 Found 58 hits with Last Name = 'cosentino' and Initial = 'l'
Found 58 hits with Last Name = 'cosentino' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tubulin beta chain
(Sus scrofa) | BDBM50485941

(CHEMBL2181004)Show SMILES COc1ccc(CN[C@H]2CCc3cc(OC)c(OC)c(OC)c3-c3ccc(OC)c(=O)cc23)cc1 |r| Show InChI InChI=1S/C28H31NO6/c1-31-19-9-6-17(7-10-19)16-29-22-12-8-18-14-25(33-3)27(34-4)28(35-5)26(18)20-11-13-24(32-2)23(30)15-21(20)22/h6-7,9-11,13-15,22,29H,8,12,16H2,1-5H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485945

(CHEMBL2181003)Show SMILES COc1cc2CC[C@H](NCc3ccc(cc3)[N+]([O-])=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28N2O7/c1-33-23-12-10-19-20(14-22(23)30)21(28-15-16-5-8-18(9-6-16)29(31)32)11-7-17-13-24(34-2)26(35-3)27(36-4)25(17)19/h5-6,8-10,12-14,21,28H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485942

(CHEMBL2181002)Show SMILES COc1cc2CC[C@H](NCc3cc(F)c(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H26F3NO5/c1-33-22-8-6-16-17(12-21(22)32)20(31-13-14-9-18(28)25(30)19(29)10-14)7-5-15-11-23(34-2)26(35-3)27(36-4)24(15)16/h6,8-12,20,31H,5,7,13H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485950
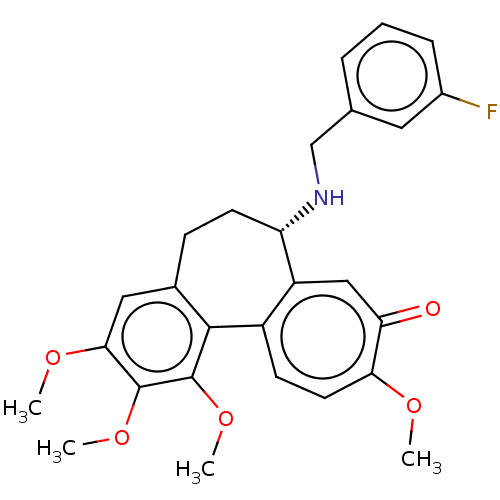
(CHEMBL2181009)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-11-9-19-20(14-22(23)30)21(29-15-16-6-5-7-18(28)12-16)10-8-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-7,9,11-14,21,29H,8,10,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485944

(CHEMBL2181006)Show SMILES COc1cc2CC[C@H](NCc3ccc(Cl)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28ClNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485943
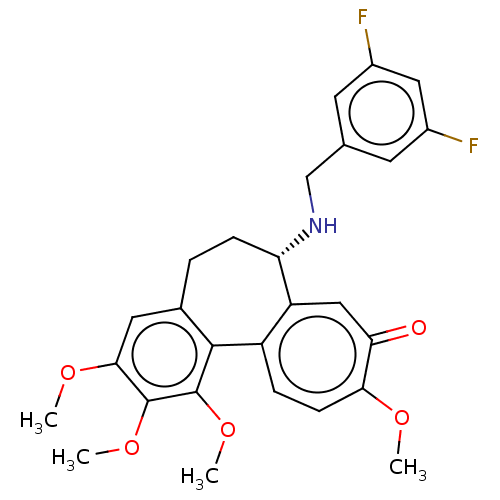
(CHEMBL2181001)Show SMILES COc1cc2CC[C@H](NCc3cc(F)cc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-8-6-19-20(13-22(23)31)21(30-14-15-9-17(28)12-18(29)10-15)7-5-16-11-24(33-2)26(34-3)27(35-4)25(16)19/h6,8-13,21,30H,5,7,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485946
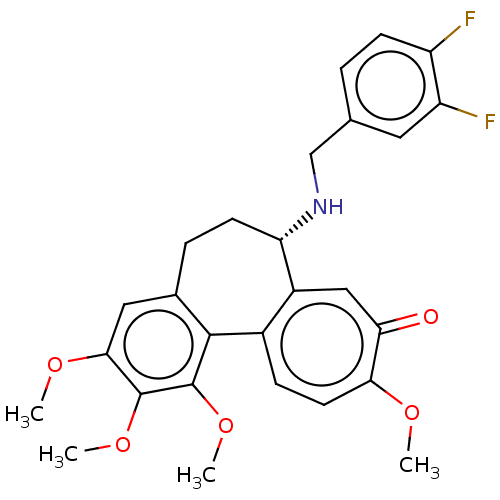
(CHEMBL2181000)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-10-7-17-18(13-22(23)31)21(30-14-15-5-8-19(28)20(29)11-15)9-6-16-12-24(33-2)26(34-3)27(35-4)25(16)17/h5,7-8,10-13,21,30H,6,9,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485947
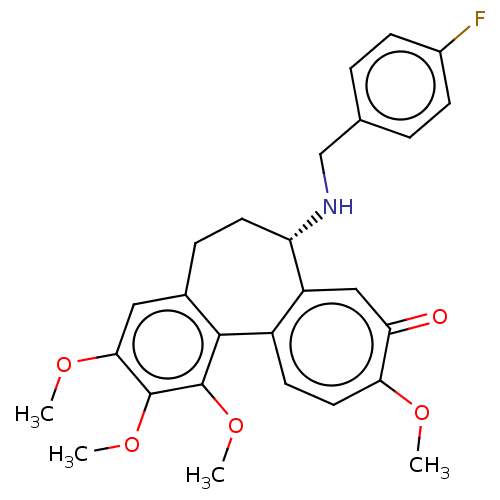
(CHEMBL2181008)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485949

