13H2,1H3/t18-,22+,24+,25-/m1/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 588 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kitasato University
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69493 from Hartley guinea pig kappa opioid receptor by liquid scintillation counter |
Bioorg Med Chem 17: 5983-8 (2009)
Article DOI: 10.1016/j.bmc.2009.06.067
BindingDB Entry DOI: 10.7270/Q2154H3Q |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(GUINEA PIG) | BDBM50060080

(2-({2-[(R)-2-Amino-3-(4-hydroxy-2,6-dimethyl-pheny...)Show SMILES N[C@@H](Cc1ccc(O)cc1)C(=O)N1Cc2ccccc2C[C@H]1C(O)=O |r| Show InChI InChI=1S/C19H20N2O4/c20-16(9-12-5-7-15(22)8-6-12)18(23)21-11-14-4-2-1-3-13(14)10-17(21)19(24)25/h1-8,16-17,22H,9-11,20H2,(H,24,25)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was evaluated for the binding affinity towards mu-opioid receptor by displacement of [3H]DAMGO radioligand from guinea pig ileum |
Bioorg Med Chem Lett 7: 3049-3052 (1997)
Article DOI: 10.1016/S0960-894X(97)10145-7
BindingDB Entry DOI: 10.7270/Q2H13211 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50039026
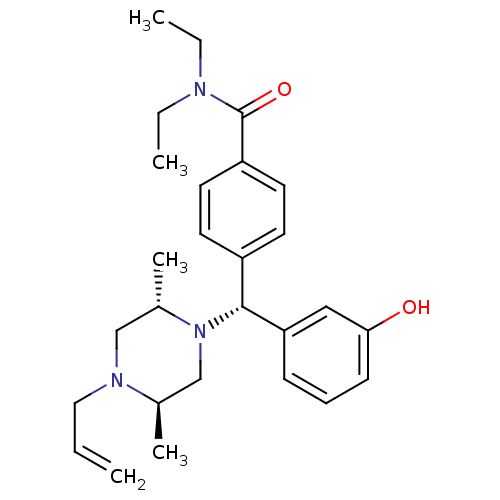
(4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(O)c1 Show InChI InChI=1S/C27H37N3O2/c1-6-16-29-18-21(5)30(19-20(29)4)26(24-10-9-11-25(31)17-24)22-12-14-23(15-13-22)27(32)28(7-2)8-3/h6,9-15,17,20-21,26,31H,1,7-8,16,18-19H2,2-5H3/t20-,21+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082445
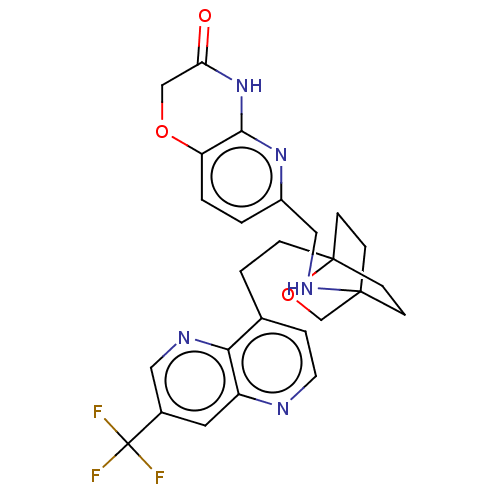
(CHEMBL3422978)Show SMILES FC(F)(F)c1cnc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2c1 |(-5.09,-.93,;-4.02,-1.54,;-4.02,-2.78,;-5.09,-2.16,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)17-11-19-22(31-12-17)16(4-10-30-19)3-5-25-8-6-24(7-9-25,15-37-25)32-13-18-1-2-20-23(33-18)34-21(35)14-36-20/h1-2,4,10-12,32H,3,5-9,13-15H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082380
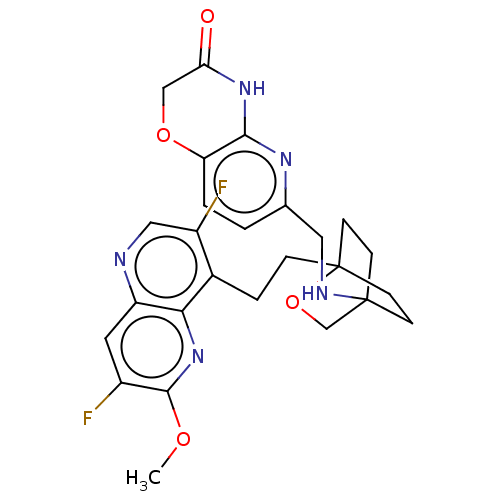
(CHEMBL3422952)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H27F2N5O4/c1-35-24-17(27)10-19-22(33-24)16(18(28)12-29-19)4-5-26-8-6-25(7-9-26,14-37-26)30-11-15-2-3-20-23(31-15)32-21(34)13-36-20/h2-3,10,12,30H,4-9,11,13-14H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082429

