 Found 130 hits with Last Name = 'carlino' and Initial = 'l'
Found 130 hits with Last Name = 'carlino' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50346552

(CHEMBL1797936)Show SMILES Cc1cc(-c2ccc(C=c3c(=C)[nH]n(-c4ccc(cc4)C(O)=O)c3=O)o2)c(cc1C)[N+]([O-])=O |w:8.7| Show InChI InChI=1S/C24H19N3O6/c1-13-10-20(21(27(31)32)11-14(13)2)22-9-8-18(33-22)12-19-15(3)25-26(23(19)28)17-6-4-16(5-7-17)24(29)30/h4-12,25H,3H2,1-2H3,(H,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Competitive inhibition of p300 HAT |
Bioorg Med Chem 19: 3605-15 (2011)
Article DOI: 10.1016/j.bmc.2011.01.029
BindingDB Entry DOI: 10.7270/Q2SN0992 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK1
(Homo sapiens (Human)) | BDBM50613682
(CHEMBL5276995)Show SMILES CC[C@H]1N(C(C)C)c2nc(Nc3ccc(cc3OC)C(=O)N[C@H]3CC[C@H](CC3)N3CCN(CC4CC4)CC3)ncc2N(C)C1=O |r,wU:25.29,22.22,2.1,(14.1,-1.92,;12.77,-2.69,;11.43,-1.92,;10.11,-2.69,;10.11,-4.23,;11.44,-5,;8.77,-5,;8.78,-1.92,;7.45,-2.7,;6.11,-1.93,;4.78,-2.7,;3.44,-1.93,;3.44,-.39,;2.11,.38,;.78,-.39,;.78,-1.93,;2.12,-2.69,;2.12,-4.23,;3.45,-5,;-.55,.38,;-.55,1.92,;-1.89,-.39,;-3.22,.38,;-4.55,-.39,;-5.89,.38,;-5.89,1.92,;-4.55,2.69,;-3.22,1.92,;-7.22,2.69,;-7.22,4.23,;-8.56,5,;-9.89,4.23,;-11.22,5,;-12.56,4.23,;-14.1,4.23,;-13.33,2.9,;-9.89,2.69,;-8.56,1.92,;6.11,-.39,;7.44,.38,;8.78,-.39,;10.1,.38,;10.1,1.92,;11.43,-.38,;12.76,.39,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50139171
(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM50139171

(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50246060
(CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM50139171

(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM50139171

(Dinaciclib | MK-7965 | SCH-727965 | US11643396, Ex...)Show SMILES CCc1cnn2c(NCc3ccc[n+]([O-])c3)cc(nc12)N1CCCC[C@H]1CCO |r| Show InChI InChI=1S/C21H28N6O2/c1-2-17-14-23-27-19(22-13-16-6-5-9-25(29)15-16)12-20(24-21(17)27)26-10-4-3-7-18(26)8-11-28/h5-6,9,12,14-15,18,22,28H,2-4,7-8,10-11,13H2,1H3/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK2
(Homo sapiens (Human)) | BDBM50613682