(CHEMBL2180999)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3F)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-22-11-9-17-18(13-21(22)31)20(30-14-16-6-5-7-19(28)25(16)29)10-8-15-12-23(33-2)26(34-3)27(35-4)24(15)17/h5-7,9,11-13,20,30H,8,10,14H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485948
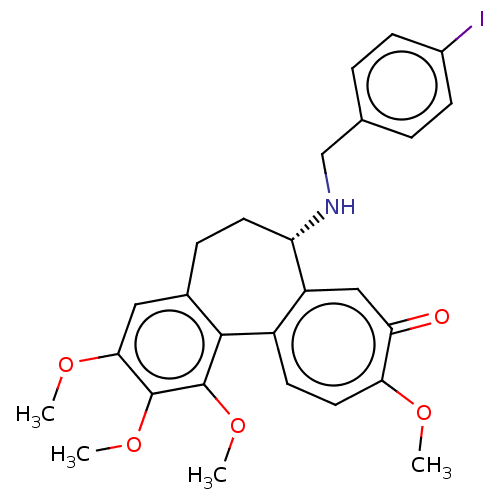
(CHEMBL2181007)Show SMILES COc1cc2CC[C@H](NCc3ccc(I)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28INO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485940

(CHEMBL2181005)Show SMILES COc1cc2CC[C@H](NCc3ccc(Br)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28BrNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50014846
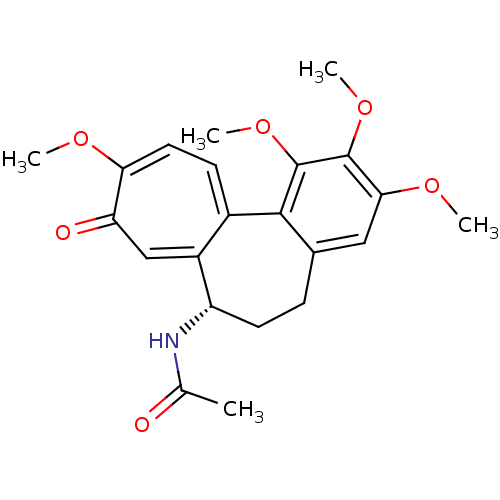
((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50030567
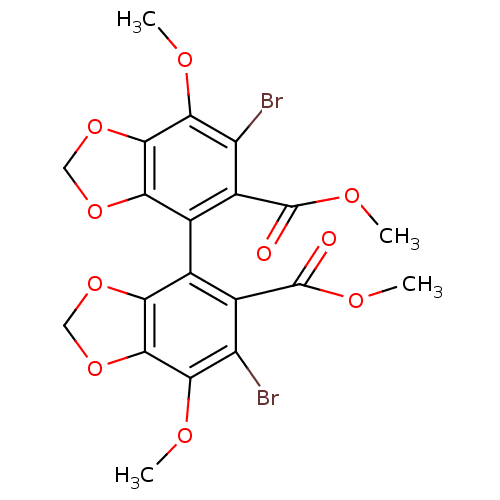
(6,6'-Dibromo-7,7'-dimethoxy-[4,4']bi[benzo[1,3]dio...)Show SMILES COC(=O)c1c(Br)c(OC)c2OCOc2c1-c1c2OCOc2c(OC)c(Br)c1C(=O)OC |(8.03,-5.65,;6.87,-4.77,;5.41,-5.35,;5.47,-6.63,;4.11,-5.09,;4.11,-3.55,;5.44,-2.78,;2.78,-2.78,;2.78,-1.24,;4.11,-.47,;1.45,-3.55,;-.02,-3.06,;-.93,-4.32,;-.02,-5.58,;1.45,-5.09,;2.78,-5.86,;2.78,-7.4,;1.45,-8.17,;-.02,-7.7,;-.93,-8.94,;-.02,-10.2,;1.45,-9.71,;2.78,-10.48,;2.78,-12.02,;1.45,-12.79,;4.11,-9.71,;5.46,-10.48,;4.11,-8.17,;5.65,-8.17,;6.42,-6.84,;6.42,-9.5,;7.96,-9.5,)| Show InChI InChI=1S/C20H16Br2O10/c1-25-15-11(21)9(19(23)27-3)7(13-17(15)31-5-29-13)8-10(20(24)28-4)12(22)16(26-2)18-14(8)30-6-32-18/h5-6H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill 27599
Curated by ChEMBL
| Assay Description
IC50 value of the compound was measured as DNA dependent DNA polymerase associated activity by using 0.05 units of radiolabeled template poly(rA)-oli... |
J Med Chem 38: 3003-8 (1995)
BindingDB Entry DOI: 10.7270/Q23J3C0R |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50030566
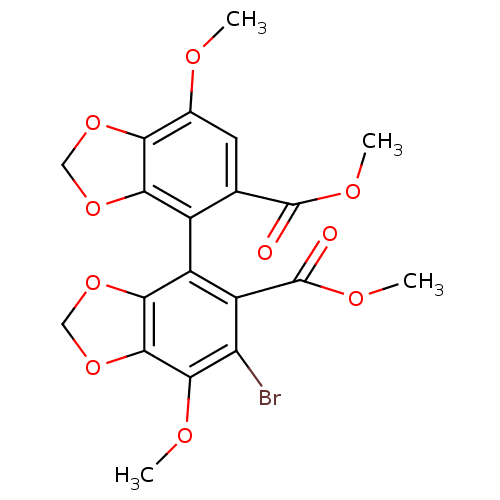
(6-Bromo-7,7'-dimethoxy-[4,4']bi[benzo[1,3]dioxolyl...)Show SMILES COC(=O)c1cc(OC)c2OCOc2c1-c1c2OCOc2c(OC)c(Br)c1C(=O)OC |(7.96,-9.5,;6.42,-9.5,;5.65,-8.17,;6.42,-6.84,;4.11,-8.17,;4.11,-9.71,;2.78,-10.48,;2.78,-12.02,;1.45,-12.79,;1.45,-9.71,;-.02,-10.2,;-.93,-8.94,;-.02,-7.7,;1.45,-8.17,;2.78,-7.4,;2.78,-5.86,;1.45,-5.09,;-.02,-5.58,;-.93,-4.32,;-.02,-3.06,;1.45,-3.55,;2.78,-2.78,;2.78,-1.24,;4.11,-.47,;4.11,-3.55,;5.44,-2.78,;4.11,-5.09,;5.41,-5.35,;5.47,-6.63,;6.87,-4.77,;8.03,-5.65,)| Show InChI InChI=1S/C20H17BrO10/c1-24-9-5-8(19(22)26-3)10(15-14(9)28-6-29-15)11-12(20(23)27-4)13(21)17(25-2)18-16(11)30-7-31-18/h5H,6-7H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill 27599
Curated by ChEMBL
| Assay Description
IC50 value of the compound was measured as DNA dependent DNA polymerase associated activity by using 0.05 units of radiolabeled template poly(rA)-oli... |
J Med Chem 38: 3003-8 (1995)
BindingDB Entry DOI: 10.7270/Q23J3C0R |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485950

