

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265453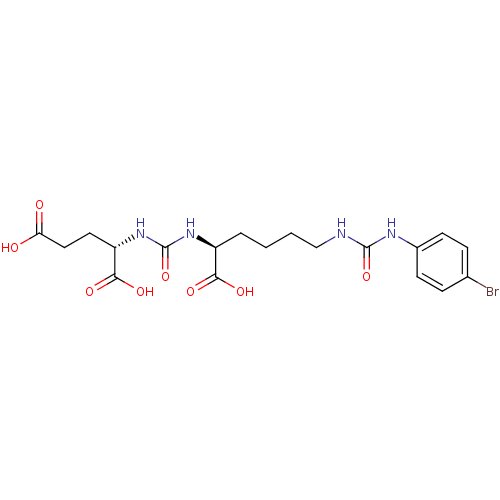 ((S)-2-(3-((S)-1-Carboxy-5-(3-(4-bromophenyl)ureido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265381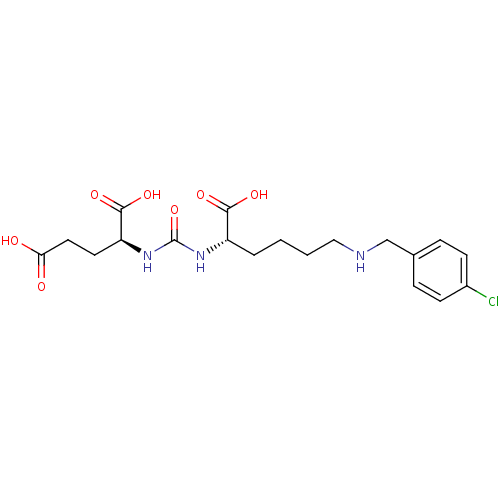 ((S)-2-(3-((S)-1-Carboxy-5-(4-chlorobenzylamino)pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265471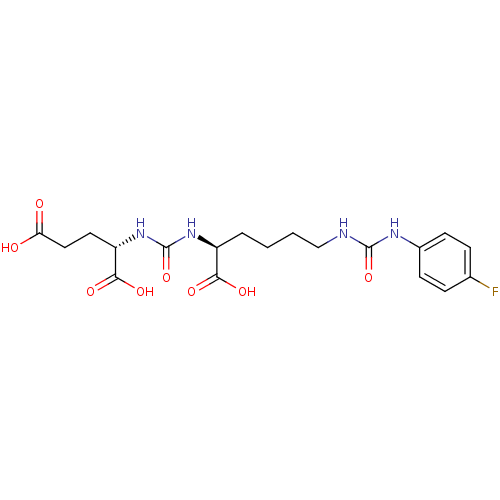 ((S)-2-(3-((S)-1-Carboxy-5-(3-(4-fluorophenyl)ureid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265454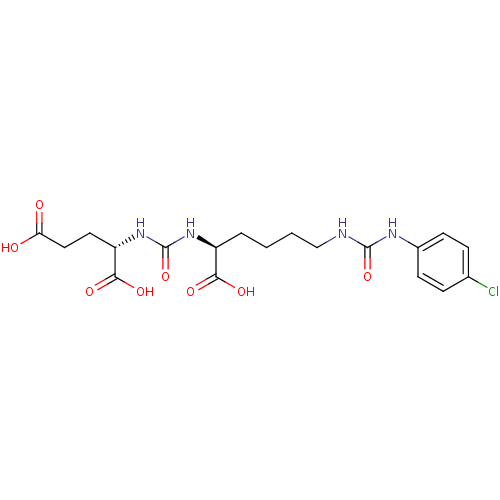 ((S)-2-(3-((S)-1-Carboxy-5-(3-(4-chlorophenyl)ureid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265783 ((S)-2-(3-((S)-1-Carboxy-5-(3-(4-iodophenyl)ureido)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265474 ((S)-2-(3-((S)-1-Carboxy-5-(4-iodophenylsulfonamido...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265472 ((S)-2-(3-((S)-1-Carboxy-5-(3-phenylureido)pentyl)u...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265473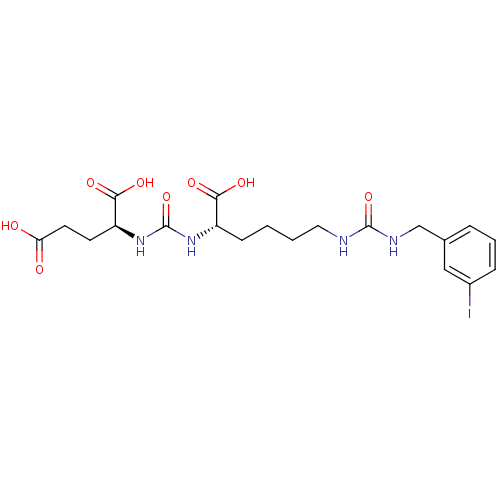 ((9S,13S)-1-(3-iodophenyl)-3,11-dioxo-2,4,10,12-tet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM103917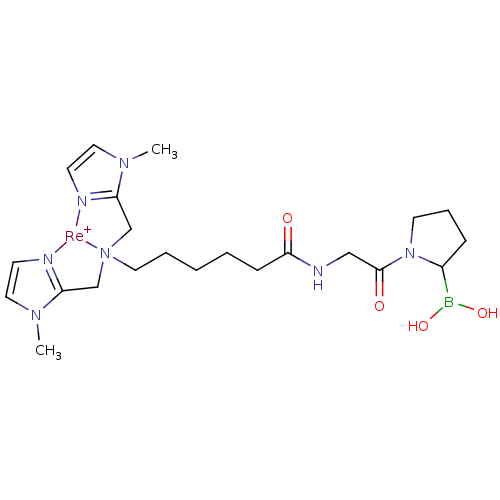 (US8562945, 253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit separase enzyme. