

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50084033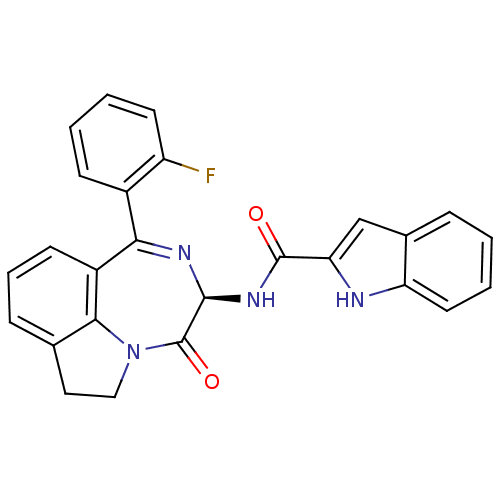 (1H-Indole-2-carboxylic acid [(R)-1-(2-fluoro-pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A1 receptor binding using [3H]DPCPX in rat cortical membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50084033 (1H-Indole-2-carboxylic acid [(R)-1-(2-fluoro-pheny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50079652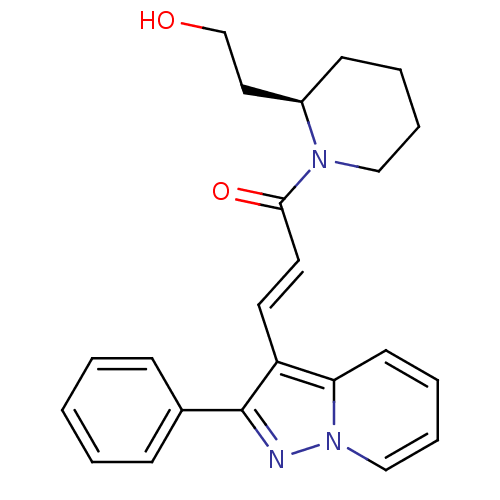 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A1 receptor binding using [3H]DPCPX in human cortical membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50079652 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A1 receptor binding using [3H]DPCOX in rat cortical membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM21147 ((3S)-3-[(2S)-2-[(2S)-2-{2-[(2S)-2-[(2S)-2-[(3S)-3-...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50079654 (4-(6-oxo-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A1 receptor using [3H]DPCOX in rat cortical membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50185261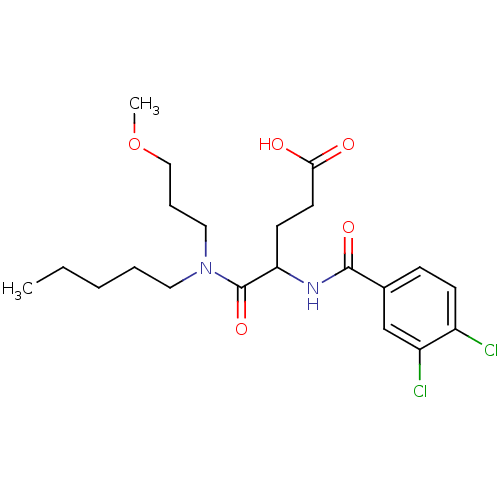 (4-(3,4-dichlorobenzamido)-5-((3-methoxypropyl)(pen...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579459 (CHEMBL4863322) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50084033 (1H-Indole-2-carboxylic acid [(R)-1-(2-fluoro-pheny...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579460 (CHEMBL4857707) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A2 receptor binding using [3H]CGS-21680 in rat striatal membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579456 (CHEMBL4863017) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 156 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579458 (CHEMBL4862554) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 164 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579455 (CHEMBL4873816) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 204 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579457 (CHEMBL4846481) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 287 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (RAT) | BDBM50185261 (4-(3,4-dichlorobenzamido)-5-((3-methoxypropyl)(pen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579461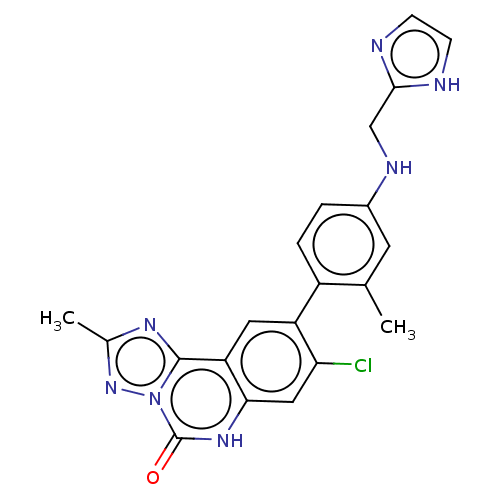 (CHEMBL4854153) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579453 (CHEMBL4875904) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled SP2 probe from human TIM-3 IgV domain (residues 22 to 130) expressed in Escherichia coli BL21 (DE3) by FPA competition ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579455 (CHEMBL4873816) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579456 (CHEMBL4863017) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579454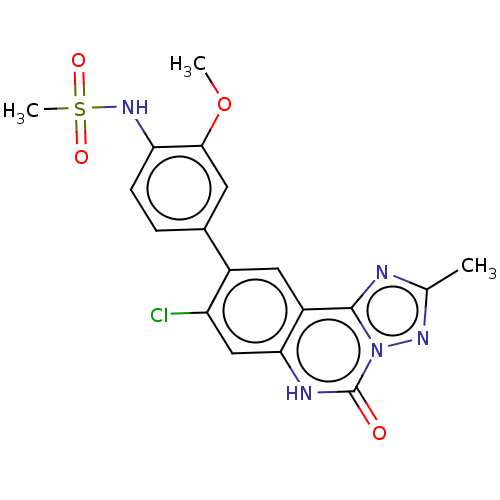 (CHEMBL4868118) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50079654 (4-(6-oxo-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)py...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A2 receptor binding using [3H]CGS-21680 in rat striatal membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a/A2b (Rattus norvegicus-Rattus norvegicus (rat)) | BDBM50079652 ((E)-1-[(R)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Company, Ltd. Curated by ChEMBL | Assay Description Adenosine A2 receptor binding using [3H]CGS-21680 in rat striatal membranes | J Med Chem 42: 779-83 (1999) Article DOI: 10.1021/jm980671w BindingDB Entry DOI: 10.7270/Q2J105WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579453 (CHEMBL4875904) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579447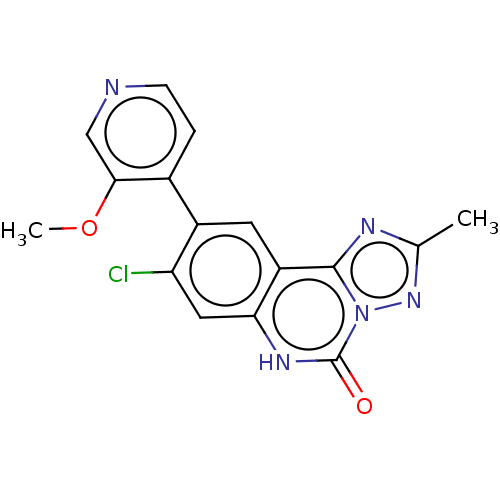 (CHEMBL4874818) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579452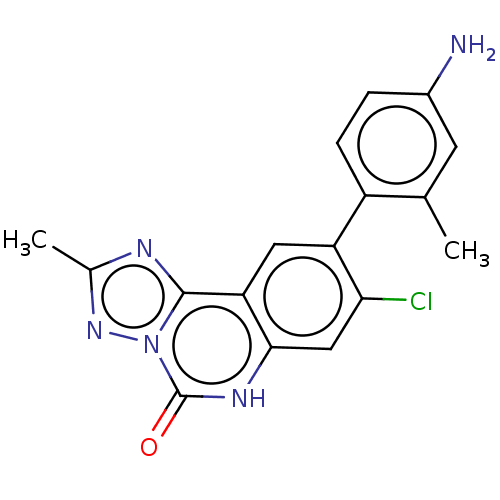 (CHEMBL4846039) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579445 (CHEMBL4859588) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579448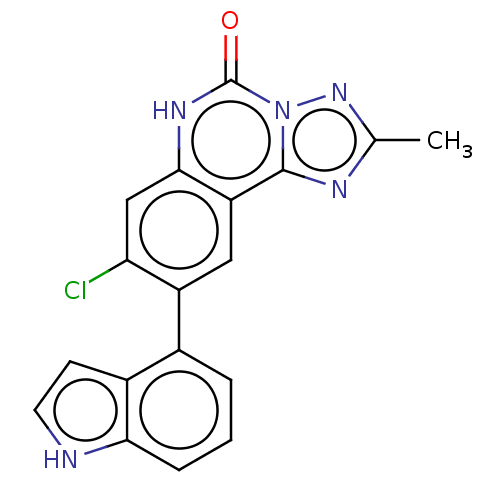 (CHEMBL4851756) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579446 (CHEMBL4860976) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579449 (CHEMBL4872496) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579450 (CHEMBL4876721) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579451 (CHEMBL4864303) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatitis A virus cellular receptor 2 (Homo sapiens) | BDBM50579435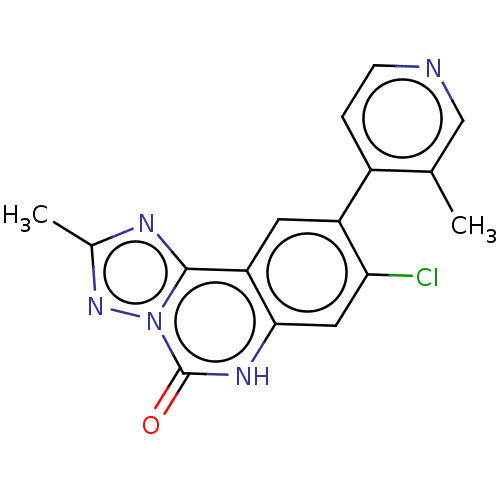 (CHEMBL4863477) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of FITC-labelled 5-(((8-Chloro-9-(3-methylpyridin-4-yl)-5-oxo-5,6-dihydro-[1,2,4]triazolo[1,5-c]quinazolin-2-yl)methyl)carbamoyl)-2-(6-h... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c01336 BindingDB Entry DOI: 10.7270/Q20V8HMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gastrin/cholecystokinin type B receptor (GUINEA PIG) | BDBM50185261 (4-(3,4-dichlorobenzamido)-5-((3-methoxypropyl)(pen...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 268: 571-5 (1994) BindingDB Entry DOI: 10.7270/Q2WQ029S | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391561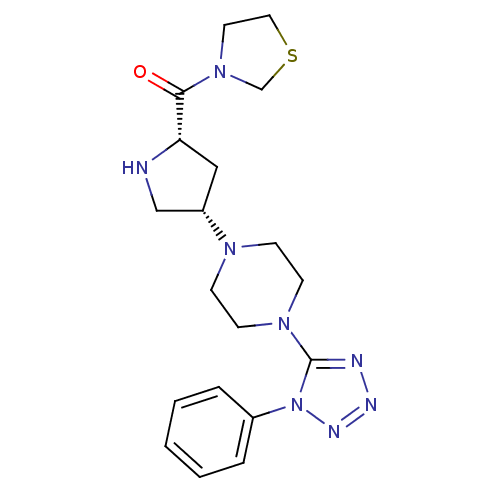 (CHEMBL2147704) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391559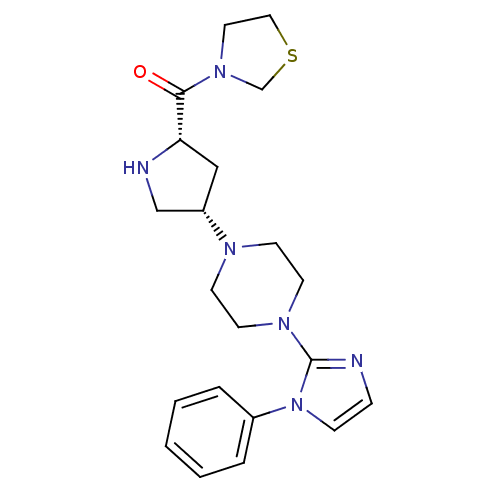 (CHEMBL2147703) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391561 (CHEMBL2147704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391559 (CHEMBL2147703) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391565 (CHEMBL2147777) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391570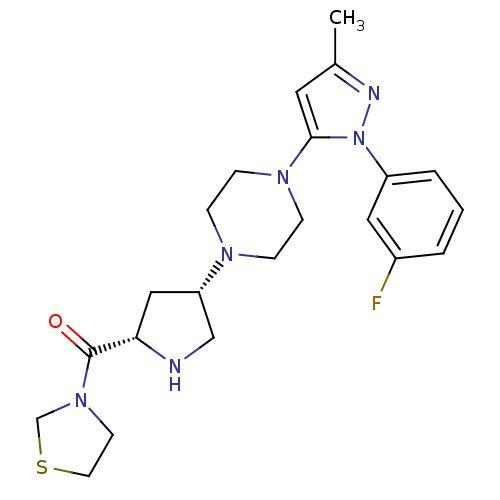 (CHEMBL2147712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391567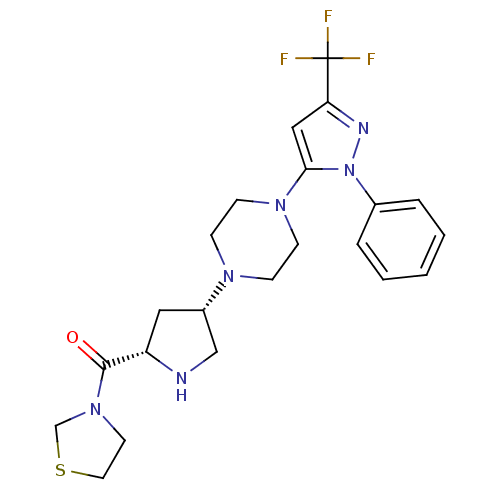 (CHEMBL2147709) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391567 (CHEMBL2147709) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50388112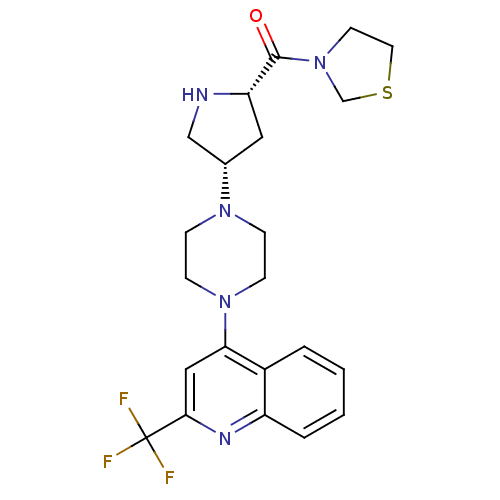 (CHEMBL2058971) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391565 (CHEMBL2147777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391570 (CHEMBL2147712) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Rattus norvegicus (rat)) | BDBM50391571 (CHEMBL2147713) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in rat plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (GUINEA PIG) | BDBM50287703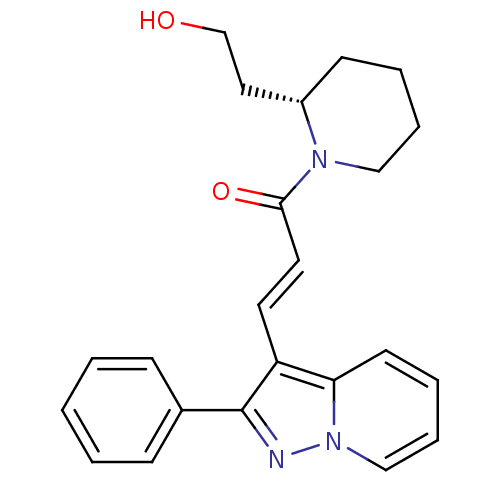 ((E)-1-[(S)-2-(2-Hydroxy-ethyl)-piperidin-1-yl]-3-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of adenosine induced negative inotropic activity against guinea-pig atria (Adenosine A1 receptor) | Bioorg Med Chem Lett 6: 2059-2062 (1996) Article DOI: 10.1016/0960-894X(96)00368-X BindingDB Entry DOI: 10.7270/Q25Q4W31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50391576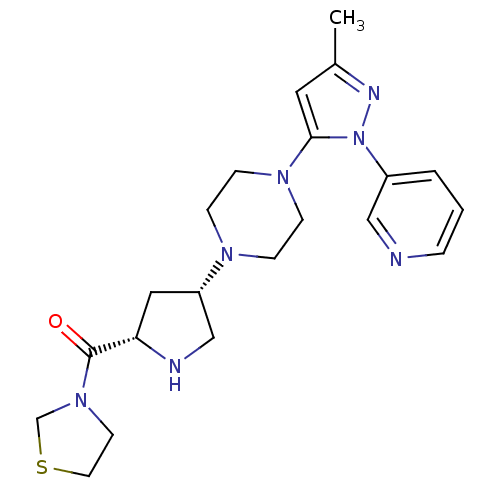 (CHEMBL2147773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Tanabe Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of DPP4 in human plasma using GLY-Pro-MCA as substrate after 60 mins by fluorescence assay | Bioorg Med Chem 20: 5705-19 (2012) Article DOI: 10.1016/j.bmc.2012.08.012 BindingDB Entry DOI: 10.7270/Q2JQ123C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 841 total ) | Next | Last >> |