

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076428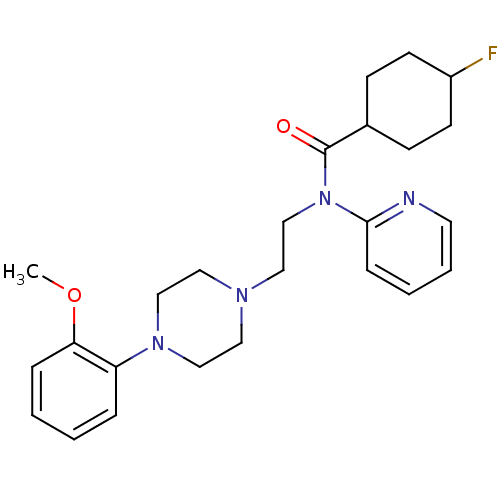 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.247 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor by Panlabs assay | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583877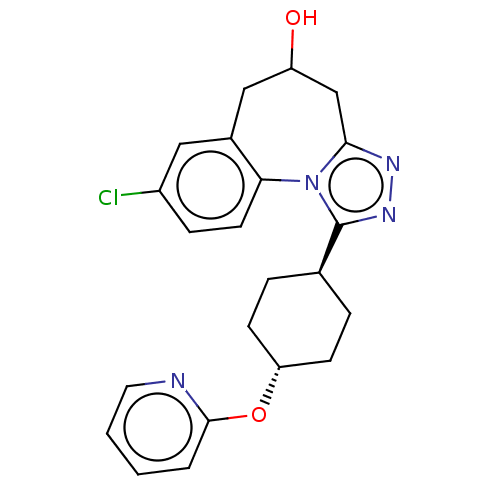 (CHEMBL5070261) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583878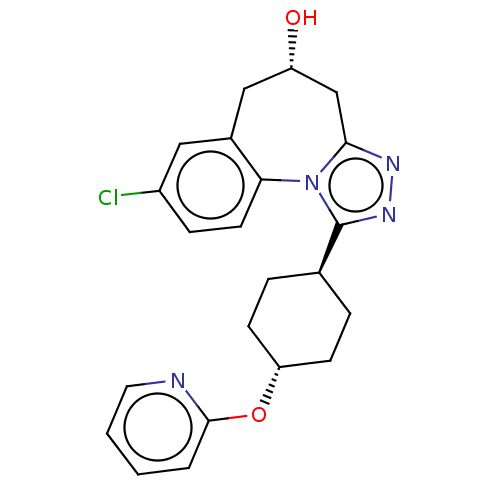 (CHEMBL5074709) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583879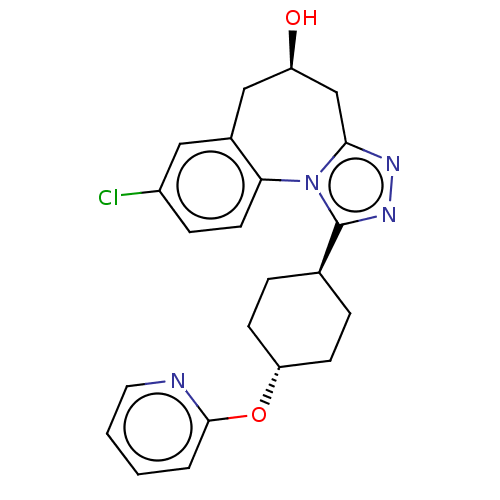 (CHEMBL5070075) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583881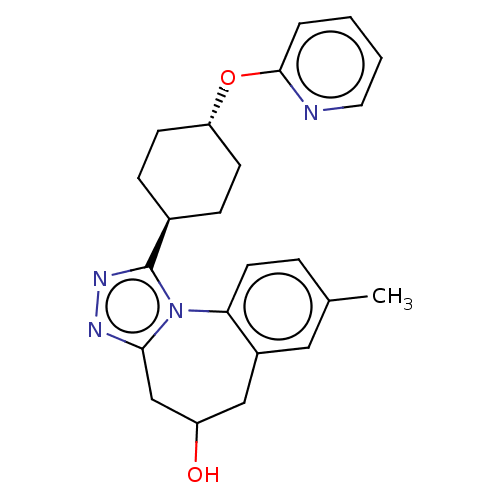 (CHEMBL5081323) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076428 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.515 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50374600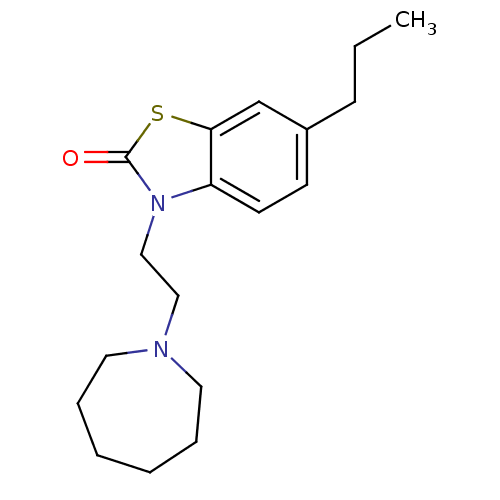 (CHEMBL272899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from Sigma1 receptor in rat liver homogenate | J Med Chem 55: 8272-82 (2012) Article DOI: 10.1021/jm300371c BindingDB Entry DOI: 10.7270/Q2V40WB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50546439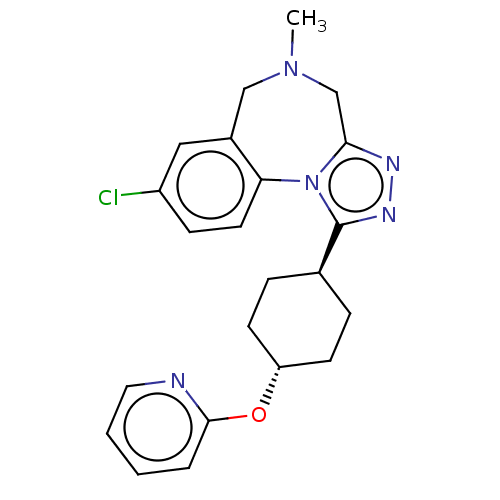 (Balovaptan | RG-7314 | RO-5285119 | RO5285119 | Rg...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076429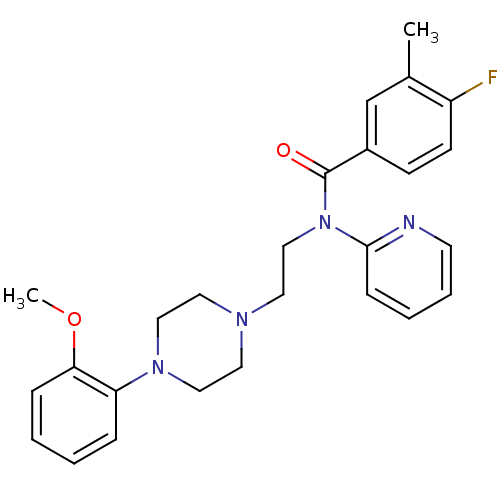 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.791 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor (Experiment 2) | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50546436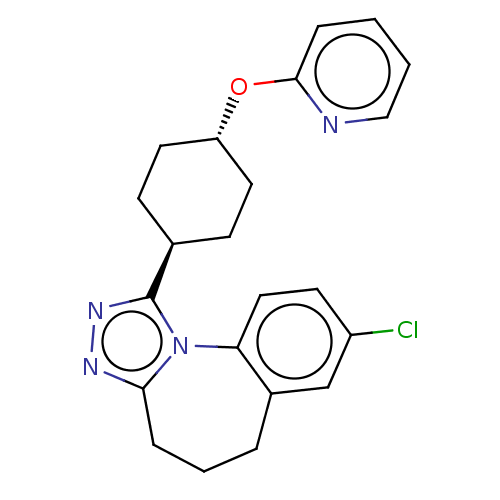 (CHEMBL4799793) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50398057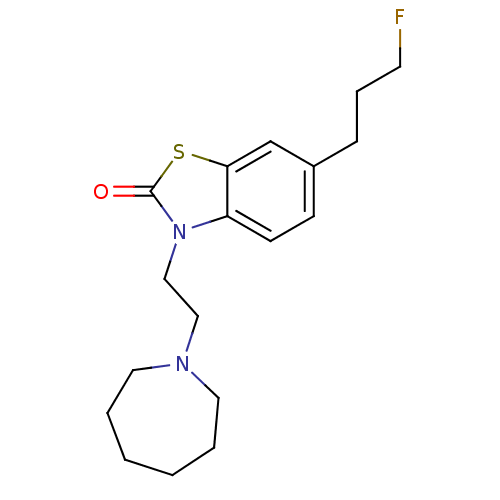 (CHEMBL2181924) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from Sigma1 receptor in rat liver homogenate | J Med Chem 55: 8272-82 (2012) Article DOI: 10.1021/jm300371c BindingDB Entry DOI: 10.7270/Q2V40WB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50398057 (CHEMBL2181924) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from Sigma1 receptor in rat liver homogenate | J Med Chem 55: 8272-82 (2012) Article DOI: 10.1021/jm300371c BindingDB Entry DOI: 10.7270/Q2V40WB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM50076428 (4-Fluoro-cyclohexanecarboxylic acid {2-[4-(2-metho...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM35723 (CHEMBL344159 | N-[4-(7-Chloro-5-hydroxy-2,3,4,5-te...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V2 receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM86708 (CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]GTP-gamma-S, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076429 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor (Expe... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-beta-hydroxysteroid-Delta(8),Delta(7)-isomerase (Homo sapiens (Human)) | BDBM50338990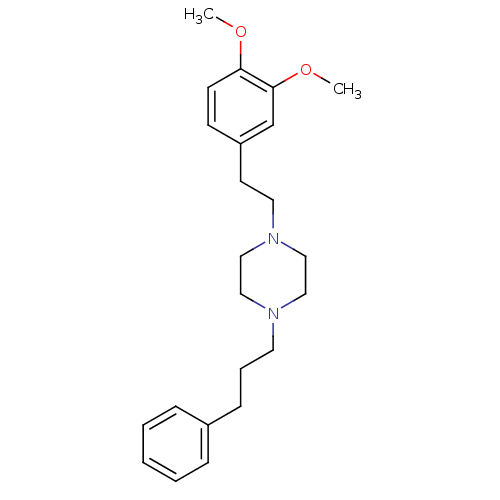 (1-(3,4-dimethoxyphenethyl)-4-(3-phenylpropyl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Binding affinity to EBP | J Med Chem 55: 8272-82 (2012) Article DOI: 10.1021/jm300371c BindingDB Entry DOI: 10.7270/Q2V40WB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50374600 (CHEMBL272899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from