(CHEMBL5276995)Show SMILES CC[C@H]1N(C(C)C)c2nc(Nc3ccc(cc3OC)C(=O)N[C@H]3CC[C@H](CC3)N3CCN(CC4CC4)CC3)ncc2N(C)C1=O |r,wU:25.29,22.22,2.1,(14.1,-1.92,;12.77,-2.69,;11.43,-1.92,;10.11,-2.69,;10.11,-4.23,;11.44,-5,;8.77,-5,;8.78,-1.92,;7.45,-2.7,;6.11,-1.93,;4.78,-2.7,;3.44,-1.93,;3.44,-.39,;2.11,.38,;.78,-.39,;.78,-1.93,;2.12,-2.69,;2.12,-4.23,;3.45,-5,;-.55,.38,;-.55,1.92,;-1.89,-.39,;-3.22,.38,;-4.55,-.39,;-5.89,.38,;-5.89,1.92,;-4.55,2.69,;-3.22,1.92,;-7.22,2.69,;-7.22,4.23,;-8.56,5,;-9.89,4.23,;-11.22,5,;-12.56,4.23,;-14.1,4.23,;-13.33,2.9,;-9.89,2.69,;-8.56,1.92,;6.11,-.39,;7.44,.38,;8.78,-.39,;10.1,.38,;10.1,1.92,;11.43,-.38,;12.76,.39,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50332294
(CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(...)Show SMILES Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N6O3S/c1-20-19-28-26(30-21-10-12-23(13-11-21)36-17-16-33-14-5-6-15-33)31-25(20)29-22-8-7-9-24(18-22)37(34,35)32-27(2,3)4/h7-13,18-19,32H,5-6,14-17H2,1-4H3,(H2,28,29,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PDB
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM192755
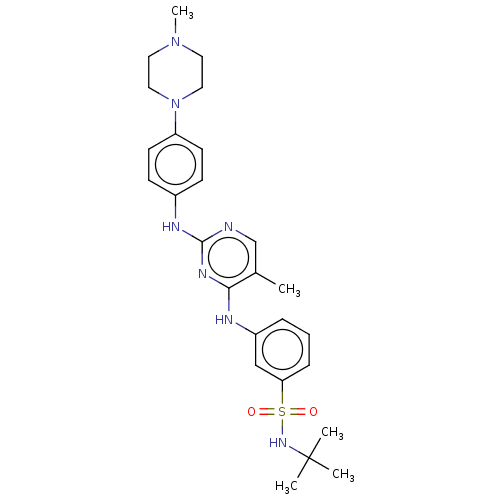
(TG101209 | US11279703, TABLE 6.171 | US11643396, E...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(C)c(Nc3cccc(c3)S(=O)(=O)NC(C)(C)C)n2)cc1 Show InChI InChI=1S/C26H35N7O2S/c1-19-18-27-25(29-20-9-11-22(12-10-20)33-15-13-32(5)14-16-33)30-24(19)28-21-7-6-8-23(17-21)36(34,35)31-26(2,3)4/h6-12,17-18,31H,13-16H2,1-5H3,(H2,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM26479
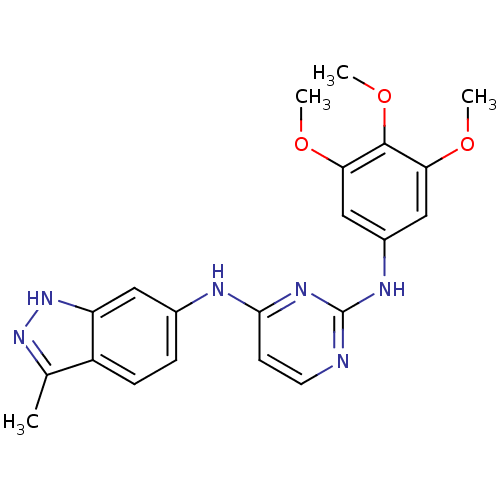
(4-N-(3-methyl-1H-indazol-6-yl)-2-N-(3,4,5-trimetho...)Show SMILES COc1cc(Nc2nccc(Nc3ccc4c(C)n[nH]c4c3)n2)cc(OC)c1OC Show InChI InChI=1S/C21H22N6O3/c1-12-15-6-5-13(9-16(15)27-26-12)23-19-7-8-22-21(25-19)24-14-10-17(28-2)20(30-4)18(11-14)29-3/h5-11H,1-4H3,(H,26,27)(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50613680
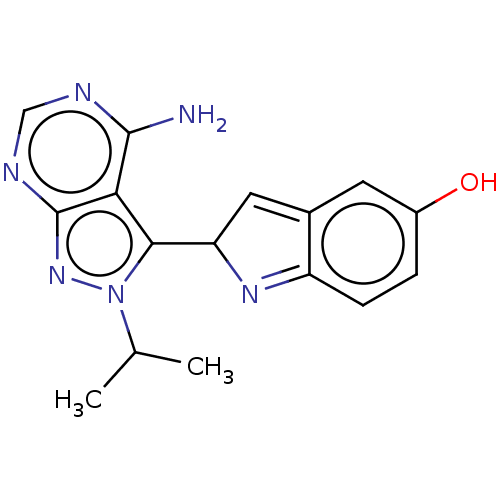
(CHEMBL5285509)Show SMILES CC(C)n1nc2ncnc(N)c2c1C1C=c2cc(O)ccc2=N1 |c:24,t:16| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
DNA-dependent protein kinase catalytic subunit
(Homo sapiens (Human)) | BDBM50427453
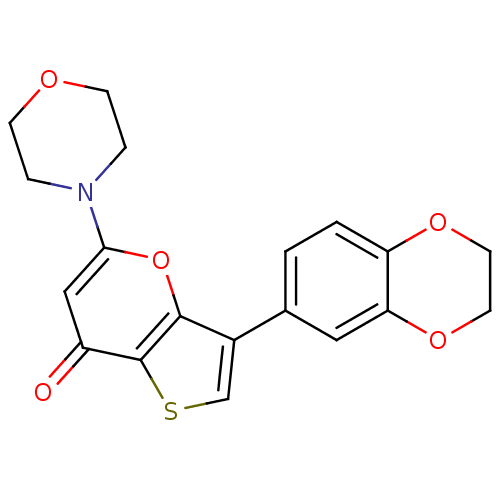
(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University
Curated by ChEMBL
| Assay Description
Inhibition of horse serum BChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method |
Bioorg Med Chem 21: 4928-37 (2013)
Article DOI: 10.1016/j.bmc.2013.06.065
BindingDB Entry DOI: 10.7270/Q21837XT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein-tyrosine kinase 2-beta
(Homo sapiens (Human)) | BDBM50246060

(CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...)Show SMILES CN(c1ccccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H21F3N6O3S/c1-31(35(2,33)34)18-6-4-3-5-13(18)11-26-20-16(22(23,24)25)12-27-21(30-20)28-15-7-8-17-14(9-15)10-19(32)29-17/h3-9,12H,10-11H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase alpha-6
(Homo sapiens (Human)) | BDBM25017
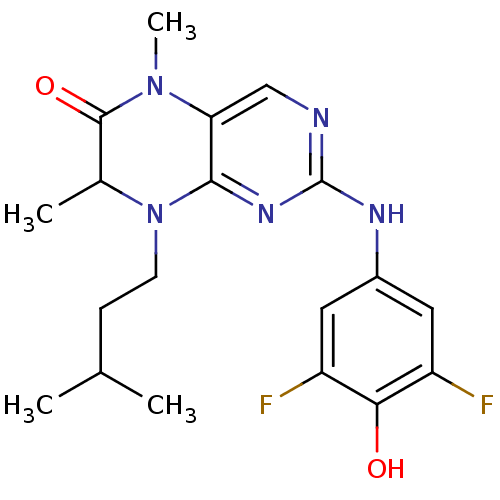
(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099349
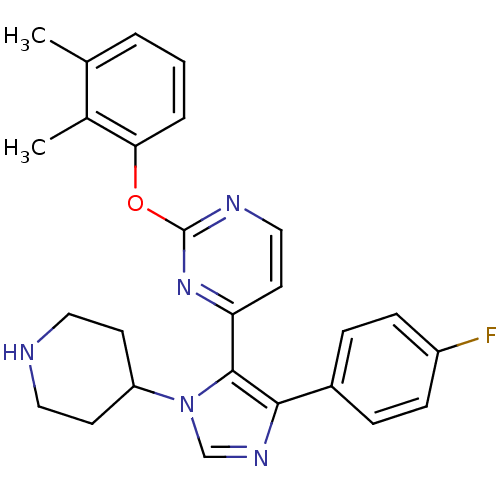
(2-(2,3-Dimethyl-phenoxy)-4-[5-(4-fluoro-phenyl)-3-...)Show SMILES Cc1cccc(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)c1C Show InChI InChI=1S/C26H26FN5O/c1-17-4-3-5-23(18(17)2)33-26-29-15-12-22(31-26)25-24(19-6-8-20(27)9-7-19)30-16-32(25)21-10-13-28-14-11-21/h3-9,12,15-16,21,28H,10-11,13-14H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50300041
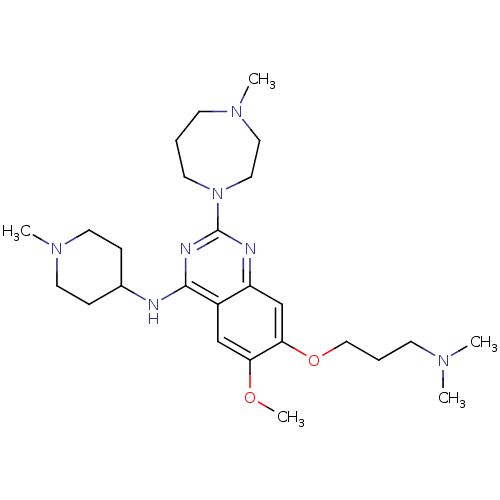
(7-(3-(dimethylamino)propoxy)-6-methoxy-2-(4-methyl...)Show SMILES COc1cc2c(NC3CCN(C)CC3)nc(nc2cc1OCCCN(C)C)N1CCCN(C)CC1 Show InChI InChI=1S/C26H43N7O2/c1-30(2)10-7-17-35-24-19-22-21(18-23(24)34-5)25(27-20-8-13-32(4)14-9-20)29-26(28-22)33-12-6-11-31(3)15-16-33/h18-20H,6-17H2,1-5H3,(H,27,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of lysine methyltransferase G9a |
Bioorg Med Chem 19: 3605-15 (2011)
Article DOI: 10.1016/j.bmc.2011.01.029
BindingDB Entry DOI: 10.7270/Q2SN0992 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50099349