(CHEMBL2181009)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28FNO5/c1-31-23-11-9-19-20(14-22(23)30)21(29-15-16-6-5-7-18(28)12-16)10-8-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-7,9,11-14,21,29H,8,10,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin polymerization measured every 1 mins of 60 mins by fluorescence assay |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485946

(CHEMBL2181000)Show SMILES COc1cc2CC[C@H](NCc3ccc(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-10-7-17-18(13-22(23)31)21(30-14-15-5-8-19(28)20(29)11-15)9-6-16-12-24(33-2)26(34-3)27(35-4)25(16)17/h5,7-8,10-13,21,30H,6,9,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin polymerization measured every 1 mins of 60 mins by fluorescence assay |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485949

(CHEMBL2180999)Show SMILES COc1cc2CC[C@H](NCc3cccc(F)c3F)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-22-11-9-17-18(13-21(22)31)20(30-14-16-6-5-7-19(28)25(16)29)10-8-15-12-23(33-2)26(34-3)27(35-4)24(15)17/h5-7,9,11-13,20,30H,8,10,14H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin polymerization measured every 1 mins of 60 mins by fluorescence assay |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485944

(CHEMBL2181006)Show SMILES COc1cc2CC[C@H](NCc3ccc(Cl)cc3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28ClNO5/c1-31-23-12-10-19-20(14-22(23)30)21(29-15-16-5-8-18(28)9-6-16)11-7-17-13-24(32-2)26(33-3)27(34-4)25(17)19/h5-6,8-10,12-14,21,29H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin polymerization measured every 1 mins of 60 mins by fluorescence assay |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50030567

(6,6'-Dibromo-7,7'-dimethoxy-[4,4']bi[benzo[1,3]dio...)Show SMILES COC(=O)c1c(Br)c(OC)c2OCOc2c1-c1c2OCOc2c(OC)c(Br)c1C(=O)OC |(8.03,-5.65,;6.87,-4.77,;5.41,-5.35,;5.47,-6.63,;4.11,-5.09,;4.11,-3.55,;5.44,-2.78,;2.78,-2.78,;2.78,-1.24,;4.11,-.47,;1.45,-3.55,;-.02,-3.06,;-.93,-4.32,;-.02,-5.58,;1.45,-5.09,;2.78,-5.86,;2.78,-7.4,;1.45,-8.17,;-.02,-7.7,;-.93,-8.94,;-.02,-10.2,;1.45,-9.71,;2.78,-10.48,;2.78,-12.02,;1.45,-12.79,;4.11,-9.71,;5.46,-10.48,;4.11,-8.17,;5.65,-8.17,;6.42,-6.84,;6.42,-9.5,;7.96,-9.5,)| Show InChI InChI=1S/C20H16Br2O10/c1-25-15-11(21)9(19(23)27-3)7(13-17(15)31-5-29-13)8-10(20(24)28-4)12(22)16(26-2)18-14(8)30-6-32-18/h5-6H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill 27599
Curated by ChEMBL
| Assay Description
IC50 value of the compound was measured as RNA dependent DNA polymerase associated activity by using 10e-3 units of radiolabeled template poly(rA)-ol... |
J Med Chem 38: 3003-8 (1995)
BindingDB Entry DOI: 10.7270/Q23J3C0R |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485945

