

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM21842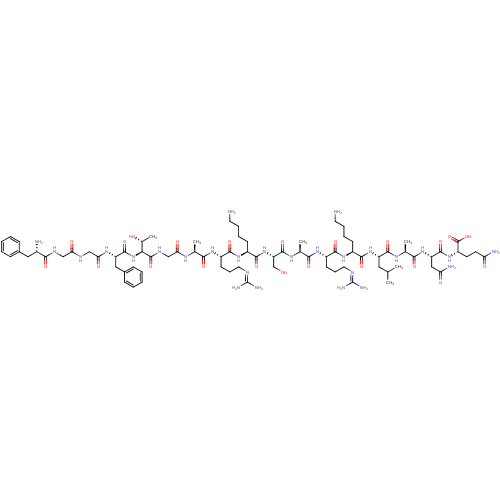 ((2S)-2-[(2S)-2-[(2S)-2-[(2S)-2-[(2S)-6-amino-2-[(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human nociceptin (NOP) receptor using [3H]N/OFQ as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137564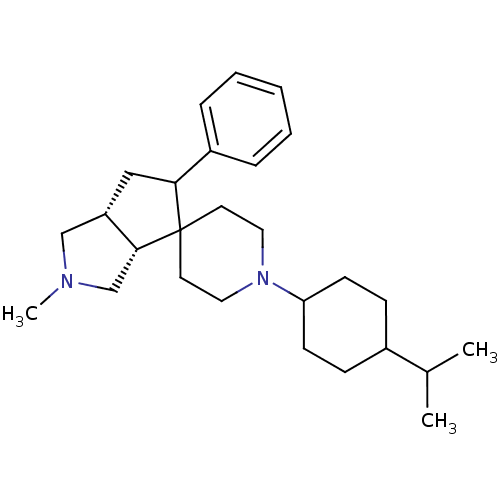 (1'-(4-isopropylcyclohexyl)-2-methyl-5-phenyl-(3aR,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human nociceptin (NOP) receptor using [3H]N/OFQ as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50137565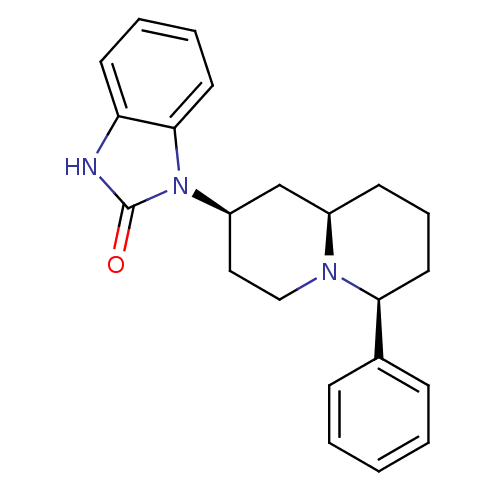 (1-((2R,6S,9aR)-6-Phenyl-octahydro-quinolizin-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor kappa 1 using [3H]- U-69,593 as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50137567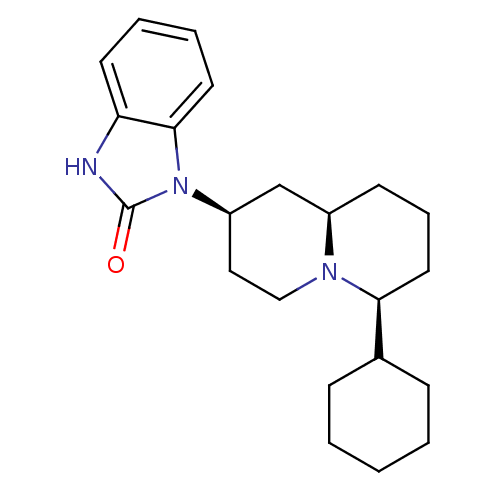 (1-((2R,6S,9aR)-6-Cyclohexyl-octahydro-quinolizin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor kappa 1 using [3H]- U-69,593 as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50137566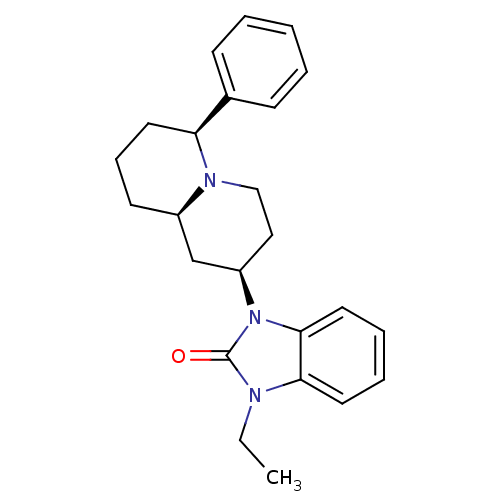 (1-Ethyl-3-((2R,6S,9aR)-6-phenyl-octahydro-quinoliz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor mu 1 using [3H]- DAMGO as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50137565 (1-((2R,6S,9aR)-6-Phenyl-octahydro-quinolizin-2-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor mu 1 using [3H]- DAMGO as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137565 (1-((2R,6S,9aR)-6-Phenyl-octahydro-quinolizin-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human nociceptin (NOP) receptor using [3H]N/OFQ as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50137563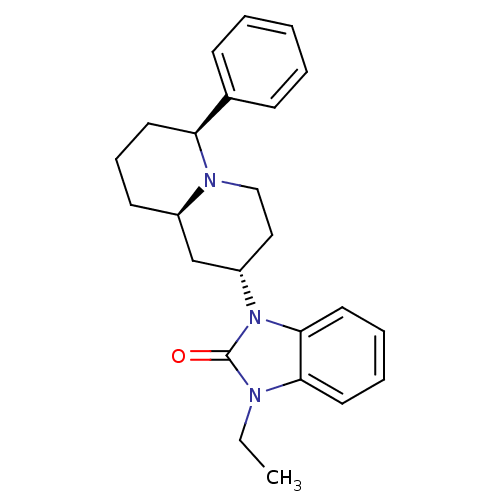 (1-Ethyl-3-((2S,6S,9aR)-6-phenyl-octahydro-quinoliz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor mu 1 using [3H]- DAMGO as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137566 (1-Ethyl-3-((2R,6S,9aR)-6-phenyl-octahydro-quinoliz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human nociceptin (NOP) receptor using [3H]N/OFQ as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50137566 (1-Ethyl-3-((2R,6S,9aR)-6-phenyl-octahydro-quinoliz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor kappa 1 using [3H]- U-69,593 as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137567 (1-((2R,6S,9aR)-6-Cyclohexyl-octahydro-quinolizin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human nociceptin (NOP) receptor using [3H]N/OFQ as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50137567 (1-((2R,6S,9aR)-6-Cyclohexyl-octahydro-quinolizin-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor mu 1 using [3H]- DAMGO as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50137561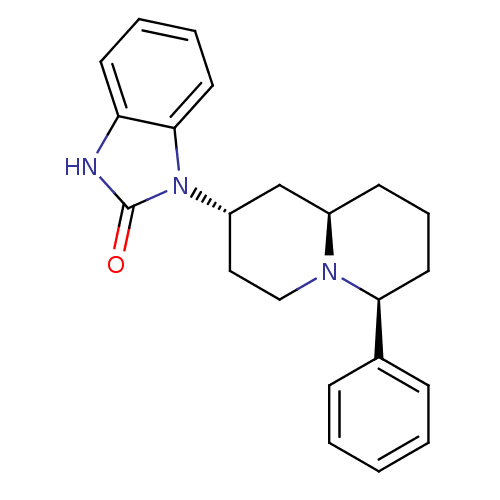 (1-((2S,6S,9aR)-6-Phenyl-octahydro-quinolizin-2-yl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor mu 1 using [3H]- DAMGO as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50137568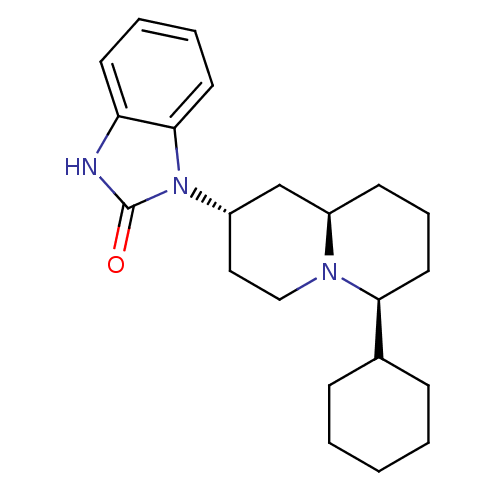 (1-((2S,6S,9aR)-6-Cyclohexyl-octahydro-quinolizin-2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor mu 1 using [3H]- DAMGO as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137568 (1-((2S,6S,9aR)-6-Cyclohexyl-octahydro-quinolizin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human nociceptin (NOP) receptor using [3H]N/OFQ as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50137568 (1-((2S,6S,9aR)-6-Cyclohexyl-octahydro-quinolizin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor kappa 1 using [3H]- U-69,593 as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50137561 (1-((2S,6S,9aR)-6-Phenyl-octahydro-quinolizin-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 271 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor kappa 1 using [3H]- U-69,593 as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137563 (1-Ethyl-3-((2S,6S,9aR)-6-phenyl-octahydro-quinoliz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 535 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human nociceptin (NOP) receptor using [3H]N/OFQ as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50137561 (1-((2S,6S,9aR)-6-Phenyl-octahydro-quinolizin-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 786 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human nociceptin (NOP) receptor using [3H]N/OFQ as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50137563 (1-Ethyl-3-((2S,6S,9aR)-6-phenyl-octahydro-quinoliz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 987 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor kappa 1 using [3H]- U-69,593 as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50137566 (1-Ethyl-3-((2R,6S,9aR)-6-phenyl-octahydro-quinoliz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor delta 1 using [3H]- CL-DPDPE as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50137565 (1-((2R,6S,9aR)-6-Phenyl-octahydro-quinolizin-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor delta 1 using [3H]- CL-DPDPE as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50137563 (1-Ethyl-3-((2S,6S,9aR)-6-phenyl-octahydro-quinoliz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor delta 1 using [3H]- CL-DPDPE as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50137567 (1-((2R,6S,9aR)-6-Cyclohexyl-octahydro-quinolizin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor delta 1 using [3H]- CL-DPDPE as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate racemase (Helicobacter pylori) | BDBM26431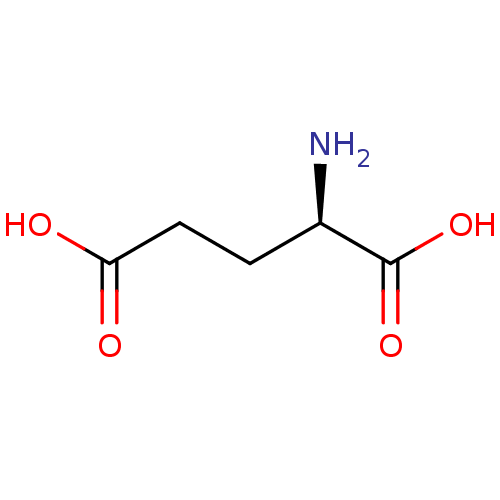 ((2R)-2-aminopentanedioic acid | CHEMBL76232 | D-Gl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Global Structural Chemistry Curated by ChEMBL | Assay Description Inhibition of Helicobacter pylori glutamate racemase | Nature 447: 817-822 (2007) Article DOI: 10.1038/nature05689 BindingDB Entry DOI: 10.7270/Q2HX1DJ3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50137568 (1-((2S,6S,9aR)-6-Cyclohexyl-octahydro-quinolizin-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor delta 1 using [3H]- CL-DPDPE as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50137561 (1-((2S,6S,9aR)-6-Phenyl-octahydro-quinolizin-2-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity for human opioid receptor delta 1 using [3H]- CL-DPDPE as radioligand transfected into CHO cells | Bioorg Med Chem Lett 14: 181-5 (2003) BindingDB Entry DOI: 