Sigma1 receptor in rat brain homogenate | J Med Chem 55: 8272-82 (2012) Article DOI: 10.1021/jm300371c BindingDB Entry DOI: 10.7270/Q2V40WB0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM50076429 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]-GTP-gammaS, binding (Experi... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295040 (5-(4-Cyclopropylphenyl)-1-(2-chloro-4-bromophenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295029 (5-(4-Cyclopropylphenyl)-1-(2,4-dichlorophenyl)-4-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295024 (5-(4-Cyclopropylphenyl)-1-(2,4-dichlorophenyl)-4-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from Sigma1 receptor in rat liver homogenate | J Med Chem 55: 8272-82 (2012) Article DOI: 10.1021/jm300371c BindingDB Entry DOI: 10.7270/Q2V40WB0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from Sigma1 receptor in rat brain homogenate | J Med Chem 55: 8272-82 (2012) Article DOI: 10.1021/jm300371c BindingDB Entry DOI: 10.7270/Q2V40WB0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50354894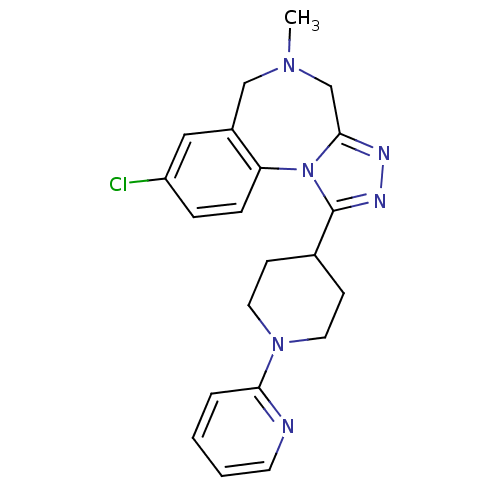 (CHEMBL1837037) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM50076429 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Receptor-linked G protein activation at 5-hydroxytryptamine receptor was determined by measuring the stimulation of [35S]GTP-gamma-S, binding | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295039 (5-(4-Cyclopropylphenyl)-1-(2,4-dichlorophenyl)-4-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50076429 (4-Fluoro-N-{2-[4-(2-methoxy-phenyl)-piperazin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Compound