(CHEMBL2181003)Show SMILES COc1cc2CC[C@H](NCc3ccc(cc3)[N+]([O-])=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H28N2O7/c1-33-23-12-10-19-20(14-22(23)30)21(28-15-16-5-8-18(9-6-16)29(31)32)11-7-17-13-24(34-2)26(35-3)27(36-4)25(17)19/h5-6,8-10,12-14,21,28H,7,11,15H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin polymerization measured every 1 mins of 60 mins by fluorescence assay |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin alpha-1A chain
(Sus scrofa (Pig)) | BDBM50470858
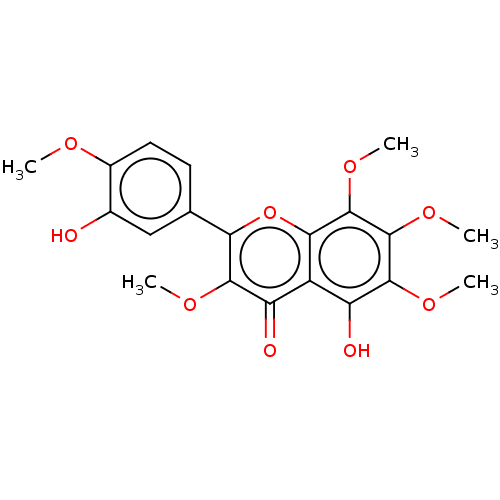
(CHEMBL18351)Show SMILES COc1ccc(cc1O)-c1oc2c(OC)c(OC)c(OC)c(O)c2c(=O)c1OC Show InChI InChI=1S/C20H20O9/c1-24-11-7-6-9(8-10(11)21)15-17(25-2)13(22)12-14(23)18(26-3)20(28-5)19(27-4)16(12)29-15/h6-8,21,23H,1-5H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against tubulin polymerization |
J Med Chem 39: 1975-80 (1996)
Article DOI: 10.1021/jm960008c
BindingDB Entry DOI: 10.7270/Q2MC92R3 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50030566

(6-Bromo-7,7'-dimethoxy-[4,4']bi[benzo[1,3]dioxolyl...)Show SMILES COC(=O)c1cc(OC)c2OCOc2c1-c1c2OCOc2c(OC)c(Br)c1C(=O)OC |(7.96,-9.5,;6.42,-9.5,;5.65,-8.17,;6.42,-6.84,;4.11,-8.17,;4.11,-9.71,;2.78,-10.48,;2.78,-12.02,;1.45,-12.79,;1.45,-9.71,;-.02,-10.2,;-.93,-8.94,;-.02,-7.7,;1.45,-8.17,;2.78,-7.4,;2.78,-5.86,;1.45,-5.09,;-.02,-5.58,;-.93,-4.32,;-.02,-3.06,;1.45,-3.55,;2.78,-2.78,;2.78,-1.24,;4.11,-.47,;4.11,-3.55,;5.44,-2.78,;4.11,-5.09,;5.41,-5.35,;5.47,-6.63,;6.87,-4.77,;8.03,-5.65,)| Show InChI InChI=1S/C20H17BrO10/c1-24-9-5-8(19(22)26-3)10(15-14(9)28-6-29-15)11-12(20(23)27-4)13(21)17(25-2)18-16(11)30-7-31-18/h5H,6-7H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill 27599
Curated by ChEMBL
| Assay Description
IC50 value of the compound was measured as RNA dependent DNA polymerase associated activity by using 1*10e-3 units of radiolabeled template poly(rC)-... |
J Med Chem 38: 3003-8 (1995)
BindingDB Entry DOI: 10.7270/Q23J3C0R |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485943

(CHEMBL2181001)Show SMILES COc1cc2CC[C@H](NCc3cc(F)cc(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H27F2NO5/c1-32-23-8-6-19-20(13-22(23)31)21(30-14-15-9-17(28)12-18(29)10-15)7-5-16-11-24(33-2)26(34-3)27(35-4)25(16)19/h6,8-13,21,30H,5,7,14H2,1-4H3/t21-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin polymerization measured every 1 mins of 60 mins by fluorescence assay |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50030566

(6-Bromo-7,7'-dimethoxy-[4,4']bi[benzo[1,3]dioxolyl...)Show SMILES COC(=O)c1cc(OC)c2OCOc2c1-c1c2OCOc2c(OC)c(Br)c1C(=O)OC |(7.96,-9.5,;6.42,-9.5,;5.65,-8.17,;6.42,-6.84,;4.11,-8.17,;4.11,-9.71,;2.78,-10.48,;2.78,-12.02,;1.45,-12.79,;1.45,-9.71,;-.02,-10.2,;-.93,-8.94,;-.02,-7.7,;1.45,-8.17,;2.78,-7.4,;2.78,-5.86,;1.45,-5.09,;-.02,-5.58,;-.93,-4.32,;-.02,-3.06,;1.45,-3.55,;2.78,-2.78,;2.78,-1.24,;4.11,-.47,;4.11,-3.55,;5.44,-2.78,;4.11,-5.09,;5.41,-5.35,;5.47,-6.63,;6.87,-4.77,;8.03,-5.65,)| Show InChI InChI=1S/C20H17BrO10/c1-24-9-5-8(19(22)26-3)10(15-14(9)28-6-29-15)11-12(20(23)27-4)13(21)17(25-2)18-16(11)30-7-31-18/h5H,6-7H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill 27599
Curated by ChEMBL
| Assay Description
IC50 value of the compound was measured as RNA dependent DNA polymerase associated activity by using 1*10e-3 units of radiolabeled template poly(rA)-... |
J Med Chem 38: 3003-8 (1995)
BindingDB Entry DOI: 10.7270/Q23J3C0R |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485942