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM103918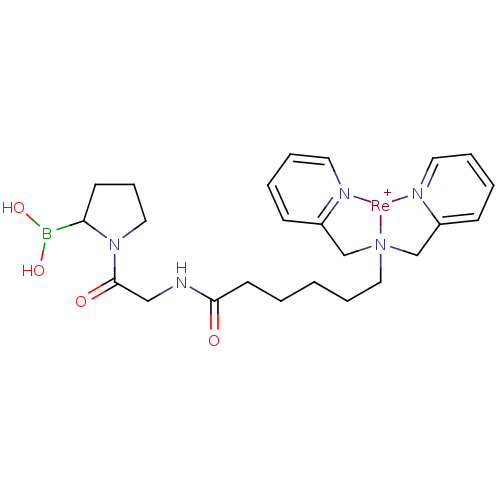 (US8562945, 254) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit separase enzyme. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265377 ((S)-2-(3-((S)-1-Carboxy-5-(4-iodobenzylamino)penty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103909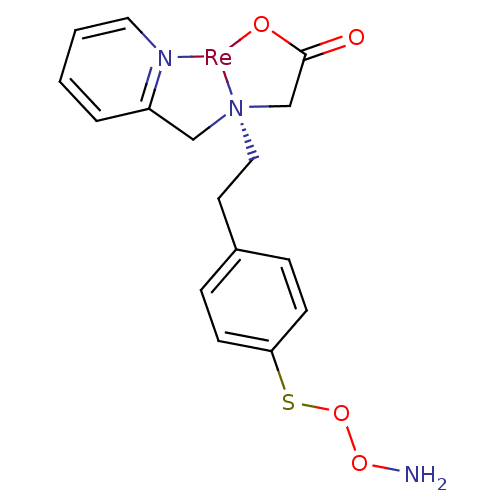 (JNK3 inhibitor 6 | US8562945, 242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265378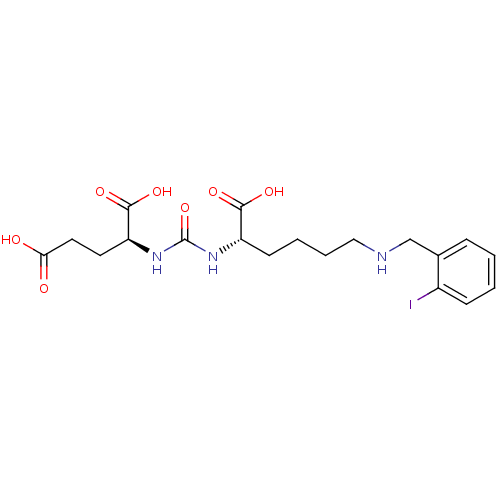 ((S)-2-(3-((S)-1-Carboxy-5-(2-iodobenzylamino)penty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103908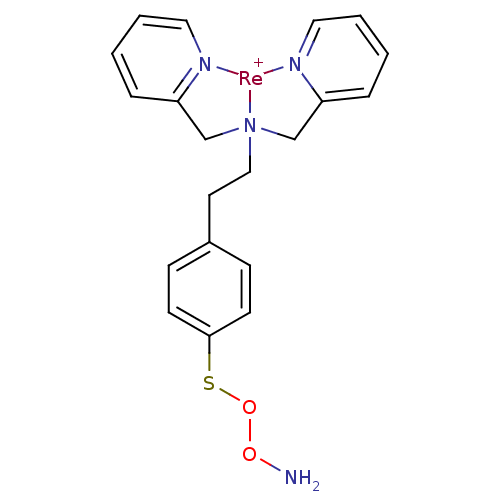 (JNK3 inhibitor 5 | US8562945, 241) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103911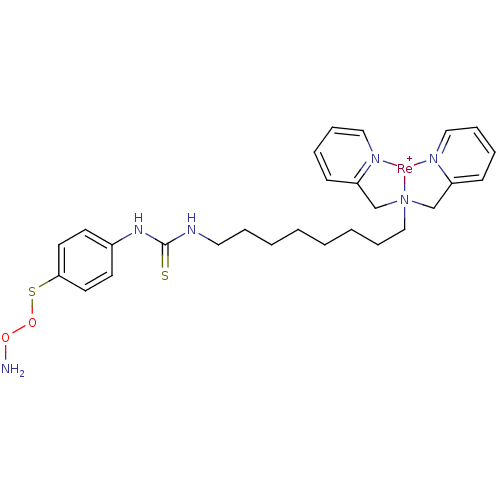 (JNK3 inhibitor 8 | US8562945, 246) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265380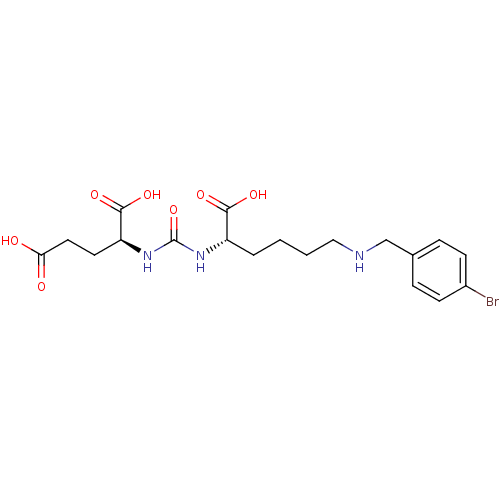 ((S)-2-(3-((S)-1-Carboxy-5-(4-bromobenzylamino)pent...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103915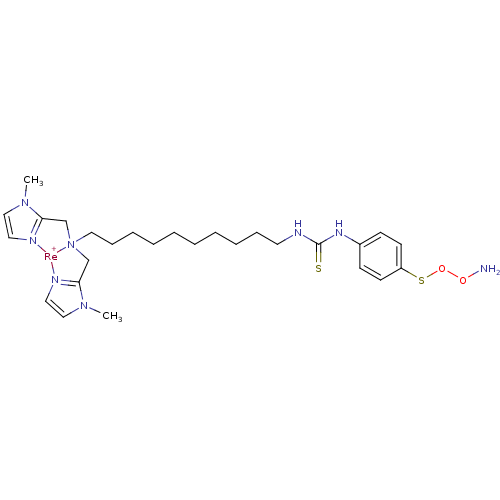 (US8562945, 250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM103911 (JNK3 inhibitor 8 | US8562945, 246) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265489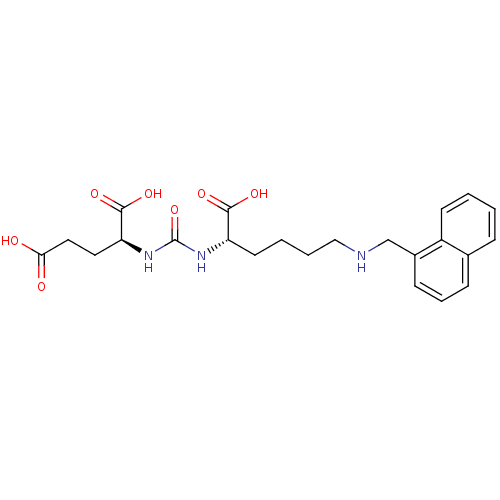 ((S)-2-(3-((S)-1-Carboxy-5-(naphthalen-1-ylmethylam...