(CHEMBL3422970)Show SMILES Cc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-3.75,1.39,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-19(13-28)12-21-24(31-17)18(5-11-29-21)4-6-27-9-7-26(8-10-27,16-36-27)30-14-20-2-3-22-25(32-20)33-23(34)15-35-22/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082385
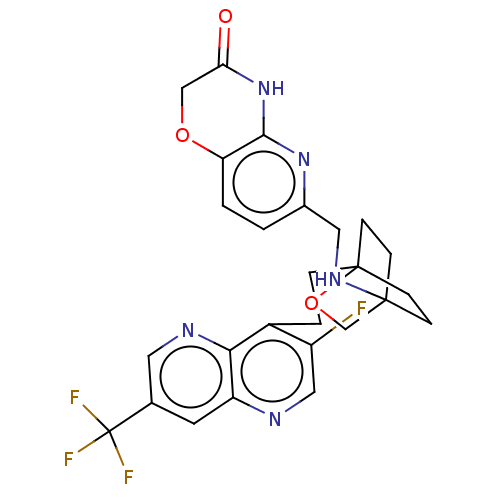
(CHEMBL3422959)Show SMILES Fc1cnc2cc(cnc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1)C(F)(F)F Show InChI InChI=1S/C26H25F4N5O3/c27-18-12-31-19-9-15(26(28,29)30)10-32-22(19)17(18)3-4-25-7-5-24(6-8-25,14-38-25)33-11-16-1-2-20-23(34-16)35-21(36)13-37-20/h1-2,9-10,12,33H,3-8,11,13-14H2,(H,34,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082440

(CHEMBL3422977)Show SMILES FC(F)(F)c1ccc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,.93,;-4.02,1.54,;-4.02,2.78,;-5.09,2.16,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C26H26F3N5O3/c27-26(28,29)20-4-2-18-22(33-20)16(6-12-30-18)5-7-25-10-8-24(9-11-25,15-37-25)31-13-17-1-3-19-23(32-17)34-21(35)14-36-19/h1-4,6,12,31H,5,7-11,13-15H2,(H,32,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082435
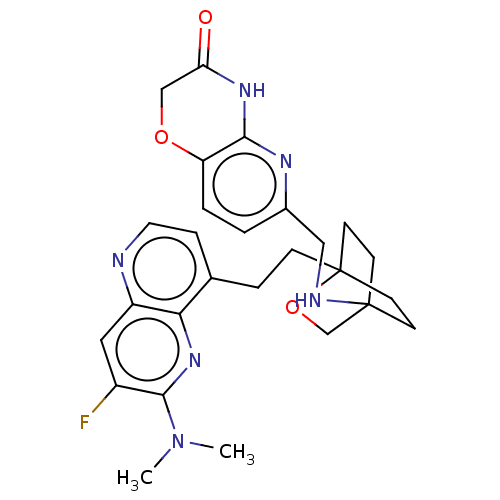
(CHEMBL3422976)Show SMILES CN(C)c1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C27H31FN6O3/c1-34(2)25-19(28)13-20-23(33-25)17(6-12-29-20)5-7-27-10-8-26(9-11-27,16-37-27)30-14-18-3-4-21-24(31-18)32-22(35)15-36-21/h3-4,6,12-13,30H,5,7-11,14-16H2,1-2H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082382
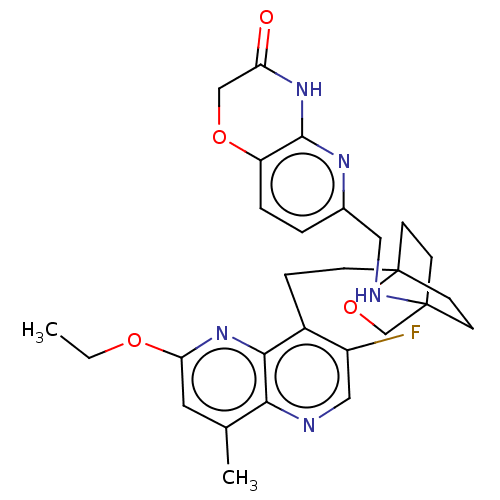
(CHEMBL3422954)Show SMILES CCOc1cc(C)c2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-5.09,3.71,;-4.02,3.09,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-2.77,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C28H32FN5O4/c1-3-36-23-12-17(2)24-25(34-23)19(20(29)14-30-24)6-7-28-10-8-27(9-11-28,16-38-28)31-13-18-4-5-21-26(32-18)33-22(35)15-37-21/h4-5,12,14,31H,3,6-11,13,15-16H2,1-2H3,(H,32,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082390
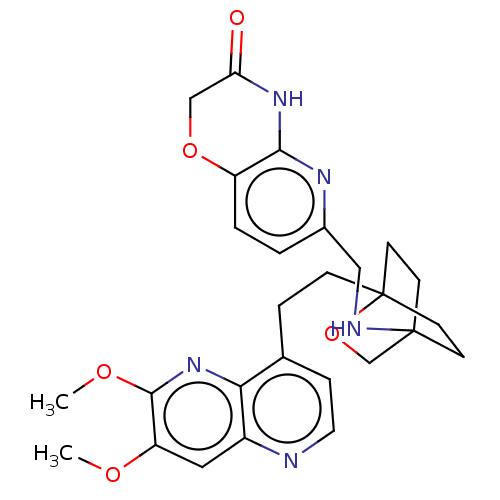
(CHEMBL3422964)Show SMILES COc1cc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2nc1OC |(-4.02,-2.78,;-4.02,-1.54,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,;-2.69,.77,;-4.02,1.54,;-4.02,2.78,)| Show InChI InChI=1S/C27H31N5O5/c1-34-21-13-19-23(32-25(21)35-2)17(6-12-28-19)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-20-24(30-18)31-22(33)15-36-20/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50068133
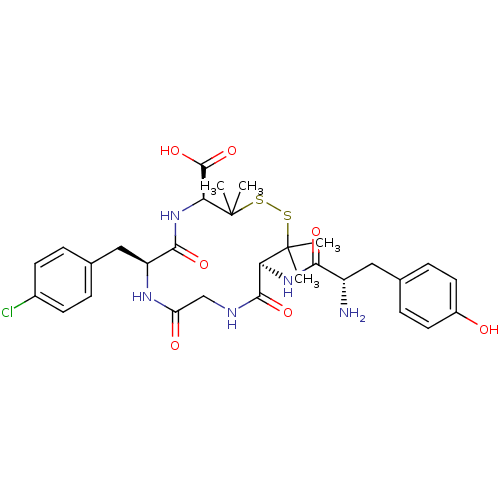
((4S,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(Cl)cc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H38ClN5O7S2/c1-29(2)23(35-25(39)20(32)13-16-7-11-19(37)12-8-16)27(41)33-15-22(38)34-21(14-17-5-9-18(31)10-6-17)26(40)36-24(28(42)43)30(3,4)45-44-29/h5-12,20-21,23-24,37H,13-15,32H2,1-4H3,(H,33,41)(H,34,38)(H,35,39)(H,36,40)(H,42,43)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against cloned human Opioid receptor delta 1 (wild-type,Wt) |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50068133