(CHEMBL2181002)Show SMILES COc1cc2CC[C@H](NCc3cc(F)c(F)c(F)c3)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C27H26F3NO5/c1-33-22-8-6-16-17(12-21(22)32)20(31-13-14-9-18(28)25(30)19(29)10-14)7-5-15-11-23(34-2)26(35-3)27(36-4)24(15)16/h6,8-12,20,31H,5,7,13H2,1-4H3/t20-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin polymerization measured every 1 mins of 60 mins by fluorescence assay |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50485941

(CHEMBL2181004)Show SMILES COc1ccc(CN[C@H]2CCc3cc(OC)c(OC)c(OC)c3-c3ccc(OC)c(=O)cc23)cc1 |r| Show InChI InChI=1S/C28H31NO6/c1-31-19-9-6-17(7-10-19)16-29-22-12-8-18-14-25(33-3)27(34-4)28(35-5)26(18)20-11-13-24(32-2)23(30)15-21(20)22/h6-7,9-11,13-15,22,29H,8,12,16H2,1-5H3/t22-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin polymerization measured every 1 mins of 60 mins by fluorescence assay |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | |
Tubulin beta chain
(Sus scrofa) | BDBM50014846

((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford
Curated by ChEMBL
| Assay Description
Binding affinity to pig brain tubulin polymerization measured every 1 mins of 60 mins by fluorescence assay |
J Med Chem 55: 11062-6 (2012)
Article DOI: 10.1021/jm301151t
BindingDB Entry DOI: 10.7270/Q22V2K0N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50030567

(6,6'-Dibromo-7,7'-dimethoxy-[4,4']bi[benzo[1,3]dio...)Show SMILES COC(=O)c1c(Br)c(OC)c2OCOc2c1-c1c2OCOc2c(OC)c(Br)c1C(=O)OC |(8.03,-5.65,;6.87,-4.77,;5.41,-5.35,;5.47,-6.63,;4.11,-5.09,;4.11,-3.55,;5.44,-2.78,;2.78,-2.78,;2.78,-1.24,;4.11,-.47,;1.45,-3.55,;-.02,-3.06,;-.93,-4.32,;-.02,-5.58,;1.45,-5.09,;2.78,-5.86,;2.78,-7.4,;1.45,-8.17,;-.02,-7.7,;-.93,-8.94,;-.02,-10.2,;1.45,-9.71,;2.78,-10.48,;2.78,-12.02,;1.45,-12.79,;4.11,-9.71,;5.46,-10.48,;4.11,-8.17,;5.65,-8.17,;6.42,-6.84,;6.42,-9.5,;7.96,-9.5,)| Show InChI InChI=1S/C20H16Br2O10/c1-25-15-11(21)9(19(23)27-3)7(13-17(15)31-5-29-13)8-10(20(24)28-4)12(22)16(26-2)18-14(8)30-6-32-18/h5-6H2,1-4H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill 27599
Curated by ChEMBL
| Assay Description
IC50 value of the compound was measured as RNA dependent DNA polymerase associated activity by using 10e-3 units of radiolabeled template poly(rC)-ol... |
J Med Chem 38: 3003-8 (1995)
BindingDB Entry DOI: 10.7270/Q23J3C0R |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50292433
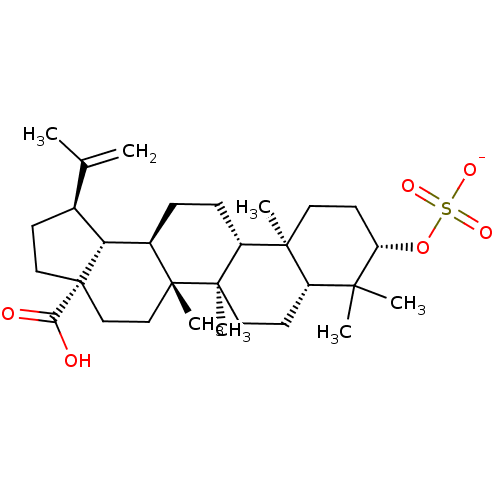
(CHEMBL502585 | betulinic acid 3-O-sulfonate potass...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OS([O-])(=O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C30H48O6S/c1-18(2)19-10-15-30(25(31)32)17-16-28(6)20(24(19)30)8-9-22-27(5)13-12-23(36-37(33,34)35)26(3,4)21(27)11-14-29(22,28)7/h19-24H,1,8-17H2,2-7H3,(H,31,32)(H,33,34,35)/p-1/t19-,20+,21-,22+,23-,24+,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC gamma |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C delta type
(Homo sapiens (Human)) | BDBM50292431
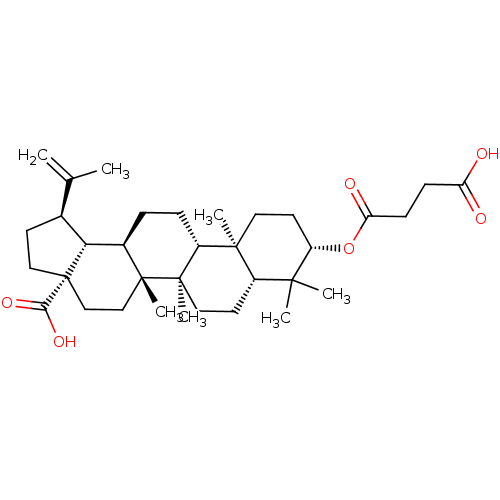
((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-(3-c...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)CCC(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C34H52O6/c1-20(2)21-12-17-34(29(38)39)19-18-32(6)22(28(21)34)8-9-24-31(5)15-14-25(40-27(37)11-10-26(35)36)30(3,4)23(31)13-16-33(24,32)7/h21-25,28H,1,8-19H2,2-7H3,(H,35,36)(H,38,39)/t21-,22+,23-,24+,25-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC delta |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM50292432
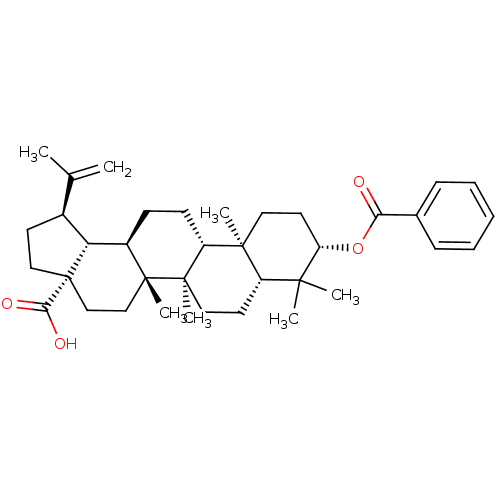
(Betulinic acid 3-O-benzoate | CHEMBL509553)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)c6ccccc6)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C37H52O4/c1-23(2)25-15-20-37(32(39)40)22-21-35(6)26(30(25)37)13-14-28-34(5)18-17-29(41-31(38)24-11-9-8-10-12-24)33(3,4)27(34)16-19-36(28,35)7/h8-12,25-30H,1,13-22H2,2-7H3,(H,39,40)/t25-,26+,27-,28+,29-,30+,34-,35+,36+,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC gamma |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292432