(2-(2,3-Dimethyl-phenoxy)-4-[5-(4-fluoro-phenyl)-3-...)Show SMILES Cc1cccc(Oc2nccc(n2)-c2c(ncn2C2CCNCC2)-c2ccc(F)cc2)c1C Show InChI InChI=1S/C26H26FN5O/c1-17-4-3-5-23(18(17)2)33-26-29-15-12-22(31-26)25-24(19-6-8-20(27)9-7-19)30-16-32(25)21-10-13-28-14-11-21/h3-9,12,15-16,21,28H,10-11,13-14H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM50431381
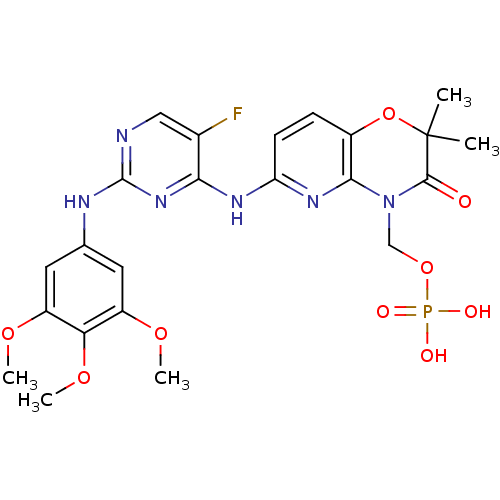
(FOSTAMATINIB | R-788 Free acid | R-935788 Free aci...)Show SMILES COc1cc(Nc2ncc(F)c(Nc3ccc4OC(C)(C)C(=O)N(COP(O)(O)=O)c4n3)n2)cc(OC)c1OC Show InChI InChI=1S/C23H26FN6O9P/c1-23(2)21(31)30(11-38-40(32,33)34)20-14(39-23)6-7-17(28-20)27-19-13(24)10-25-22(29-19)26-12-8-15(35-3)18(37-5)16(9-12)36-4/h6-10H,11H2,1-5H3,(H2,32,33,34)(H2,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM192755