((4S,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(Cl)cc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H38ClN5O7S2/c1-29(2)23(35-25(39)20(32)13-16-7-11-19(37)12-8-16)27(41)33-15-22(38)34-21(14-17-5-9-18(31)10-6-17)26(40)36-24(28(42)43)30(3,4)45-44-29/h5-12,20-21,23-24,37H,13-15,32H2,1-4H3,(H,33,41)(H,34,38)(H,35,39)(H,36,40)(H,42,43)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against mutated human Opioid receptor delta 1 (W248L) |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082394

(CHEMBL3422966)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C#N |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-2.16,)| Show InChI InChI=1S/C27H28N6O4/c1-35-25-18(13-28)12-20-23(33-25)17(5-11-29-20)4-6-27-9-7-26(8-10-27,16-37-27)30-14-19-2-3-21-24(31-19)32-22(34)15-36-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50001683

(13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@H](Cc2ccccc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H39N5O7S2/c1-29(2)23(34-25(38)20(31)14-18-10-12-19(36)13-11-18)27(40)32-16-22(37)33-21(15-17-8-6-5-7-9-17)26(39)35-24(28(41)42)30(3,4)44-43-29/h5-13,20-21,23-24,36H,14-16,31H2,1-4H3,(H,32,40)(H,33,37)(H,34,38)(H,35,39)(H,41,42)/t20-,21+,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50068133

((4S,7S,13S)-13-[(S)-2-Amino-3-(4-hydroxy-phenyl)-p...)Show SMILES CC1(C)SSC(C)(C)[C@@H](NC(=O)[C@@H](N)Cc2ccc(O)cc2)C(=O)NCC(=O)N[C@@H](Cc2ccc(Cl)cc2)C(=O)N[C@H]1C(O)=O Show InChI InChI=1S/C30H38ClN5O7S2/c1-29(2)23(35-25(39)20(32)13-16-7-11-19(37)12-8-16)27(41)33-15-22(38)34-21(14-17-5-9-18(31)10-6-17)26(40)36-24(28(42)43)30(3,4)45-44-29/h5-12,20-21,23-24,37H,13-15,32H2,1-4H3,(H,33,41)(H,34,38)(H,35,39)(H,36,40)(H,42,43)/t20-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082388
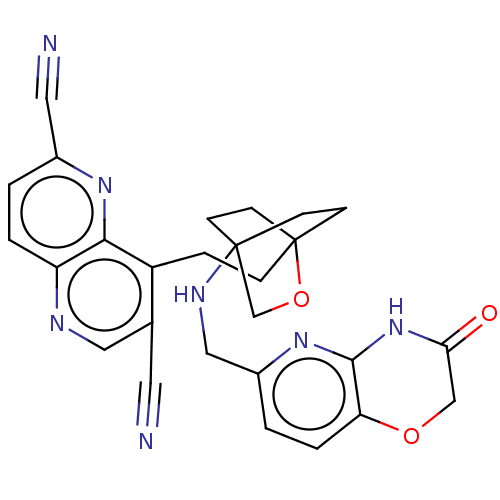
(CHEMBL3422962)Show SMILES O=C1COc2ccc(CNC34CCC(CCc5c(cnc6ccc(nc56)C#N)C#N)(CC3)OC4)nc2N1 |(10.96,15.37,;9.76,15.09,;8.71,16.22,;7.21,15.87,;6.76,14.39,;5.26,14.04,;4.8,12.56,;5.86,11.44,;5.41,9.96,;3.9,9.61,;3.46,8.16,;4.56,7.06,;4.17,5.57,;2.68,5.4,;2.67,3.85,;1.34,3.08,;1.33,1.54,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;-4.02,1.54,;-5.09,2.16,;4,1.54,;5.07,2.16,;2.27,6.67,;3.81,6.67,;1.58,6.27,;1.98,7.74,;7.37,11.8,;7.81,13.27,;9.31,13.62,)| Show InChI InChI=1S/C27H25N7O3/c28-11-17-13-30-21-3-1-18(12-29)32-24(21)20(17)5-6-27-9-7-26(8-10-27,16-37-27)31-14-19-2-4-22-25(33-19)34-23(35)15-36-22/h1-4,13,31H,5-10,14-16H2,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082383

