

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179201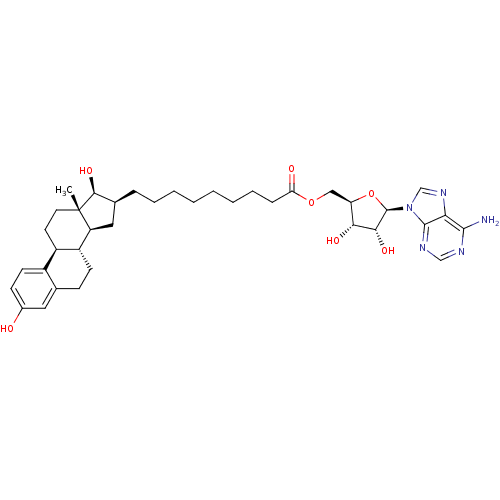 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50237104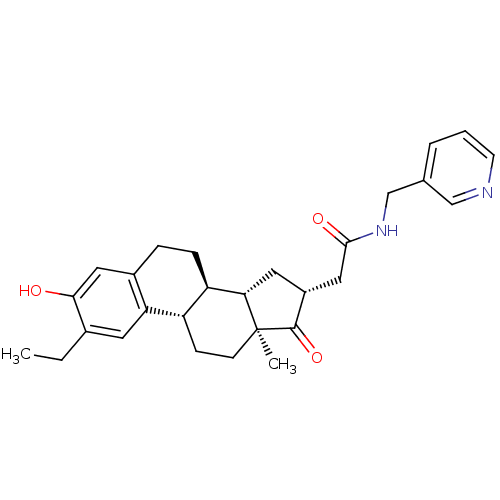 (2-((8R,9S,13S,14S,16R)-2-ethyl-3-hydroxy-13-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50179201 (((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188387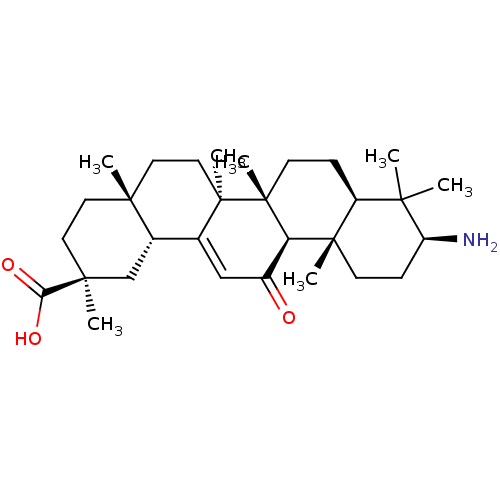 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-amino-2...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188390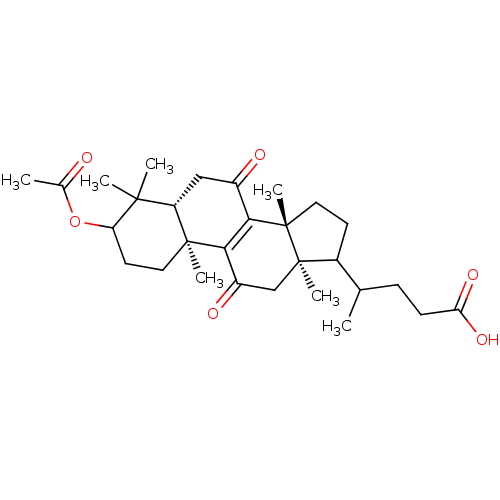 (4-((5R,10S,13R,14R)-3-acetoxy-4,4,10,13,14-pentame...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50241159 (CHEMBL373383 | N-butyl-6-((6R,8R,9S,13S,14S,17S)-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188387 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-amino-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188385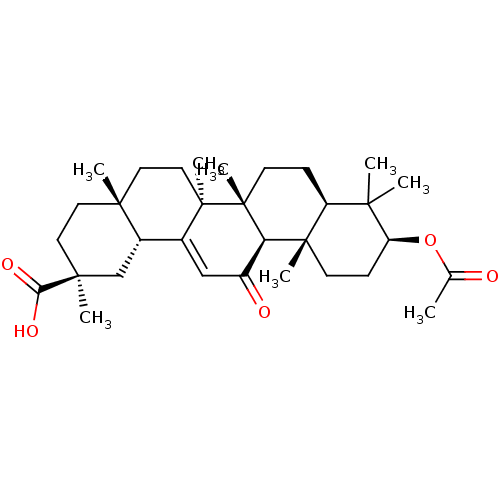 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-acetoxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 201 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188386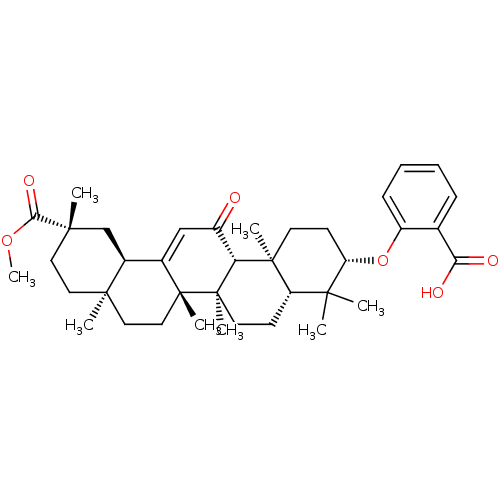 (2-((3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-(met...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD2 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188390 (4-((5R,10S,13R,14R)-3-acetoxy-4,4,10,13,14-pentame...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11-beta-HSD1 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188386 (2-((3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-(met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188385 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-acetoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188387 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-amino-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11-beta-HSD1 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD2 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188389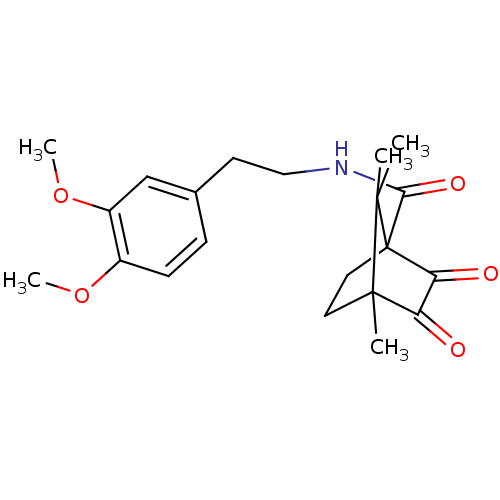 (CHEMBL210465 | N-(3,4-dimethoxyphenethyl)-4,7,7-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50188390 (4-((5R,10S,13R,14R)-3-acetoxy-4,4,10,13,14-pentame...