

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50369223 (CHEMBL1907788) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 in guinea pig brain | J Med Chem 39: 1729-35 (1996) Article DOI: 10.1021/jm950813b BindingDB Entry DOI: 10.7270/Q27S7PFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50369224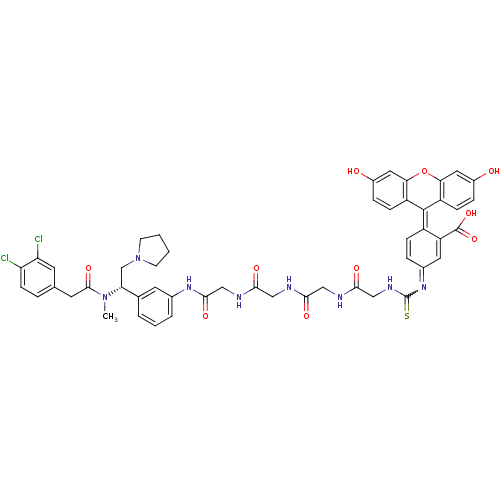 (CHEMBL1907787) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 in guinea pig brain | J Med Chem 39: 1729-35 (1996) Article DOI: 10.1021/jm950813b BindingDB Entry DOI: 10.7270/Q27S7PFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50369223 (CHEMBL1907788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards delta-1 opioid receptor in guinea pig brain | J Med Chem 39: 1729-35 (1996) Article DOI: 10.1021/jm950813b BindingDB Entry DOI: 10.7270/Q27S7PFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50369223 (CHEMBL1907788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards delta-2 opioid receptor in guinea pig brain | J Med Chem 39: 1729-35 (1996) Article DOI: 10.1021/jm950813b BindingDB Entry DOI: 10.7270/Q27S7PFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50064015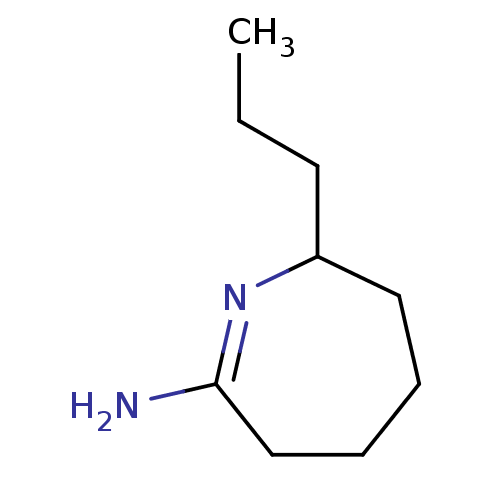 (7-Propyl-azepan-(2Z)-ylideneamine; hydrochloride |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against L-arginine binding to Inducible nitric oxide synthase | J Med Chem 41: 1361-6 (1998) Article DOI: 10.1021/jm9704715 BindingDB Entry DOI: 10.7270/Q2348M29 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50369224 (CHEMBL1907787) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards delta-1 opioid receptor in guinea pig brain | J Med Chem 39: 1729-35 (1996) Article DOI: 10.1021/jm950813b BindingDB Entry DOI: 10.7270/Q27S7PFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50369224 (CHEMBL1907787) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 264 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards delta-2 opioid receptor in guinea pig brain | J Med Chem 39: 1729-35 (1996) Article DOI: 10.1021/jm950813b BindingDB Entry DOI: 10.7270/Q27S7PFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50369223 (CHEMBL1907788) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 286 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 in guinea pig brain | J Med Chem 39: 1729-35 (1996) Article DOI: 10.1021/jm950813b BindingDB Entry DOI: 10.7270/Q27S7PFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50369224 (CHEMBL1907787) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 in guinea pig brain | J Med Chem 39: 1729-35 (1996) Article DOI: 10.1021/jm950813b BindingDB Entry DOI: 10.7270/Q27S7PFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50612500 (CHEMBL5287761) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | 5.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13433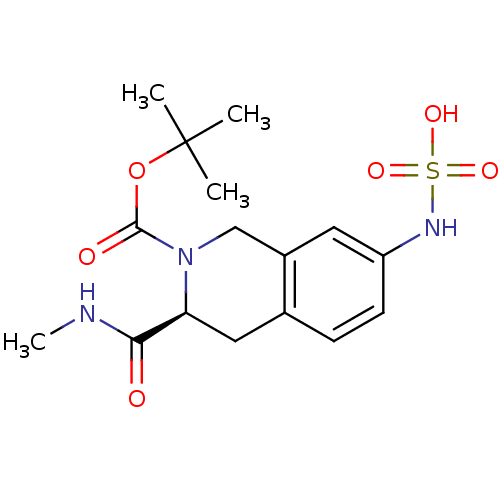 (N-[(3S)-2-[(tert-butoxy)carbonyl]-3-(methylcarbamo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 2.40E+4 | -26.1 | n/a | n/a | n/a | n/a | n/a | 7.0 | 22 |