(CHEMBL3422957)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c(F)cnc2cc1C(F)(F)F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;3.74,1.39,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.93,;-4.02,-2.78,;-5.09,-2.16,)| Show InChI InChI=1S/C27H27F4N5O4/c1-38-24-17(27(29,30)31)10-19-22(36-24)16(18(28)12-32-19)4-5-26-8-6-25(7-9-26,14-40-26)33-11-15-2-3-20-23(34-15)35-21(37)13-39-20/h2-3,10,12,33H,4-9,11,13-14H2,1H3,(H,34,35,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082427
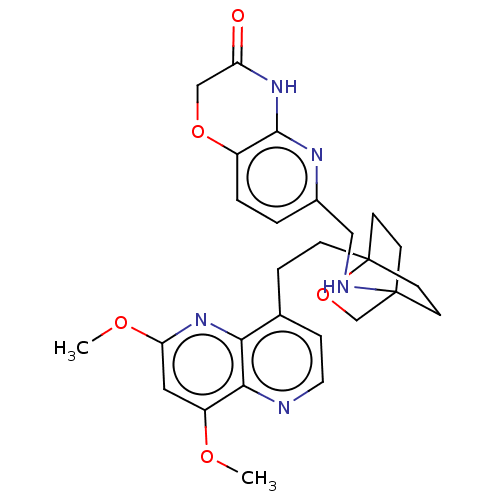
(CHEMBL3422968)Show SMILES COc1cc(OC)c2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-3.08,;-2.39,-3.71,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31N5O5/c1-34-20-13-22(35-2)32-23-17(6-12-28-24(20)23)5-7-27-10-8-26(9-11-27,16-37-27)29-14-18-3-4-19-25(30-18)31-21(33)15-36-19/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082430
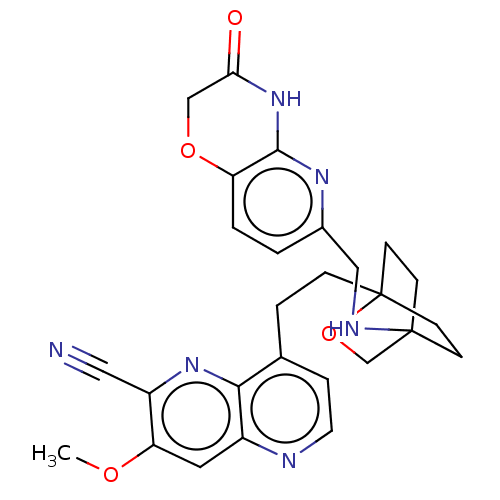
(CHEMBL3422971)Show SMILES COc1cc2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2nc1C#N |(-4.02,-2.78,;-4.02,-1.54,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,;-2.69,.77,;-4.02,1.54,;-5.09,2.16,)| Show InChI InChI=1S/C27H28N6O4/c1-35-22-12-19-24(32-20(22)13-28)17(5-11-29-19)4-6-27-9-7-26(8-10-27,16-37-27)30-14-18-2-3-21-25(31-18)33-23(34)15-36-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,31,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082432
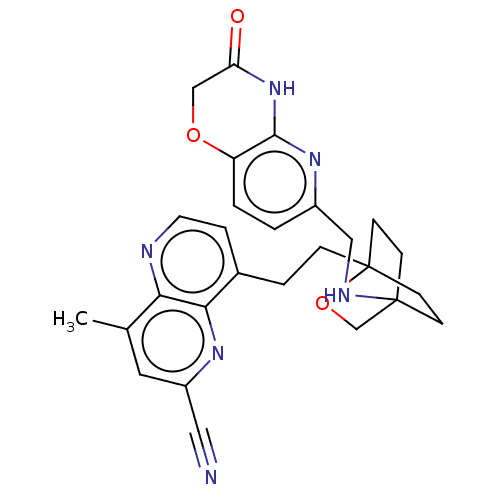
(CHEMBL3422973)Show SMILES Cc1cc(nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc12)C#N |(-1.33,-2.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-4.02,1.54,;-5.09,2.16,)| Show InChI InChI=1S/C27H28N6O3/c1-17-12-20(13-28)31-24-18(5-11-29-23(17)24)4-6-27-9-7-26(8-10-27,16-36-27)30-14-19-2-3-21-25(32-19)33-22(34)15-35-21/h2-3,5,11-12,30H,4,6-10,14-16H2,1H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082520

(CHEMBL3422983)Show SMILES COc1ccc2ncc(C#N)c(CC(O)C34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;4,1.54,;5.07,2.16,;1.33,1.54,;1.34,3.08,;2.67,3.85,;3.74,3.23,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H28N6O5/c1-36-23-5-3-19-24(33-23)18(16(11-28)12-29-19)10-21(34)27-8-6-26(7-9-27,15-38-27)30-13-17-2-4-20-25(31-17)32-22(35)14-37-20/h2-5,12,21,30,34H,6-10,13-15H2,1H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082520