(TG101209 | US11279703, TABLE 6.171 | US11643396, E...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(C)c(Nc3cccc(c3)S(=O)(=O)NC(C)(C)C)n2)cc1 Show InChI InChI=1S/C26H35N7O2S/c1-19-18-27-25(29-20-9-11-22(12-10-20)33-15-13-32(5)14-16-33)30-24(19)28-21-7-6-8-23(17-21)36(34,35)31-26(2,3)4/h6-12,17-18,31H,13-16H2,1-5H3,(H2,27,28,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50332294

(CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(...)Show SMILES Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N6O3S/c1-20-19-28-26(30-21-10-12-23(13-11-21)36-17-16-33-14-5-6-15-33)31-25(20)29-22-8-7-9-24(18-22)37(34,35)32-27(2,3)4/h7-13,18-19,32H,5-6,14-17H2,1-4H3,(H2,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-2
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-2
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-3
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50099334
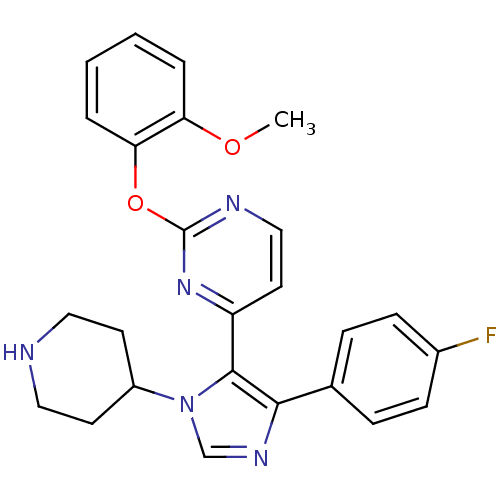
(4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...)Show SMILES COc1ccccc1Oc1nccc(n1)-c1c(ncn1C1CCNCC1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H24FN5O2/c1-32-21-4-2-3-5-22(21)33-25-28-15-12-20(30-25)24-23(17-6-8-18(26)9-7-17)29-16-31(24)19-10-13-27-14-11-19/h2-9,12,15-16,19,27H,10-11,13-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50099334

(4-[5-(4-Fluoro-phenyl)-3-piperidin-4-yl-3H-imidazo...)Show SMILES COc1ccccc1Oc1nccc(n1)-c1c(ncn1C1CCNCC1)-c1ccc(F)cc1 Show InChI InChI=1S/C25H24FN5O2/c1-32-21-4-2-3-5-22(21)33-25-28-15-12-20(30-25)24-23(17-6-8-18(26)9-7-17)29-16-31(24)19-10-13-27-14-11-19/h2-9,12,15-16,19,27H,10-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50332294

(CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(...)Show SMILES Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N6O3S/c1-20-19-28-26(30-21-10-12-23(13-11-21)36-17-16-33-14-5-6-15-33)31-25(20)29-22-8-7-9-24(18-22)37(34,35)32-27(2,3)4/h7-13,18-19,32H,5-6,14-17H2,1-4H3,(H2,28,29,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50427457

(CHEMBL2326952 | US10308662, Compound 25 | US950578...)Show SMILES CCOC(=O)c1ccc(cc1)-c1csc2c1oc(cc2=O)N1CCOCC1 Show InChI InChI=1S/C20H19NO5S/c1-2-25-20(23)14-5-3-13(4-6-14)15-12-27-19-16(22)11-17(26-18(15)19)21-7-9-24-10-8-21/h3-6,11-12H,2,7-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 alpha
(Homo sapiens (Human)) | BDBM8246
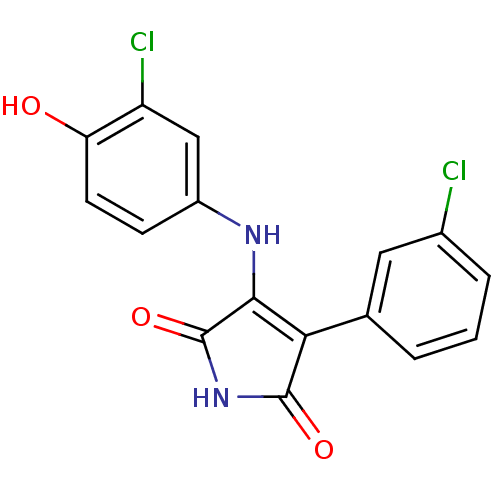
(3-[(3-chloro-4-hydroxyphenyl)amino]-4-(3-chlorophe...)Show SMILES Oc1ccc(NC2=C(C(=O)NC2=O)c2cccc(Cl)c2)cc1Cl |t:6| Show InChI InChI=1S/C16H10Cl2N2O3/c17-9-3-1-2-8(6-9)13-14(16(23)20-15(13)22)19-10-4-5-12(21)11(18)7-10/h1-7,21H,(H2,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM5655