Procter & Gamble Pharmaceuticals | Assay Description The activity of PTP1B enzyme was assayed with DiFMUP as substrate. Hydrolysis of substrate was monitored on a Victor V plate reader (Wallac). Kinetic... | Bioorg Med Chem Lett 16: 1574-8 (2006) Article DOI: 10.1016/j.bmcl.2005.12.051 BindingDB Entry DOI: 10.7270/Q2PN93WP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 [1-298] (Homo sapiens (Human)) | BDBM13529 (N-(1-methyl-3-phenyl-1H-pyrazol-5-yl)sulfamic acid...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank KEGG MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals | Assay Description The activity of PTP1B enzyme was assayed with DiFMUP as substrate. Hydrolysis of substrate was monitored on a Victor V plate reader (Wallac). Kinetic... | Bioorg Med Chem Lett 16: 1574-8 (2006) Article DOI: 10.1016/j.bmcl.2005.12.051 BindingDB Entry DOI: 10.7270/Q2PN93WP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359101 (US10220048, Compound AA11 | US10952992, No. AA11 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO PHARMACEUTICALS, INC. US Patent | Assay Description Non-limiting examples of the HPTP-β IC50 (μM) activity. | US Patent US10952992 (2021) BindingDB Entry DOI: 10.7270/Q2FR00R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359101 (US10220048, Compound AA11 | US10952992, No. AA11 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-limiting examples of the HPTPβ IC50 (μM) activity. | Citation and Details BindingDB Entry DOI: 10.7270/Q2668HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359101 (US10220048, Compound AA11 | US10952992, No. AA11 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2RV0SPJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359101 (US10220048, Compound AA11 | US10952992, No. AA11 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO THERAPEUTICS, INC. US Patent | Assay Description HPTP-β inhibition can be tested by any method chosen by the formulator, for example, Amarasinge K. K. et al., “Design and Synthesis of Potent, N... | US Patent US10220048 (2019) BindingDB Entry DOI: 10.7270/Q2VT1VC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [669-770,'SVD',771-1210] (Homo sapiens (Human)) | BDBM555110 (3-[(3-fluorophenyl)amino]-2-[3-(2-hydroxy-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H70K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [669-770,'SVD',771-1210] (Homo sapiens (Human)) | BDBM555116 (3-[(3,5-difluorophenyl)amino]-2-[3-(2-methoxy-2-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H70K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [669-770,'SVD',771-1210] (Homo sapiens (Human)) | BDBM555115 (3-[(3,5-difluorophenyl)amino]-2-[3-(2-methoxyethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H70K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [669-770,'SVD',771-1210] (Homo sapiens (Human)) | BDBM555112 (3-[(3-fluorophenyl)amino]-2-[3-(2-methoxy-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.0720 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H70K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359106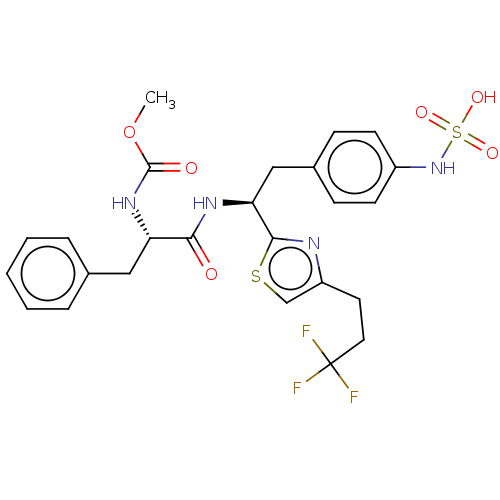 (US10220048, Compound