(CHEMBL3422983)Show SMILES COc1ccc2ncc(C#N)c(CC(O)C34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;4,1.54,;5.07,2.16,;1.33,1.54,;1.34,3.08,;2.67,3.85,;3.74,3.23,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H28N6O5/c1-36-23-5-3-19-24(33-23)18(16(11-28)12-29-19)10-21(34)27-8-6-26(7-9-27,15-38-27)30-13-17-2-4-20-25(31-17)32-22(35)14-37-20/h2-5,12,21,30,34H,6-10,13-15H2,1H3,(H,31,32,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082434

(CHEMBL3422975)Show SMILES CN(C)c1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1C(F)(F)F |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.93,;-4.02,-2.78,;-5.09,-2.16,)| Show InChI InChI=1S/C28H31F3N6O3/c1-37(2)25-19(28(29,30)31)13-20-23(36-25)17(6-12-32-20)5-7-27-10-8-26(9-11-27,16-40-27)33-14-18-3-4-21-24(34-18)35-22(38)15-39-21/h3-4,6,12-13,33H,5,7-11,14-16H2,1-2H3,(H,34,35,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082391
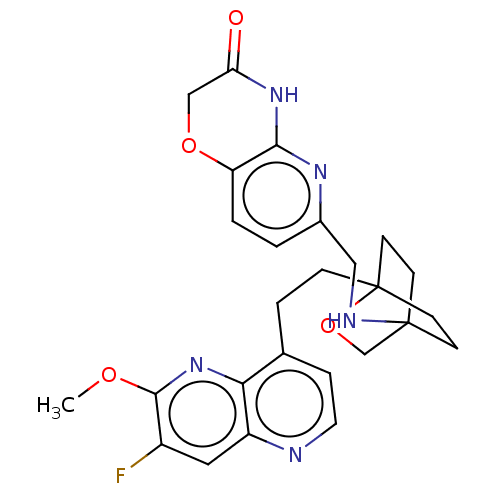
(CHEMBL3422965)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1F |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H28FN5O4/c1-34-24-18(27)12-19-22(32-24)16(5-11-28-19)4-6-26-9-7-25(8-10-26,15-36-26)29-13-17-2-3-20-23(30-17)31-21(33)14-35-20/h2-3,5,11-12,29H,4,6-10,13-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082381

(CHEMBL3422953)Show SMILES COc1cc(C)c2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-2.77,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H30FN5O4/c1-16-11-22(35-2)33-24-18(19(28)13-29-23(16)24)5-6-27-9-7-26(8-10-27,15-37-27)30-12-17-3-4-20-25(31-17)32-21(34)14-36-20/h3-4,11,13,30H,5-10,12,14-15H2,1-2H3,(H,31,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039029

((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@@H](N1C[C@@H](C)N(CC=C)C[C@@H]1C)c1cccc(OC)c1 |r| Show InChI InChI=1S/C28H39N3O2/c1-7-17-30-19-22(5)31(20-21(30)4)27(25-11-10-12-26(18-25)33-6)23-13-15-24(16-14-23)28(32)29(8-2)9-3/h7,10-16,18,21-22,27H,1,8-9,17,19-20H2,2-6H3/t21-,22+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against cloned human Opioid receptor delta 1 (wild-type,Wt) |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082376

(CHEMBL3422948)Show SMILES Nc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-3.75,1.39,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H27FN6O3/c26-17-12-28-18-2-4-20(27)31-22(18)16(17)5-6-25-9-7-24(8-10-25,14-35-25)29-11-15-1-3-19-23(30-15)32-21(33)13-34-19/h1-4,12,29H,5-11,13-14H2,(H2,27,31)(H,30,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082387

(CHEMBL3422961)Show SMILES Fc1cnc2cc(Cl)cnc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1 |(3.74,1.39,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,)| Show InChI InChI=1S/C25H25ClFN5O3/c26-15-9-19-22(29-10-15)17(18(27)12-28-19)3-4-25-7-5-24(6-8-25,14-35-25)30-11-16-1-2-20-23(31-16)32-21(33)13-34-20/h1-2,9-10,12,30H,3-8,11,13-14H2,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082340
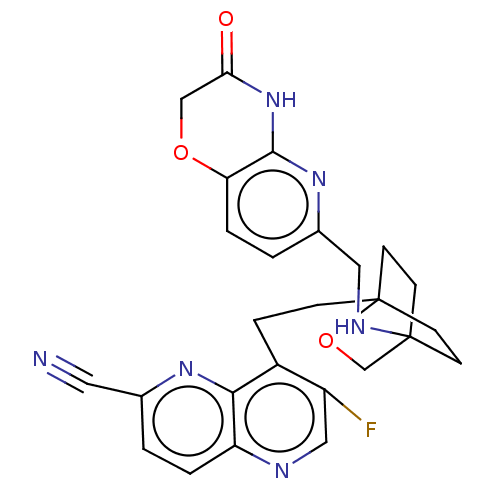
(CHEMBL3422947)Show SMILES Fc1cnc2ccc(nc2c1CCC12CCC(CC1)(CO2)NCc1ccc2OCC(=O)Nc2n1)C#N |(-12.24,-4.65,;-12.56,-5.84,;-14.04,-6.24,;-14.44,-7.72,;-13.36,-8.81,;-13.75,-10.3,;-12.66,-11.41,;-11.17,-11.01,;-10.78,-9.5,;-11.87,-8.41,;-11.47,-6.93,;-9.98,-6.52,;-9.58,-5.03,;-8.11,-4.64,;-7.75,-3.13,;-6.28,-2.7,;-5.35,-3.86,;-5.52,-5.26,;-6.99,-5.7,;-6.24,-4.82,;-7.05,-3.51,;-4.02,-3.09,;-4.02,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.67,.77,;2.67,-.77,;3.74,-1.39,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-10.08,-12.1,;-9.21,-12.97,)| Show InChI InChI=1S/C26H25FN6O3/c27-19-13-29-20-3-1-16(11-28)31-23(20)18(19)5-6-26-9-7-25(8-10-26,15-36-26)30-12-17-2-4-21-24(32-17)33-22(34)14-35-21/h1-4,13,30H,5-10,12,14-15H2,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082389