(2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...)Show SMILES CN1CC[C@@H]([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl |r| Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12-,17+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-1
(Homo sapiens (Human)) | BDBM25017

(2-[(3,5-difluoro-4-hydroxyphenyl)amino]-5,7-dimeth...)Show SMILES CC(C)CCN1C(C)C(=O)N(C)c2cnc(Nc3cc(F)c(O)c(F)c3)nc12 Show InChI InChI=1S/C19H23F2N5O2/c1-10(2)5-6-26-11(3)18(28)25(4)15-9-22-19(24-17(15)26)23-12-7-13(20)16(27)14(21)8-12/h7-11,27H,5-6H2,1-4H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Mus musculus (mouse)) | BDBM50365463

(CHEMBL1232461)Show SMILES CCNC(=O)C[C@@H]1N=C(c2ccc(Cl)cc2)c2cc(OC)ccc2-n2c(C)nnc12 |r,t:7| Show InChI InChI=1S/C22H22ClN5O2/c1-4-24-20(29)12-18-22-27-26-13(2)28(22)19-10-9-16(30-3)11-17(19)21(25-18)14-5-7-15(23)8-6-14/h5-11,18H,4,12H2,1-3H3,(H,24,29)/t18-/m0/s1 | MMDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50427453

(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein
(Homo sapiens (Human)) | BDBM50365262

((+)-JQ1 | (S)-JQ1 (1) | CHEMBL1957266 | JQ1 | US10...)Show SMILES Cc1nnc2[C@H](CC(=O)OC(C)(C)C)N=C(c3c(C)c(C)sc3-n12)c1ccc(Cl)cc1 |r,c:14| Show InChI InChI=1S/C23H25ClN4O2S/c1-12-13(2)31-22-19(12)20(15-7-9-16(24)10-8-15)25-17(11-18(29)30-23(4,5)6)21-27-26-14(3)28(21)22/h7-10,17H,11H2,1-6H3/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50613683
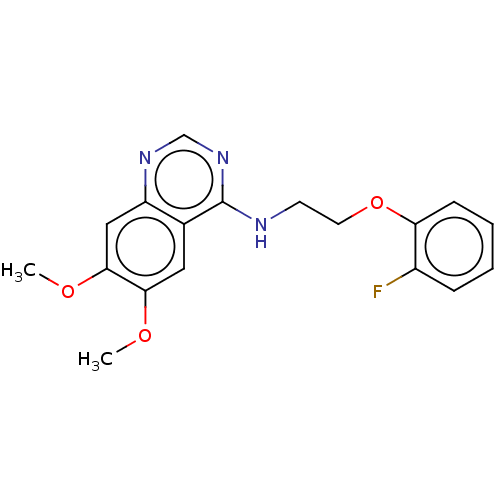
(CHEMBL5270270) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50044784
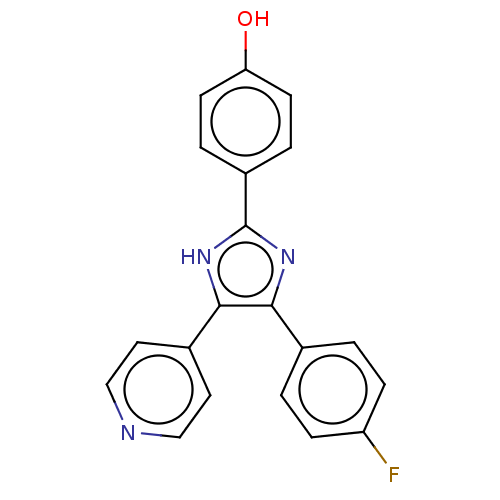
(CHEBI:79090 | SB-202190 | US10865384, Compound SB2...)Show SMILES Oc1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H14FN3O/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(25)8-4-15/h1-12,25H,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase PLK3
(Homo sapiens (Human)) | BDBM50613682