(Betulinic acid 3-O-benzoate | CHEMBL509553)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)c6ccccc6)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C37H52O4/c1-23(2)25-15-20-37(32(39)40)22-21-35(6)26(30(25)37)13-14-28-34(5)18-17-29(41-31(38)24-11-9-8-10-12-24)33(3,4)27(34)16-19-36(28,35)7/h8-12,25-30H,1,13-22H2,2-7H3,(H,39,40)/t25-,26+,27-,28+,29-,30+,34-,35+,36+,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292435
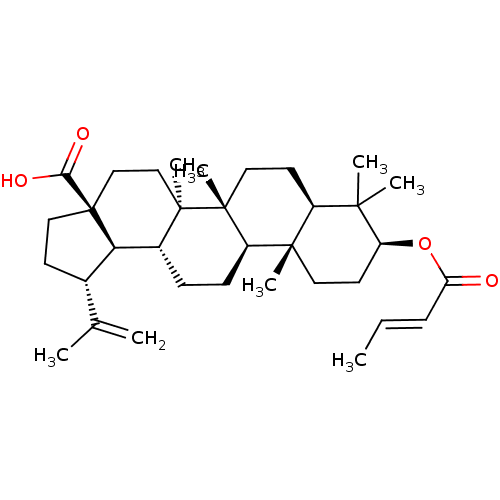
((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-((E)...)Show SMILES C\C=C\C(=O)O[C@H]1CC[C@@]2(C)[C@@H](CC[C@]3(C)[C@@H]2CC[C@@H]2[C@H]4[C@@H](CC[C@@]4(CC[C@@]32C)C(O)=O)C(C)=C)C1(C)C |r| Show InChI InChI=1S/C34H52O4/c1-9-10-27(35)38-26-15-16-31(6)24(30(26,4)5)14-17-33(8)25(31)12-11-23-28-22(21(2)3)13-18-34(28,29(36)37)20-19-32(23,33)7/h9-10,22-26,28H,2,11-20H2,1,3-8H3,(H,36,37)/b10-9+/t22-,23+,24-,25+,26-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292433

(CHEMBL502585 | betulinic acid 3-O-sulfonate potass...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OS([O-])(=O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C30H48O6S/c1-18(2)19-10-15-30(25(31)32)17-16-28(6)20(24(19)30)8-9-22-27(5)13-12-23(36-37(33,34)35)26(3,4)21(27)11-14-29(22,28)7/h19-24H,1,8-17H2,2-7H3,(H,31,32)(H,33,34,35)/p-1/t19-,20+,21-,22+,23-,24+,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292431

((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-(3-c...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)CCC(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C34H52O6/c1-20(2)21-12-17-34(29(38)39)19-18-32(6)22(28(21)34)8-9-24-31(5)15-14-25(40-27(37)11-10-26(35)36)30(3,4)23(31)13-16-33(24,32)7/h21-25,28H,1,8-19H2,2-7H3,(H,35,36)(H,38,39)/t21-,22+,23-,24+,25-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292432