AA16 | US10952992, No. AA16 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO THERAPEUTICS, INC. US Patent | Assay Description HPTP-β inhibition can be tested by any method chosen by the formulator, for example, Amarasinge K. K. et al., “Design and Synthesis of Potent, N... | US Patent US10220048 (2019) BindingDB Entry DOI: 10.7270/Q2VT1VC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359121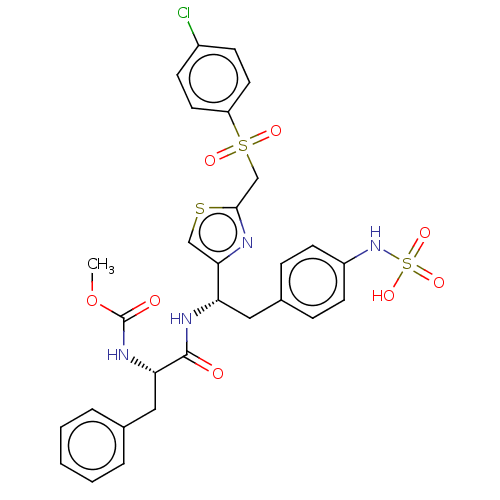 (US10220048, Compound AA31 | US10952992, No. AA31 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO THERAPEUTICS, INC. US Patent | Assay Description HPTP-β inhibition can be tested by any method chosen by the formulator, for example, Amarasinge K. K. et al., “Design and Synthesis of Potent, N... | US Patent US10220048 (2019) BindingDB Entry DOI: 10.7270/Q2VT1VC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359121 (US10220048, Compound AA31 | US10952992, No. AA31 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO PHARMACEUTICALS, INC. US Patent | Assay Description Non-limiting examples of the HPTP-β IC50 (μM) activity. | US Patent US10952992 (2021) BindingDB Entry DOI: 10.7270/Q2FR00R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359106 (US10220048, Compound AA16 | US10952992, No. AA16 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO PHARMACEUTICALS, INC. US Patent | Assay Description Non-limiting examples of the HPTP-β IC50 (μM) activity. | US Patent US10952992 (2021) BindingDB Entry DOI: 10.7270/Q2FR00R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359121 (US10220048, Compound AA31 | US10952992, No. AA31 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-limiting examples of the HPTPβ IC50 (μM) activity. | Citation and Details BindingDB Entry DOI: 10.7270/Q2668HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359121 (US10220048, Compound AA31 | US10952992, No. AA31 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2RV0SPJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359106 (US10220048, Compound AA16 | US10952992, No. AA16 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2RV0SPJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359106 (US10220048, Compound AA16 | US10952992, No. AA16 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-limiting examples of the HPTPβ IC50 (μM) activity. | Citation and Details BindingDB Entry DOI: 10.7270/Q2668HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [669-770,'SVD',771-1210] (Homo sapiens (Human)) | BDBM555118 (3-[(2-bromo-3-fluorophenyl)amino]-2-[3-(2-methoxye...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H70K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359113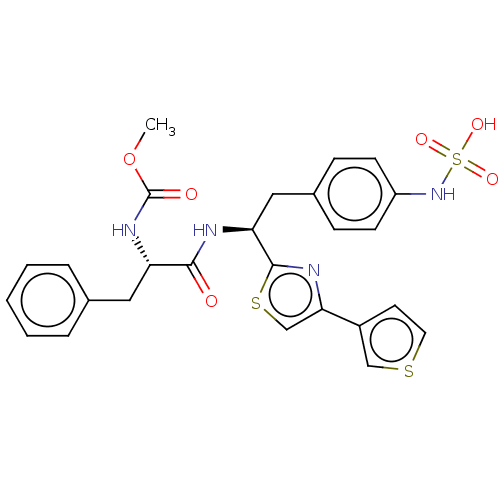 (US10220048, Compound AA23 | US10952992, No. AA23 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO THERAPEUTICS, INC. US Patent | Assay Description HPTP-β inhibition can be tested by any method chosen by the formulator, for example, Amarasinge K. K. et al., “Design and Synthesis of Potent, N... | US Patent US10220048 (2019) BindingDB Entry DOI: 