(CHEMBL5276995)Show SMILES CC[C@H]1N(C(C)C)c2nc(Nc3ccc(cc3OC)C(=O)N[C@H]3CC[C@H](CC3)N3CCN(CC4CC4)CC3)ncc2N(C)C1=O |r,wU:25.29,22.22,2.1,(14.1,-1.92,;12.77,-2.69,;11.43,-1.92,;10.11,-2.69,;10.11,-4.23,;11.44,-5,;8.77,-5,;8.78,-1.92,;7.45,-2.7,;6.11,-1.93,;4.78,-2.7,;3.44,-1.93,;3.44,-.39,;2.11,.38,;.78,-.39,;.78,-1.93,;2.12,-2.69,;2.12,-4.23,;3.45,-5,;-.55,.38,;-.55,1.92,;-1.89,-.39,;-3.22,.38,;-4.55,-.39,;-5.89,.38,;-5.89,1.92,;-4.55,2.69,;-3.22,1.92,;-7.22,2.69,;-7.22,4.23,;-8.56,5,;-9.89,4.23,;-11.22,5,;-12.56,4.23,;-14.1,4.23,;-13.33,2.9,;-9.89,2.69,;-8.56,1.92,;6.11,-.39,;7.44,.38,;8.78,-.39,;10.1,.38,;10.1,1.92,;11.43,-.38,;12.76,.39,)| | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Ege University
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 5 mins before substrate addition by Ellman's method |
Bioorg Med Chem 21: 4928-37 (2013)
Article DOI: 10.1016/j.bmc.2013.06.065
BindingDB Entry DOI: 10.7270/Q21837XT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8246

(3-[(3-chloro-4-hydroxyphenyl)amino]-4-(3-chlorophe...)Show SMILES Oc1ccc(NC2=C(C(=O)NC2=O)c2cccc(Cl)c2)cc1Cl |t:6| Show InChI InChI=1S/C16H10Cl2N2O3/c17-9-3-1-2-8(6-9)13-14(16(23)20-15(13)22)19-10-4-5-12(21)11(18)7-10/h1-7,21H,(H2,19,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50044784

(CHEBI:79090 | SB-202190 | US10865384, Compound SB2...)Show SMILES Oc1ccc(cc1)-c1nc(c([nH]1)-c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C20H14FN3O/c21-16-5-1-13(2-6-16)18-19(14-9-11-22-12-10-14)24-20(23-18)15-3-7-17(25)8-4-15/h1-12,25H,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50613680

(CHEMBL5285509)Show SMILES CC(C)n1nc2ncnc(N)c2c1C1C=c2cc(O)ccc2=N1 |c:24,t:16| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM5655