10.7270/Q24X58BV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548859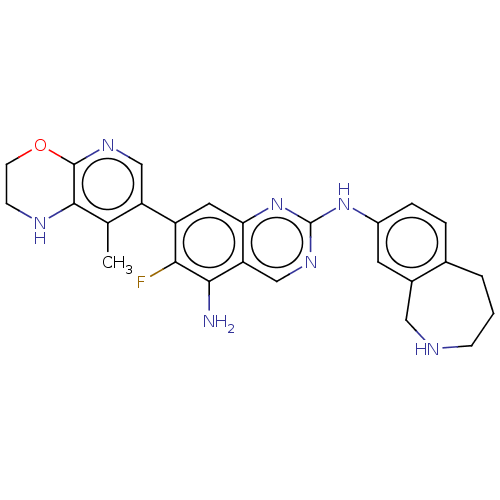 (WO2022098806, Compound 2-120) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548828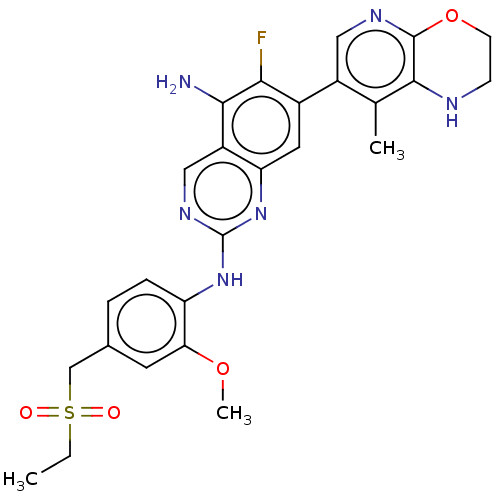 (WO2022098806, Compound 2-89) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548847 (WO2022098806, Compound 2-108) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548860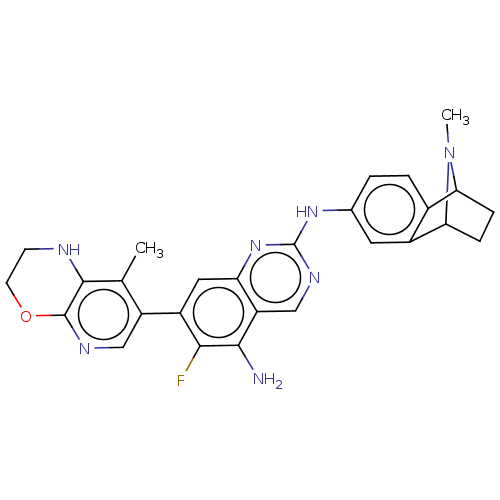 (WO2022098806, Compound 2-121) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548834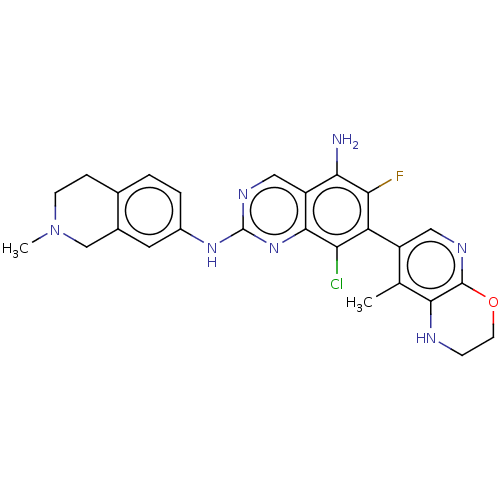 (WO2022098806, Compound 2-180 | WO2022098806, Compo...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548817 (WO2022098806, Compound 2-78) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548823 (WO2022098806, Compound 2-84) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548829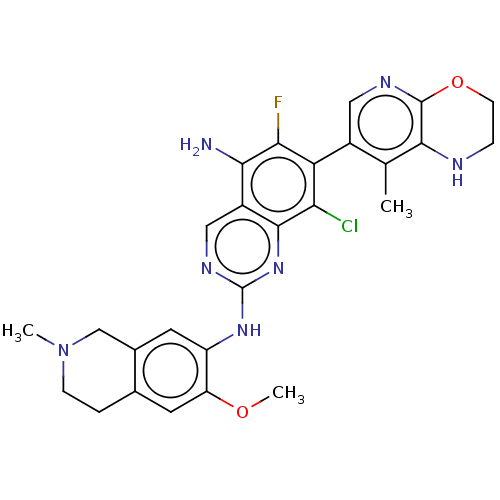 (WO2022098806, Compound 2-179 | WO2022098806, Compo...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548850 (WO2022098806, Compound 2-111) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548727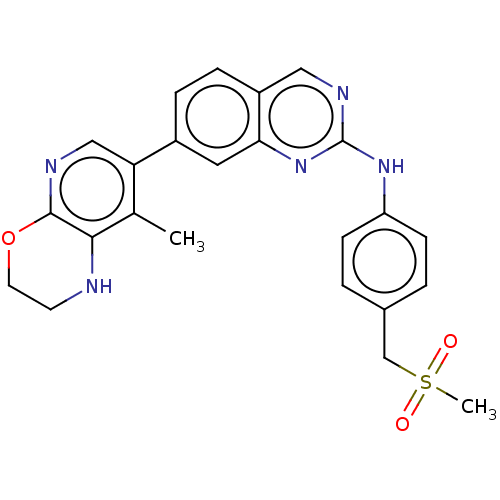 (WO2022098806, Compound 1-123) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548846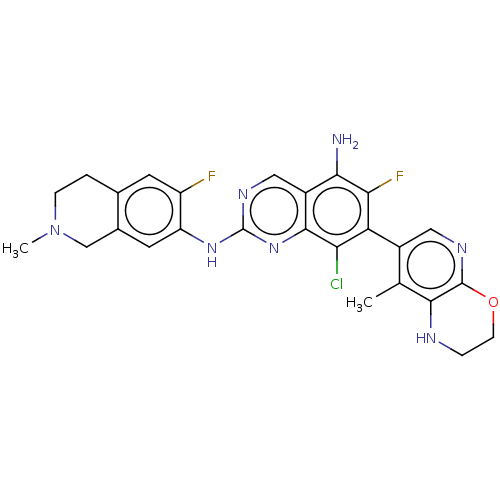 (WO2022098806, Compound 2-107 | WO2022098806, Compo...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548867 (WO2022098806, Compound 2-128) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548897 (WO2022098806, Compound 2-158) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548863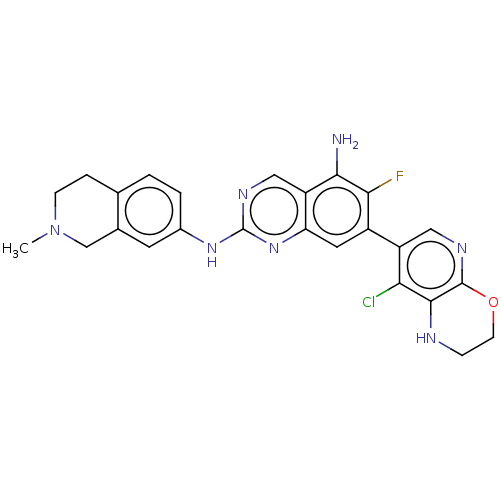 (WO2022098806, Compound 2-124 | WO2022098806, Compo...