(Betulinic acid 3-O-benzoate | CHEMBL509553)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)c6ccccc6)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C37H52O4/c1-23(2)25-15-20-37(32(39)40)22-21-35(6)26(30(25)37)13-14-28-34(5)18-17-29(41-31(38)24-11-9-8-10-12-24)33(3,4)27(34)16-19-36(28,35)7/h8-12,25-30H,1,13-22H2,2-7H3,(H,39,40)/t25-,26+,27-,28+,29-,30+,34-,35+,36+,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50103967
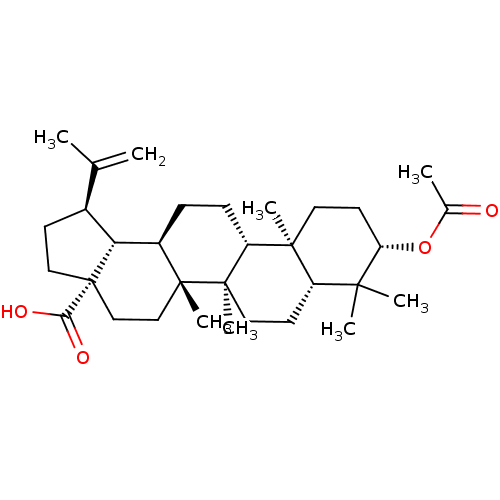
((3-O-acetyl)betulinic acid | 3-acetylbetulinic aci...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C32H50O4/c1-19(2)21-11-16-32(27(34)35)18-17-30(7)22(26(21)32)9-10-24-29(6)14-13-25(36-20(3)33)28(4,5)23(29)12-15-31(24,30)8/h21-26H,1,9-18H2,2-8H3,(H,34,35)/t21-,22+,23-,24+,25-,26+,29-,30+,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292434
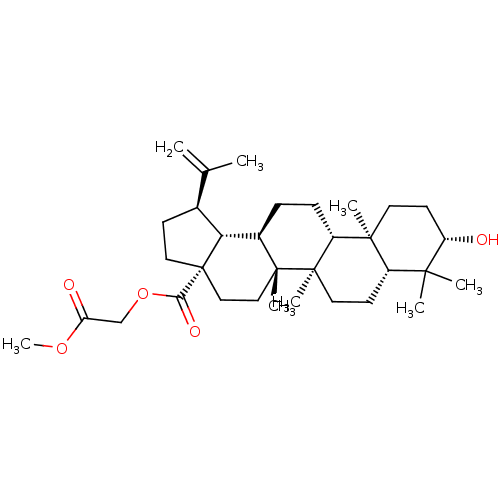
(CHEMBL509422 | betulinic acid 28-O-carboxymethylme...)Show SMILES COC(=O)COC(=O)[C@]12CC[C@H]([C@@H]1[C@H]1CC[C@@H]3[C@@]4(C)CC[C@H](O)C(C)(C)[C@@H]4CC[C@@]3(C)[C@]1(C)CC2)C(C)=C |r| Show InChI InChI=1S/C33H52O5/c1-20(2)21-11-16-33(28(36)38-19-26(35)37-8)18-17-31(6)22(27(21)33)9-10-24-30(5)14-13-25(34)29(3,4)23(30)12-15-32(24,31)7/h21-25,27,34H,1,9-19H2,2-8H3/t21-,22+,23-,24+,25-,27+,30-,31+,32+,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM23207
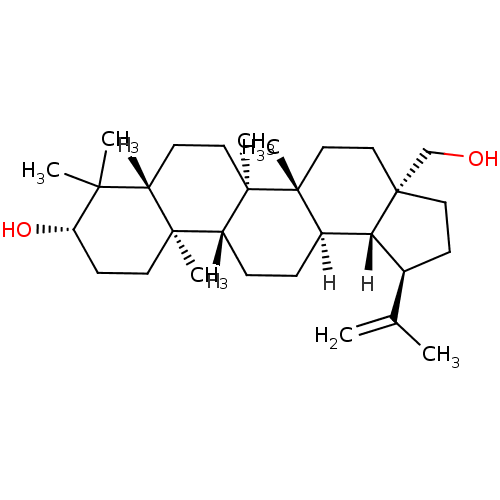
((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-5-(hydroxymet...)Show SMILES [H][C@]12[C@@H](CC[C@]1(CO)CC[C@]1(C)[C@]2([H])CC[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)[C@]3([H])CC[C@@]12C)C(C)=C Show InChI InChI=1S/C30H50O2/c1-19(2)20-10-15-30(18-31)17-16-28(6)21(25(20)30)8-9-23-27(5)13-12-24(32)26(3,4)22(27)11-14-29(23,28)7/h20-25,31-32H,1,8-18H2,2-7H3/t20-,21+,22-,23+,24-,25+,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50103962
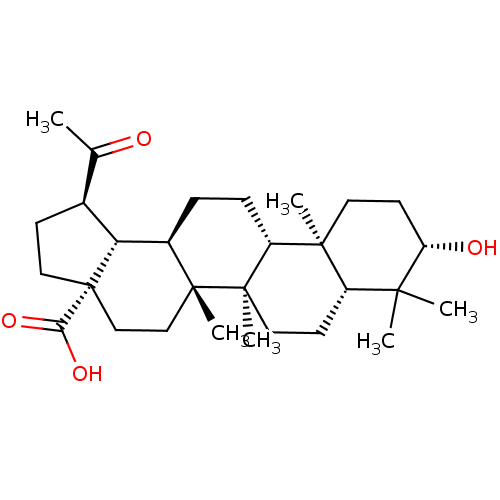
((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bS)-1-Acet...)Show SMILES CC(=O)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C29H46O4/c1-17(30)18-9-14-29(24(32)33)16-15-27(5)19(23(18)29)7-8-21-26(4)12-11-22(31)25(2,3)20(26)10-13-28(21,27)6/h18-23,31H,7-16H2,1-6H3,(H,32,33)/t18-,19+,20-,21+,22-,23+,26-,27+,28+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50103967

((3-O-acetyl)betulinic acid | 3-acetylbetulinic aci...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(C)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C32H50O4/c1-19(2)21-11-16-32(27(34)35)18-17-30(7)22(26(21)32)9-10-24-29(6)14-13-25(36-20(3)33)28(4,5)23(29)12-15-31(24,30)8/h21-26H,1,9-18H2,2-8H3,(H,34,35)/t21-,22+,23-,24+,25-,26+,29-,30+,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50292434