(2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...)Show SMILES CN1CC[C@@H]([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl |r| Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EHMT2
(Homo sapiens (Human)) | BDBM50346553
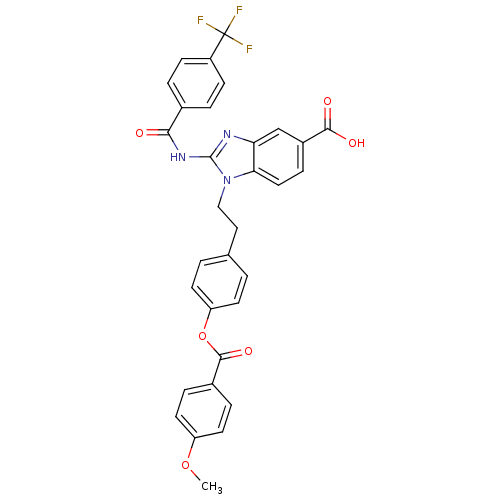
(CHEMBL1797937)Show SMILES COc1ccc(cc1)C(=O)Oc1ccc(CCn2c(NC(=O)c3ccc(cc3)C(F)(F)F)nc3cc(ccc23)C(O)=O)cc1 Show InChI InChI=1S/C32H24F3N3O6/c1-43-24-13-6-21(7-14-24)30(42)44-25-11-2-19(3-12-25)16-17-38-27-15-8-22(29(40)41)18-26(27)36-31(38)37-28(39)20-4-9-23(10-5-20)32(33,34)35/h2-15,18H,16-17H2,1H3,(H,40,41)(H,36,37,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University of Halle-Wittenberg
Curated by ChEMBL
| Assay Description
Inhibition of lysine methyltransferase G9a |
Bioorg Med Chem 19: 3605-15 (2011)
Article DOI: 10.1016/j.bmc.2011.01.029
BindingDB Entry DOI: 10.7270/Q2SN0992 |
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein
(Homo sapiens (Human)) | BDBM192755

(TG101209 | US11279703, TABLE 6.171 | US11643396, E...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(C)c(Nc3cccc(c3)S(=O)(=O)NC(C)(C)C)n2)cc1 Show InChI InChI=1S/C26H35N7O2S/c1-19-18-27-25(29-20-9-11-22(12-10-20)33-15-13-32(5)14-16-33)30-24(19)28-21-7-6-8-23(17-21)36(34,35)31-26(2,3)4/h6-12,17-18,31H,13-16H2,1-5H3,(H2,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50427453

(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM192755

(TG101209 | US11279703, TABLE 6.171 | US11643396, E...)Show SMILES CN1CCN(CC1)c1ccc(Nc2ncc(C)c(Nc3cccc(c3)S(=O)(=O)NC(C)(C)C)n2)cc1 Show InChI InChI=1S/C26H35N7O2S/c1-19-18-27-25(29-20-9-11-22(12-10-20)33-15-13-32(5)14-16-33)30-24(19)28-21-7-6-8-23(17-21)36(34,35)31-26(2,3)4/h6-12,17-18,31H,13-16H2,1-5H3,(H2,27,28,29,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| | n/a | n/a | 169 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM5655

(2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydr...)Show SMILES CN1CC[C@@H]([C@H](O)C1)c1c(O)cc(O)c2c1oc(cc2=O)-c1ccccc1Cl |r| Show InChI InChI=1S/C21H20ClNO5/c1-23-7-6-12(17(27)10-23)19-14(24)8-15(25)20-16(26)9-18(28-21(19)20)11-4-2-3-5-13(11)22/h2-5,8-9,12,17,24-25,27H,6-7,10H2,1H3/t12-,17+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50427453

(CHEMBL2322228 | US10308662, Compound 28 | US950578...)Show InChI InChI=1S/C19H17NO5S/c21-14-10-17(20-3-5-22-6-4-20)25-18-13(11-26-19(14)18)12-1-2-15-16(9-12)24-8-7-23-15/h1-2,9-11H,3-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Bromodomain testis-specific protein
(Homo sapiens (Human)) | BDBM50332294

(CHEMBL1287853 | N-tert-butyl-3-(5-methyl-2-(4-(2-(...)Show SMILES Cc1cnc(Nc2ccc(OCCN3CCCC3)cc2)nc1Nc1cccc(c1)S(=O)(=O)NC(C)(C)C Show InChI InChI=1S/C27H36N6O3S/c1-20-19-28-26(30-21-10-12-23(13-11-21)36-17-16-33-14-5-6-15-33)31-25(20)29-22-8-7-9-24(18-22)37(34,35)32-27(2,3)4/h7-13,18-19,32H,5-6,14-17H2,1-4H3,(H2,28,29,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data