10.7270/Q2VT1VC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359113 (US10220048, Compound AA23 | US10952992, No. AA23 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO PHARMACEUTICALS, INC. US Patent | Assay Description Non-limiting examples of the HPTP-β IC50 (μM) activity. | US Patent US10952992 (2021) BindingDB Entry DOI: 10.7270/Q2FR00R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359113 (US10220048, Compound AA23 | US10952992, No. AA23 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-limiting examples of the HPTPβ IC50 (μM) activity. | Citation and Details BindingDB Entry DOI: 10.7270/Q2668HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359113 (US10220048, Compound AA23 | US10952992, No. AA23 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2RV0SPJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [669-770,'SVD',771-1210] (Homo sapiens (Human)) | BDBM555136 (3-[(3,5-difluoro-2-methoxyphenyl)amino]-2-[3-(2-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H70K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [669-770,'SVD',771-1210] (Homo sapiens (Human)) | BDBM555137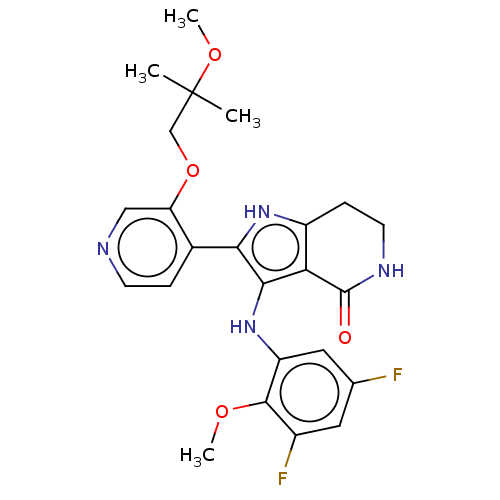 (3-[(3,5-difluoro-2-methoxyphenyl)amino]-2-[3-(2-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.0990 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H70K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359118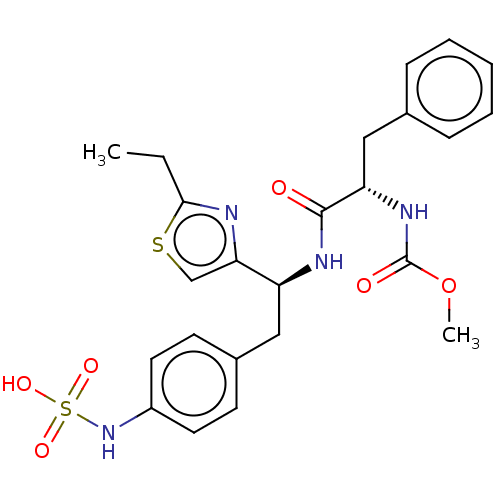 (US10220048, Compound AA28 | US10952992, No. AA28 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO THERAPEUTICS, INC. US Patent | Assay Description HPTP-β inhibition can be tested by any method chosen by the formulator, for example, Amarasinge K. K. et al., “Design and Synthesis of Potent, N... | US Patent US10220048 (2019) BindingDB Entry DOI: 10.7270/Q2VT1VC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359099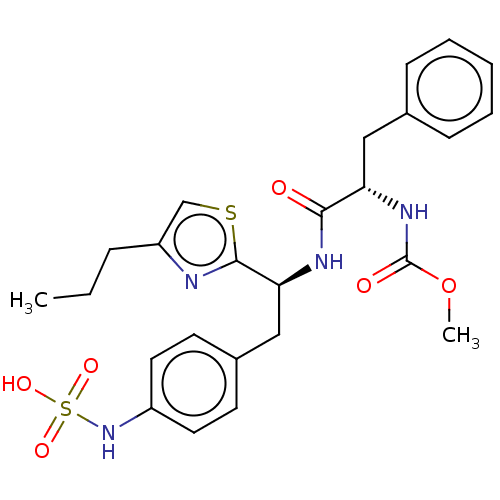 (US10220048, Compound AA9 | US10952992, No. AA9 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO THERAPEUTICS, INC. US Patent | Assay Description HPTP-β inhibition can be tested by any method chosen by the formulator, for example, Amarasinge K. K. et al., “Design and Synthesis of Potent, N... | US Patent US10220048 (2019) BindingDB Entry DOI: 10.7270/Q2VT1VC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359099 (US10220048, Compound AA9 | US10952992, No. AA9 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO PHARMACEUTICALS, INC. US Patent | Assay Description Non-limiting examples of the HPTP-β IC50 (μM) activity. | US Patent US10952992 (2021) BindingDB Entry DOI: 10.7270/Q2FR00R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359104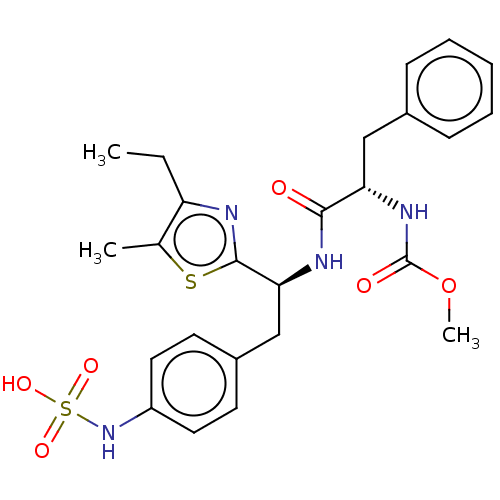 (US10220048, Compound AA14 | US10952992, No. AA14 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO PHARMACEUTICALS, INC. US Patent | Assay Description Non-limiting examples of the HPTP-β IC50 (μM) activity. | US Patent US10952992 (2021) BindingDB Entry DOI: 10.7270/Q2FR00R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359118 (US10220048, Compound AA28 | US10952992, No. AA28 