(CHEMBL3422963)Show SMILES COc1nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2cc1Cl |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.69,-.77,;-3.75,-1.39,)| Show InChI InChI=1S/C26H28ClN5O4/c1-34-24-18(27)12-19-22(32-24)16(5-11-28-19)4-6-26-9-7-25(8-10-26,15-36-26)29-13-17-2-3-20-23(30-17)31-21(33)14-35-20/h2-3,5,11-12,29H,4,6-10,13-15H2,1H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082428
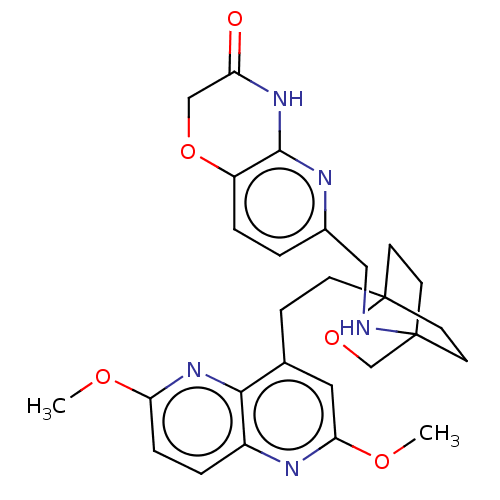
(CHEMBL3422969)Show SMILES COc1ccc2nc(OC)cc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;4.01,-1.54,;5.07,-.92,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31N5O5/c1-34-22-6-4-19-24(32-22)17(13-23(30-19)35-2)7-8-27-11-9-26(10-12-27,16-37-27)28-14-18-3-5-20-25(29-18)31-21(33)15-36-20/h3-6,13,28H,7-12,14-16H2,1-2H3,(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082332
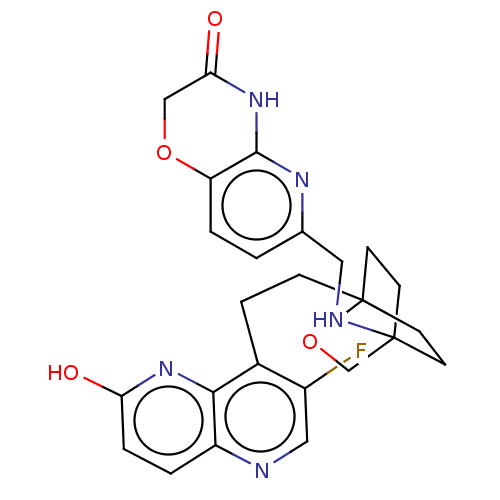
(CHEMBL3422765)Show SMILES Oc1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-3.75,1.39,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C25H26FN5O4/c26-17-12-27-18-2-4-20(32)30-22(18)16(17)5-6-25-9-7-24(8-10-25,14-35-25)28-11-15-1-3-19-23(29-15)31-21(33)13-34-19/h1-4,12,28H,5-11,13-14H2,(H,30,32)(H,29,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082517

(CHEMBL3422980)Show SMILES Clc1cnc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc2c1 |(-3.75,-1.39,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-1.33,-1.54,)| Show InChI InChI=1S/C25H26ClN5O3/c26-17-11-19-22(28-12-17)16(4-10-27-19)3-5-25-8-6-24(7-9-25,15-34-25)29-13-18-1-2-20-23(30-18)31-21(32)14-33-20/h1-2,4,10-12,29H,3,5-9,13-15H2,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50084127

((4S,7S,13S)-13-[(2S,3R)-2-Amino-3-(4-hydroxy-2,6-d...)Show SMILES C[C@@H]([C@H](N)C(=O)N[C@H]1C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(O)=O)C(C)(C)SSC1(C)C)c1c(C)cc(O)cc1C Show InChI InChI=1S/C33H45N5O7S2/c1-17-13-21(39)14-18(2)24(17)19(3)25(34)29(42)37-26-30(43)35-16-23(40)36-22(15-20-11-9-8-10-12-20)28(41)38-27(31(44)45)33(6,7)47-46-32(26,4)5/h8-14,19,22,25-27,39H,15-16,34H2,1-7H3,(H,35,43)(H,36,40)(H,37,42)(H,38,41)(H,44,45)/t19-,22+,25+,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50084127