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 154 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM103909 (JNK3 inhibitor 6 | US8562945, 242) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103914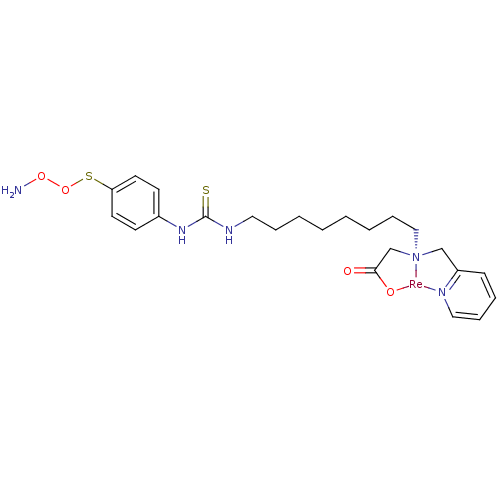 (US8562945, 249) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 189 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265391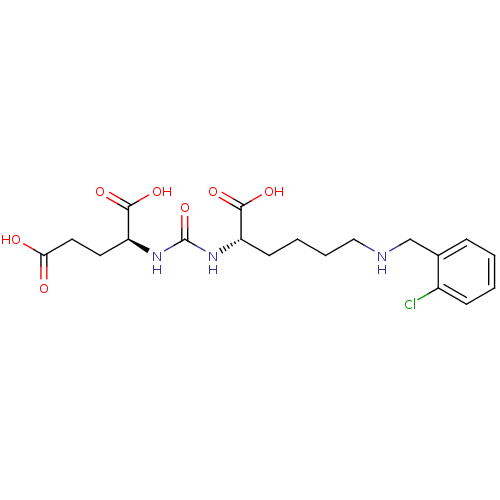 ((S)-2-(3-((S)-1-Carboxy-5-(2-chlorobenzylamino)pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 245 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103913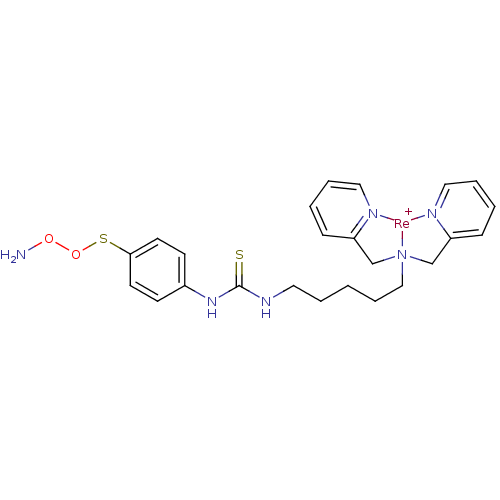 (US8562945, 248) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265451 ((S)-2-(3-((S)-1-Carboxy-5-(3-chlorobenzylamino)pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103912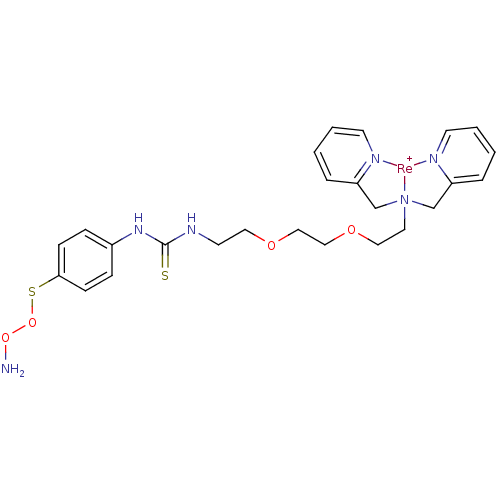 (US8562945, 247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 305 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM103914 (US8562945, 249) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 405 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265379 ((S)-2-(3-((S)-1-Carboxy-5-(3-iodobenzylamino)penty...