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO PHARMACEUTICALS, INC. US Patent | Assay Description Non-limiting examples of the HPTP-β IC50 (μM) activity. | US Patent US10952992 (2021) BindingDB Entry DOI: 10.7270/Q2FR00R3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359118 (US10220048, Compound AA28 | US10952992, No. AA28 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-limiting examples of the HPTPβ IC50 (μM) activity. | Citation and Details BindingDB Entry DOI: 10.7270/Q2668HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359118 (US10220048, Compound AA28 | US10952992, No. AA28 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2RV0SPJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359099 (US10220048, Compound AA9 | US10952992, No. AA9 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2RV0SPJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359104 (US10220048, Compound AA14 | US10952992, No. AA14 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2RV0SPJ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359099 (US10220048, Compound AA9 | US10952992, No. AA9 | U...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-limiting examples of the HPTPβ IC50 (μM) activity. | Citation and Details BindingDB Entry DOI: 10.7270/Q2668HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359104 (US10220048, Compound AA14 | US10952992, No. AA14 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non-limiting examples of the HPTPβ IC50 (μM) activity. | Citation and Details BindingDB Entry DOI: 10.7270/Q2668HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein phosphatase beta (Homo sapiens (Human)) | BDBM359104 (US10220048, Compound AA14 | US10952992, No. AA14 |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AERPIO THERAPEUTICS, INC. US Patent | Assay Description HPTP-β inhibition can be tested by any method chosen by the formulator, for example, Amarasinge K. K. et al., “Design and Synthesis of Potent, N... | US Patent US10220048 (2019) BindingDB Entry DOI: 10.7270/Q2VT1VC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [669-770,'SVD',771-1210] (Homo sapiens (Human)) | BDBM555123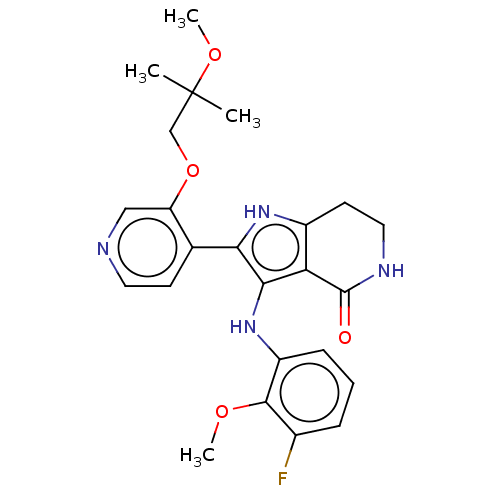 (3-[(3-fluoro-2-methoxyphenyl)amino]-2-[3-(2-methox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.113 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H70K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [669-770,'SVD',771-1210] (Homo sapiens (Human)) | BDBM555124 (3-{[2-(difluoromethoxy)-3-fluorophenyl]amino}-2-[3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2H70K0Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50035476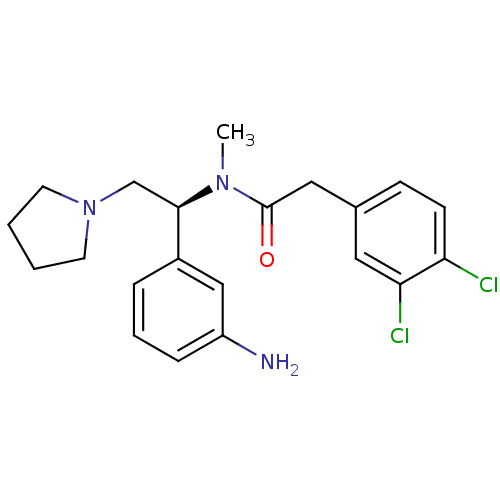 (CHEMBL413530 | N-[(S)-1-(3-Amino-phenyl)-2-pyrroli...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory activity against Opioid receptor kappa 1 in electrically stimulated mouse vas deferens (MVD) preparation. | J Med Chem 39: 1729-35 (1996) Article DOI: 10.1021/jm950813b BindingDB Entry DOI: 10.7270/Q27S7PFG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3458 total ) | Next | Last >> |