((4S,7S,13S)-13-[(2S,3R)-2-Amino-3-(4-hydroxy-2,6-d...)Show SMILES C[C@@H]([C@H](N)C(=O)N[C@H]1C(=O)NCC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(O)=O)C(C)(C)SSC1(C)C)c1c(C)cc(O)cc1C Show InChI InChI=1S/C33H45N5O7S2/c1-17-13-21(39)14-18(2)24(17)19(3)25(34)29(42)37-26-30(43)35-16-23(40)36-22(15-20-11-9-8-10-12-20)28(41)38-27(31(44)45)33(6,7)47-46-32(26,4)5/h8-14,19,22,25-27,39H,15-16,34H2,1-7H3,(H,35,43)(H,36,40)(H,37,42)(H,38,41)(H,44,45)/t19-,22+,25+,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-deltorphin binding to Opioid receptor delta 1 using rat brain membranes. |
J Med Chem 42: 5359-68 (2000)
BindingDB Entry DOI: 10.7270/Q23777XM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082409

(CHEMBL3422967)Show SMILES COc1cc(C)c2nccc(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;-1.33,-2.77,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31N5O4/c1-17-13-22(34-2)32-24-18(6-12-28-23(17)24)5-7-27-10-8-26(9-11-27,16-36-27)29-14-19-3-4-20-25(30-19)31-21(33)15-35-20/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50068132
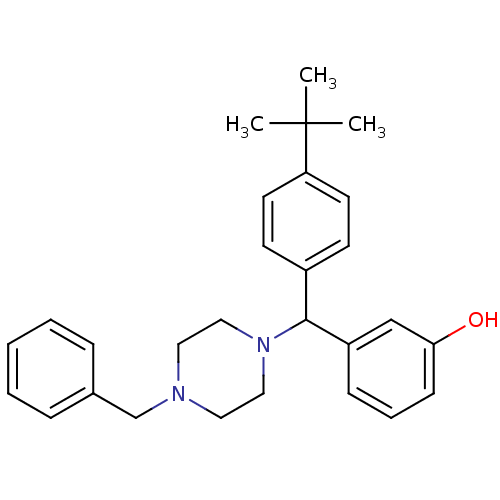
(3-[(4-Benzyl-piperazin-1-yl)-(4-tert-butyl-phenyl)...)Show SMILES CC(C)(C)c1ccc(cc1)C(N1CCN(Cc2ccccc2)CC1)c1cccc(O)c1 Show InChI InChI=1S/C28H34N2O/c1-28(2,3)25-14-12-23(13-15-25)27(24-10-7-11-26(31)20-24)30-18-16-29(17-19-30)21-22-8-5-4-6-9-22/h4-15,20,27,31H,16-19,21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against cloned human Opioid receptor delta 1 (wild-type,Wt) |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082433

(CHEMBL3422974)Show SMILES Cc1cc(nc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc12)S(C)(=O)=O |(-1.33,-2.77,;-1.33,-1.54,;-2.69,-.77,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.05,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,;-4.02,1.54,;-5.09,2.16,;-5.09,.93,;-4.02,2.78,)| Show InChI InChI=1S/C27H31N5O5S/c1-17-13-22(38(2,34)35)32-24-18(6-12-28-23(17)24)5-7-27-10-8-26(9-11-27,16-37-27)29-14-19-3-4-20-25(30-19)31-21(33)15-36-20/h3-4,6,12-13,29H,5,7-11,14-16H2,1-2H3,(H,30,31,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082519

(CHEMBL3422982)Show SMILES CCOC(=O)c1c(OC)cnc2c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)ccnc12 |(-3.72,-6.03,;-2.65,-5.41,;-2.66,-3.86,;-1.33,-3.08,;-.26,-3.7,;-1.33,-1.54,;-2.69,-.77,;-4.02,-1.54,;-5.09,-.92,;-2.69,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;2.67,.77,;2.67,-.77,;1.33,-1.54,;,-.77,)| Show InChI InChI=1S/C29H33N5O6/c1-3-38-27(36)23-21(37-2)15-31-24-18(7-13-30-25(23)24)6-8-29-11-9-28(10-12-29,17-40-29)32-14-19-4-5-20-26(33-19)34-22(35)16-39-20/h4-5,7,13,15,32H,3,6,8-12,14,16-17H2,1-2H3,(H,33,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50084125
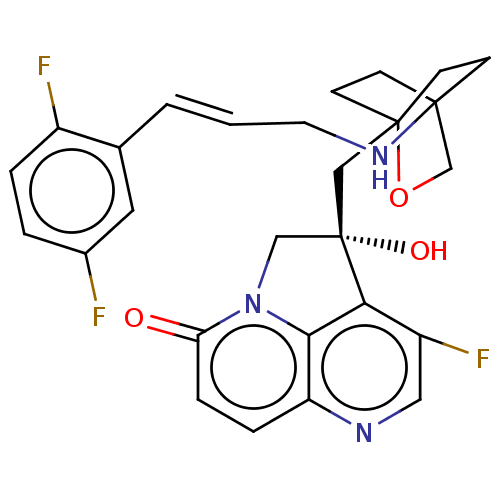
(CHEMBL3425935)Show SMILES O[C@@]1(CC23CCC(CC2)(CO3)NC\C=C\c2cc(F)ccc2F)Cn2c3c1c(F)cnc3ccc2=O |r,wD:1.0,(2.14,2.31,;.92,2.5,;.32,3.91,;-1.21,4.13,;-1.59,2.67,;-3.12,2.49,;-4.05,3.74,;-2.63,3.19,;-2.41,4.71,;-3.43,5.14,;-1.91,5.34,;-5.55,3.5,;-6.11,2.06,;-7.63,1.82,;-8.19,.38,;-9.72,.15,;-10.69,1.35,;-12.21,1.12,;-12.99,2.08,;-12.77,-.32,;-11.81,-1.53,;-10.28,-1.29,;-9.51,-2.26,;-1,2.52,;-1.3,1,;,.24,;1.32,1,;2.63,.24,;3.7,.86,;2.63,-1.26,;1.32,-2.01,;,-1.26,;-1.3,-2.01,;-2.61,-1.26,;-2.61,.24,;-3.68,.86,)| Show InChI InChI=1S/C27H26F3N3O3/c28-18-3-4-19(29)17(12-18)2-1-11-32-25-7-9-26(10-8-25,36-16-25)14-27(35)15-33-22(34)6-5-21-24(33)23(27)20(30)13-31-21/h1-6,12-13,32,35H,7-11,14-16H2/b2-1+/t25?,26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of MK499 from human ERG |
Bioorg Med Chem Lett 25: 2473-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.063
BindingDB Entry DOI: 10.7270/Q2NZ89BD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50068132