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 443 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM103908 (JNK3 inhibitor 5 | US8562945, 241) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 445 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265784 ((S)-2-(3-((S)-5-Amino-1-carboxypentyl)ureido)penta...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 498 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM103910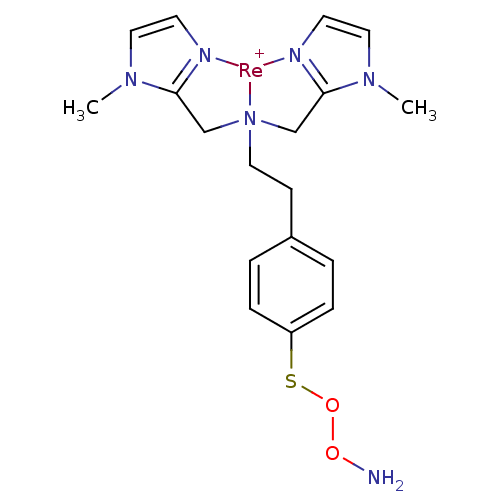 (JNK3 inhibitor 7 | US8562945, 243) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 564 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM103910 (JNK3 inhibitor 7 | US8562945, 243) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 652 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM103915 (US8562945, 250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 669 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM103913 (US8562945, 248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 770 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265452 ((S)-2-(3-((S)-1-Carboxy-5-(4-fluorobenzylamino)pen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM103912 (US8562945, 247) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit carbonic anhydrase isozymes II and IX in vito. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265782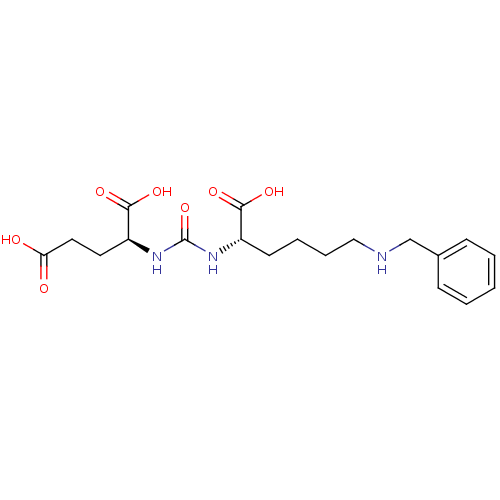 ((S)-2-(3-((S)-5-(Benzylamino)-1-carboxypentyl)urei...