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548914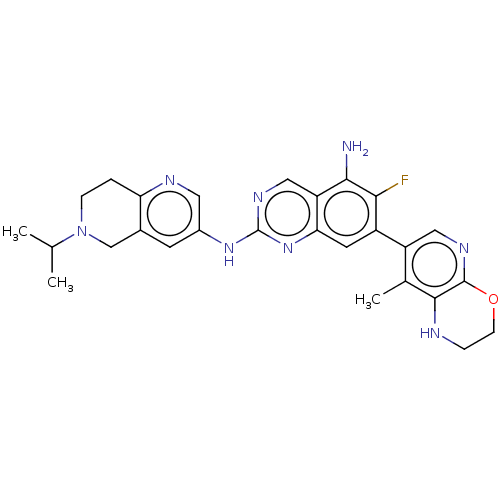 (WO2022098806, Compound 2-175) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548815 (WO2022098806, Compound 2-76) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548818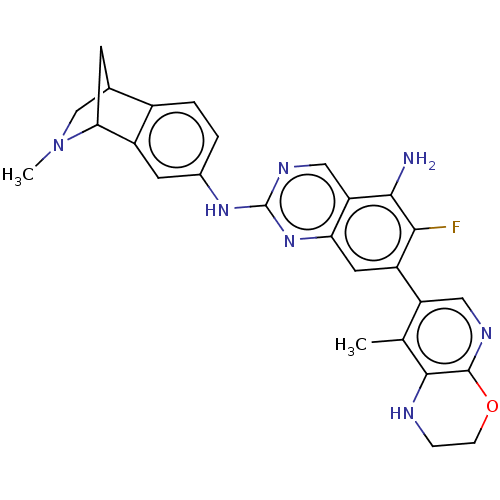 (WO2022098806, Compound 2-79) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548821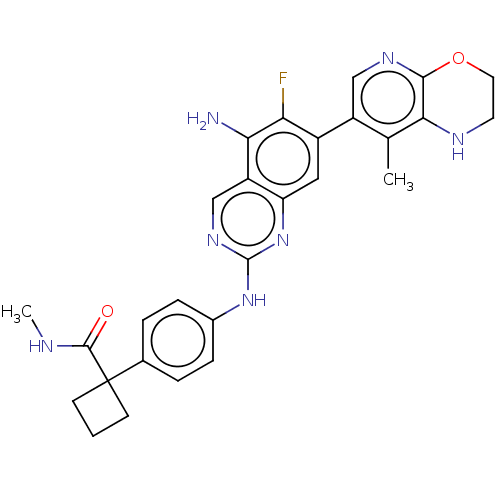 (WO2022098806, Compound 2-82) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548824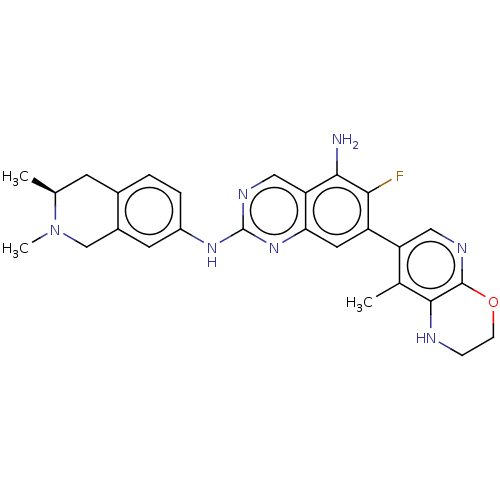 (WO2022098806, Compound 2-85) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548922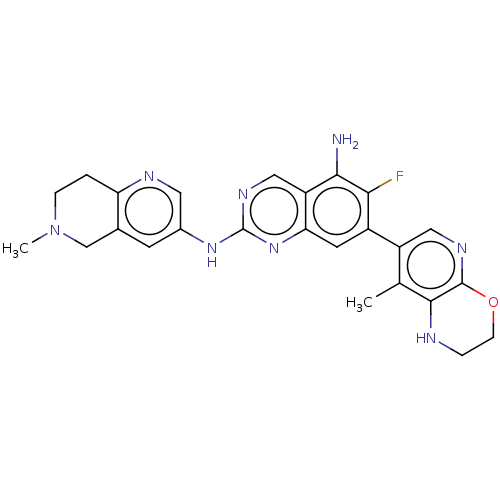 (WO2022098806, Compound 2-183) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548858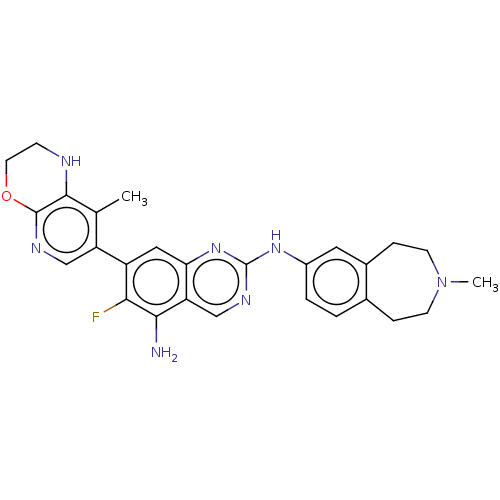 (WO2022098806, Compound 2-119) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548763 (WO2022098806, Compound 2-114 | WO2022098806, Compo...) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 1 (Homo sapiens (Human)) | BDBM548878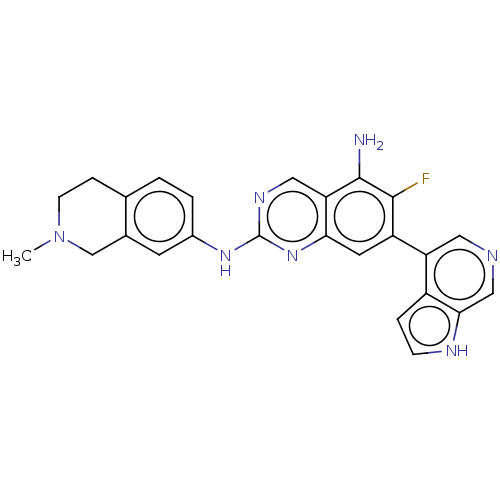 (WO2022098806, Compound 2-139) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | WIPO WO2022098806 | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description To each well of black Corning #3820384-well plate, an ECHO was used to dispense 7.5 nL of DMSO or Test compound in DMSO. A 1.5x kinase solution, 5 &... | Citation and Details BindingDB Entry DOI: 10.7270/Q2KH0RJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 645 total ) | Next | Last >> |