(3-[(4-Benzyl-piperazin-1-yl)-(4-tert-butyl-phenyl)...)Show SMILES CC(C)(C)c1ccc(cc1)C(N1CCN(Cc2ccccc2)CC1)c1cccc(O)c1 Show InChI InChI=1S/C28H34N2O/c1-28(2,3)25-14-12-23(13-15-25)27(24-10-7-11-26(31)20-24)30-18-16-29(17-19-30)21-22-8-5-4-6-9-22/h4-15,20,27,31H,16-19,21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50068132

(3-[(4-Benzyl-piperazin-1-yl)-(4-tert-butyl-phenyl)...)Show SMILES CC(C)(C)c1ccc(cc1)C(N1CCN(Cc2ccccc2)CC1)c1cccc(O)c1 Show InChI InChI=1S/C28H34N2O/c1-28(2,3)25-14-12-23(13-15-25)27(24-10-7-11-26(31)20-24)30-18-16-29(17-19-30)21-22-8-5-4-6-9-22/h4-15,20,27,31H,16-19,21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-deltorphin binding to Opioid receptor delta 1 using rat brain membranes. |
J Med Chem 42: 5359-68 (2000)
BindingDB Entry DOI: 10.7270/Q23777XM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50082377

(CHEMBL3422949)Show SMILES CN(C)c1ccc2ncc(F)c(CCC34CCC(CC3)(CO4)NCc3ccc4OCC(=O)Nc4n3)c2n1 |(-4.02,2.78,;-4.02,1.54,;-5.09,.93,;-2.69,.77,;-2.69,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;2.67,-.77,;2.67,.77,;3.74,1.39,;1.33,1.54,;1.34,3.08,;2.67,3.85,;2.68,5.4,;4.17,5.57,;4.56,7.06,;3.46,8.16,;3.81,6.67,;2.27,6.67,;1.98,7.74,;1.58,6.27,;3.9,9.61,;5.41,9.96,;5.86,11.44,;4.8,12.56,;5.26,14.04,;6.76,14.39,;7.21,15.87,;8.71,16.22,;9.76,15.09,;10.96,15.37,;9.31,13.62,;7.81,13.27,;7.37,11.8,;,.77,;-1.33,1.54,)| Show InChI InChI=1S/C27H31FN6O3/c1-34(2)22-6-4-20-24(32-22)18(19(28)14-29-20)7-8-27-11-9-26(10-12-27,16-37-27)30-13-17-3-5-21-25(31-17)33-23(35)15-36-21/h3-6,14,30H,7-13,15-16H2,1-2H3,(H,31,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of MK499 binding to human ERG |
Bioorg Med Chem Lett 25: 2409-15 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.002
BindingDB Entry DOI: 10.7270/Q2CN75MC |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50068132

(3-[(4-Benzyl-piperazin-1-yl)-(4-tert-butyl-phenyl)...)Show SMILES CC(C)(C)c1ccc(cc1)C(N1CCN(Cc2ccccc2)CC1)c1cccc(O)c1 Show InChI InChI=1S/C28H34N2O/c1-28(2,3)25-14-12-23(13-15-25)27(24-10-7-11-26(31)20-24)30-18-16-29(17-19-30)21-22-8-5-4-6-9-22/h4-15,20,27,31H,16-19,21H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Arizona
Curated by ChEMBL
| Assay Description
Binding affinity was measured against Opioid receptor delta 1 using [3H]-p-Cl-DPDPE as radioligand. |
J Med Chem 41: 4767-76 (1998)
Article DOI: 10.1021/jm980374r
BindingDB Entry DOI: 10.7270/Q2DZ07FQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data