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate carboxypeptidase 2 (Homo sapiens (Human)) | BDBM50265785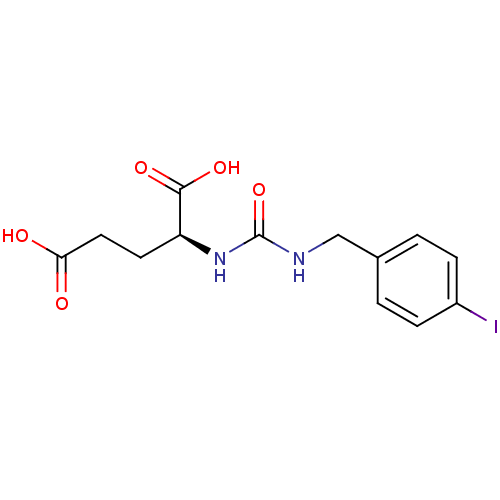 ((S)-2-(3-(4-iodobenzyl)ureido)pentanedioic acid | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of [131I]DCIT from PSMA in human LNCAP cells | J Med Chem 52: 347-57 (2009) Article DOI: 10.1021/jm800994j BindingDB Entry DOI: 10.7270/Q2V987ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM103916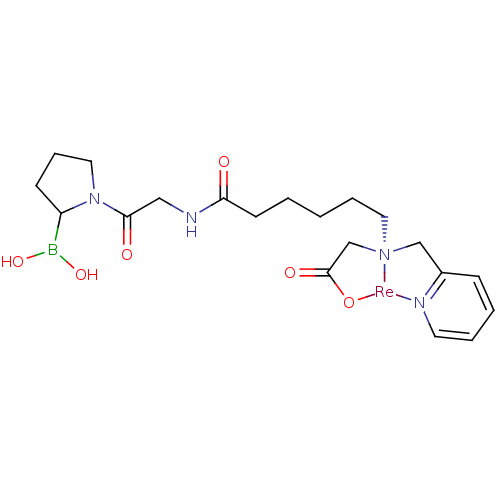 (US8562945, 252) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit separase enzyme. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM103917 (US8562945, 253) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit DPPIV enzyme. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM103916 (US8562945, 252) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit DPPIV enzyme. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM103918 (US8562945, 254) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Molecular Insight Pharmaceuticals, Inc. US Patent | Assay Description Compounds were tested for their ability to inhibit DPPIV enzyme. | US Patent US8562945 (2013) BindingDB Entry DOI: 10.7270/Q2XP73KD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||