(CHEMBL509422 | betulinic acid 28-O-carboxymethylme...)Show SMILES COC(=O)COC(=O)[C@]12CC[C@H]([C@@H]1[C@H]1CC[C@@H]3[C@@]4(C)CC[C@H](O)C(C)(C)[C@@H]4CC[C@@]3(C)[C@]1(C)CC2)C(C)=C |r| Show InChI InChI=1S/C33H52O5/c1-20(2)21-11-16-33(28(36)38-19-26(35)37-8)18-17-31(6)22(27(21)33)9-10-24-30(5)14-13-25(34)29(3,4)23(30)12-15-32(24,31)7/h21-25,27,34H,1,9-19H2,2-8H3/t21-,22+,23-,24+,25-,27+,30-,31+,32+,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM23207

((1R,2R,5S,8R,9R,10R,13R,14R,17S,19R)-5-(hydroxymet...)Show SMILES [H][C@]12[C@@H](CC[C@]1(CO)CC[C@]1(C)[C@]2([H])CC[C@]2([H])[C@@]3(C)CC[C@H](O)C(C)(C)[C@]3([H])CC[C@@]12C)C(C)=C Show InChI InChI=1S/C30H50O2/c1-19(2)20-10-15-30(18-31)17-16-28(6)21(25(20)30)8-9-23-27(5)13-12-24(32)26(3,4)22(27)11-14-29(23,28)7/h20-25,31-32H,1,8-18H2,2-7H3/t20-,21+,22-,23+,24-,25+,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C epsilon type
(Homo sapiens (Human)) | BDBM50103962

((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bS)-1-Acet...)Show SMILES CC(=O)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C29H46O4/c1-17(30)18-9-14-29(24(32)33)16-15-27(5)19(23(18)29)7-8-21-26(4)12-11-22(31)25(2,3)20(26)10-13-28(21,27)6/h18-23,31H,7-16H2,1-6H3,(H,32,33)/t18-,19+,20-,21+,22-,23+,26-,27+,28+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC epsilon |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50002692

((AZT) 1-(4-Azido-5-hydroxymethyl-tetrahydro-furan-...)Show SMILES Cc1cn([C@H]2C[C@H](N=[N+]=[N-])[C@@H](CO)O2)c(=O)[nH]c1=O |r| Show InChI InChI=1S/C10H13N5O4/c1-5-3-15(10(18)12-9(5)17)8-2-6(13-14-11)7(4-16)19-8/h3,6-8,16H,2,4H2,1H3,(H,12,17,18)/t6-,7+,8+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292435

((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-((E)...)Show SMILES C\C=C\C(=O)O[C@H]1CC[C@@]2(C)[C@@H](CC[C@]3(C)[C@@H]2CC[C@@H]2[C@H]4[C@@H](CC[C@@]4(CC[C@@]32C)C(O)=O)C(C)=C)C1(C)C |r| Show InChI InChI=1S/C34H52O4/c1-9-10-27(35)38-26-15-16-31(6)24(30(26,4)5)14-17-33(8)25(31)12-11-23-28-22(21(2)3)13-18-34(28,29(36)37)20-19-32(23,33)7/h9-10,22-26,28H,2,11-20H2,1,3-8H3,(H,36,37)/b10-9+/t22-,23+,24-,25+,26-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292433

(CHEMBL502585 | betulinic acid 3-O-sulfonate potass...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OS([O-])(=O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C30H48O6S/c1-18(2)19-10-15-30(25(31)32)17-16-28(6)20(24(19)30)8-9-22-27(5)13-12-23(36-37(33,34)35)26(3,4)21(27)11-14-29(22,28)7/h19-24H,1,8-17H2,2-7H3,(H,31,32)(H,33,34,35)/p-1/t19-,20+,21-,22+,23-,24+,27-,28+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50292431

((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-(3-c...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)CCC(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O |r| Show InChI InChI=1S/C34H52O6/c1-20(2)21-12-17-34(29(38)39)19-18-32(6)22(28(21)34)8-9-24-31(5)15-14-25(40-27(37)11-10-26(35)36)30(3,4)23(31)13-16-33(24,32)7/h21-25,28H,1,8-19H2,2-7H3,(H,35,36)(H,38,39)/t21-,22+,23-,24+,25-,28+,31-,32+,33+,34-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PKC beta2 |
J Nat Prod 57: 243-247 (1994)
Article DOI: 10.1021/np50104a008
BindingDB Entry DOI: 10.7270/Q2TT4R0Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50050957

((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-(4-c...)Show SMILES CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC[C@H](OC(=O)CC(C)(C)CC(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)C(O)=O Show InChI InChI=1S/C37H58O6/c1-22(2)23-12-17-37(31(41)42)19-18-35(8)24(30(23)37)10-11-26-34(7)15-14-27(33(5,6)25(34)13-16-36(26,35)9)43-29(40)21-32(3,4)20-28(38)39/h23-27,30H,1,10-21H2,2-9H3,(H,38,39)(H,41,42)/t23-,24+,25-,26+,27-,30+,34-,35+,36+,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.67E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against HIV-RT |
J Med Chem 39: 1016-7 (1996)
Article DOI: 10.1021/jm950922q
BindingDB Entry DOI: 10.7270/Q2PN94QV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data