was tested in vitro for the inhibition of [3H]- 8-OH-DPAT binding to cloned cell line containing human 5-hydroxytryptamine 1A receptor (Expe... | J Med Chem 42: 1576-86 (1999) Article DOI: 10.1021/jm980456f BindingDB Entry DOI: 10.7270/Q23R0S2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295041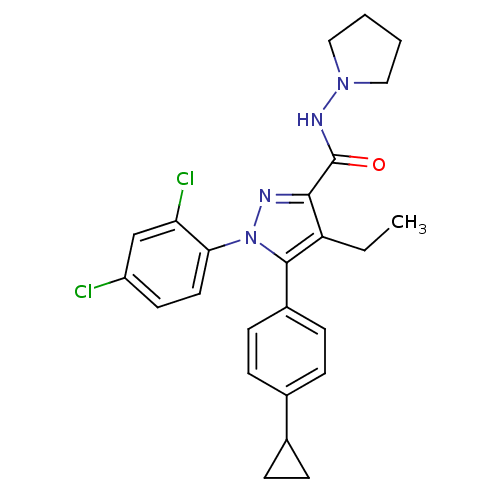 (5-(4-Cyclopropylphenyl)-1-(2,4-dichlorophenyl)-4-e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295032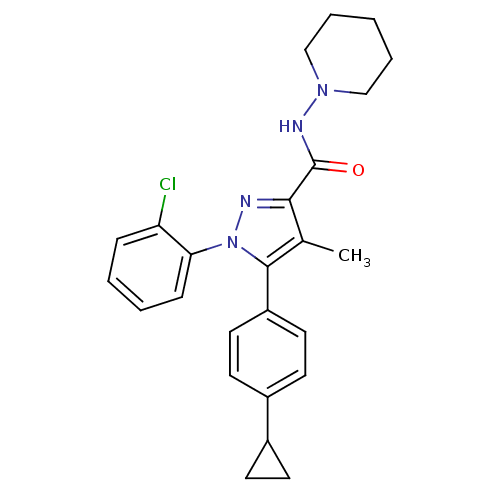 (5-(4-Cyclopropylphenyl)-1-(2-chlorophenyl)-4-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50077217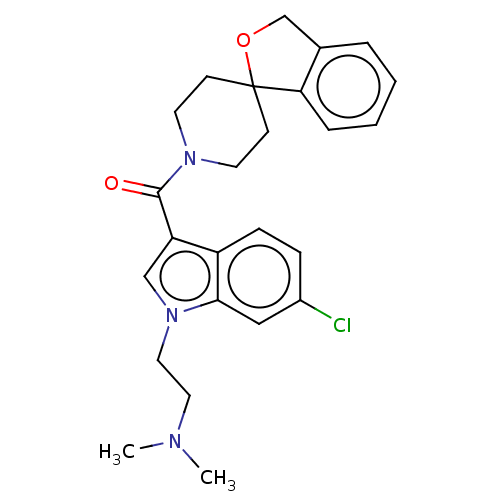 (CHEMBL3416885) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583883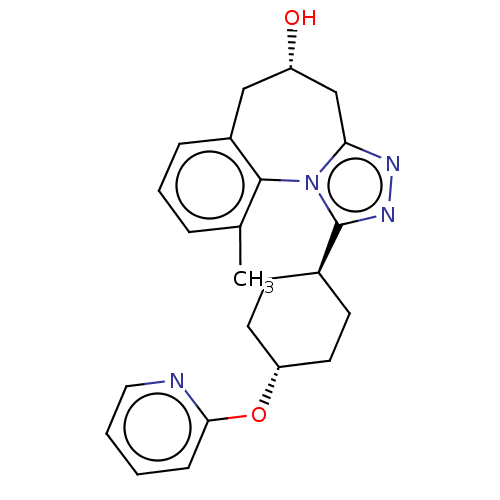 (CHEMBL5078950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583883 (CHEMBL5078950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum membrane | Bioorg Med Chem Lett 19: 3471-5 (2009) Article DOI: 10.1016/j.bmcl.2009.05.010 BindingDB Entry DOI: 10.7270/Q2P55NDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295025 (5-(4-Cyclobutylphenyl)-1-(2,4-dichlorophenyl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295033 (5-(4-Cyclopropylphenyl)-1-(2-chloro-4-fluorophenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583880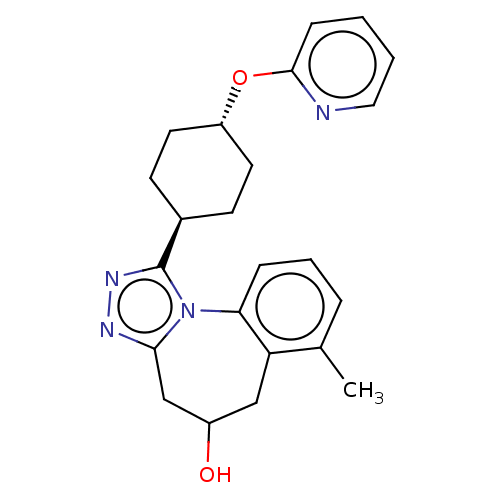 (CHEMBL5088219) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295034 (5-(4-Cyclopropylphenyl)-1-(2-fluorophenyl)-4-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50583887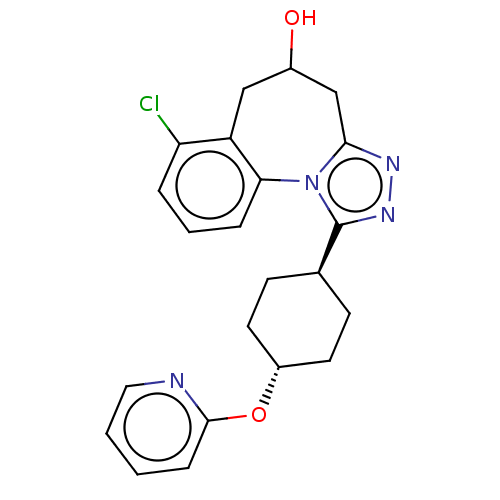 (CHEMBL5074961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-arginine-vasopressin from human V1A receptor expressed in human 1321N1 cell membranes incubated for 60 mins by radioligand bindi... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00863 BindingDB Entry DOI: 10.7270/Q2GM8C6F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552011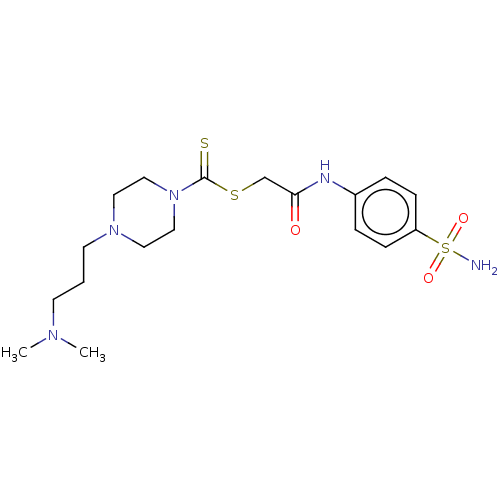 (CHEMBL4792992) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295027 (5-(4-Cyclohexylphenyl)-1-(2,4-dichlorophenyl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295037 (5-(4-Cyclopropylphenyl)-1-(2-fluoro-4-chlorophenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295026 (5-(4-Cyclopentylphenyl)-1-(2,4-dichlorophenyl)-4-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50295028 (5-(4-Cyclopropylphenyl)-1-(2,4-dichlorophenyl)-N-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gedeon Richter Plc Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from CB1 receptor in rat cerebellum by scintillation spectrometry | J Med Chem 52: 4329-37 (2009) Article DOI: 10.1021/jm900179y BindingDB Entry DOI: 10.7270/Q2NG4QN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552018 (CHEMBL4764100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552012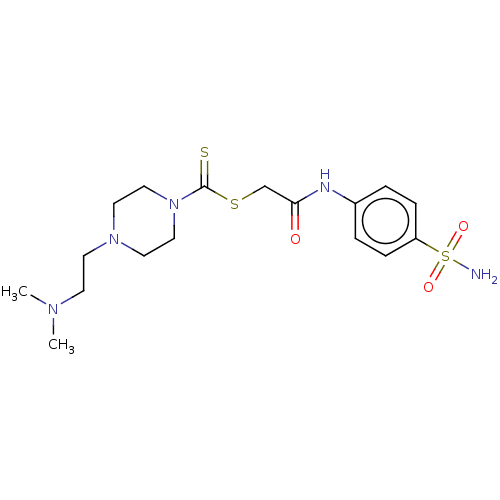 (CHEMBL4786423) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50552009 (CHEMBL4747356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte CA2 using 4-nitrophenyl acetate as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112392 BindingDB Entry DOI: 10.7270/Q2C2512X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 785 total ) | Next | Last >> |