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of GR-mediated transactivation of galactosidase reporter gene in HEK293 cells expressing 11betaHSD1 | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM19459 (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188388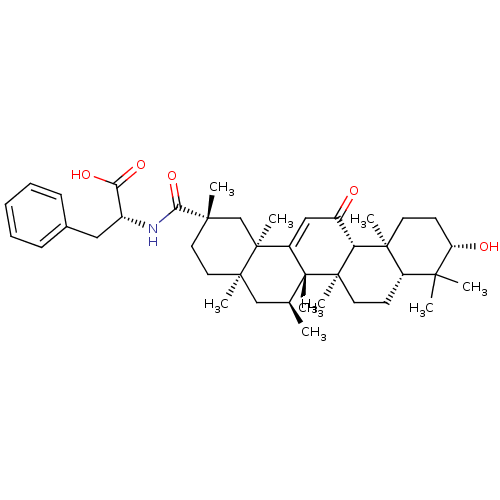 ((R)-2-((2S,4aR,6S,6aS,6bR,8aR,10S,12aS,12bR,14bR)-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188388 ((R)-2-((2S,4aR,6S,6aS,6bR,8aR,10S,12aS,12bR,14bR)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188385 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-acetoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | n/a | n/a | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11-beta-HSD1 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188385 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-acetoxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17-beta-HSD2 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50244328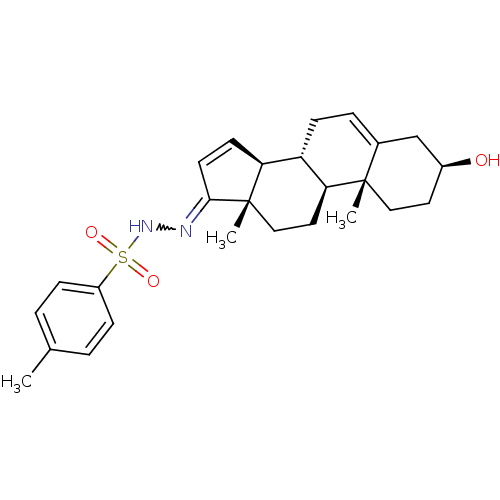 (CHEMBL469909 | N'-((3S,8R,9S,10R,13S,14S)-3-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188386 (2-((3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-(met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11-beta-HSD1 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50188389 (CHEMBL210465 | N-(3,4-dimethoxyphenethyl)-4,7,7-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 11-beta-HSD1-dependent cortisone conversion to cortisol in mouse intact 3T3 L1 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188390 (4-((5R,10S,13R,14R)-3-acetoxy-4,4,10,13,14-pentame...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50188389 (CHEMBL210465 | N-(3,4-dimethoxyphenethyl)-4,7,7-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 11-beta-HSD1-dependent cortisone conversion to cortisol in mouse intact C2C12 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM23419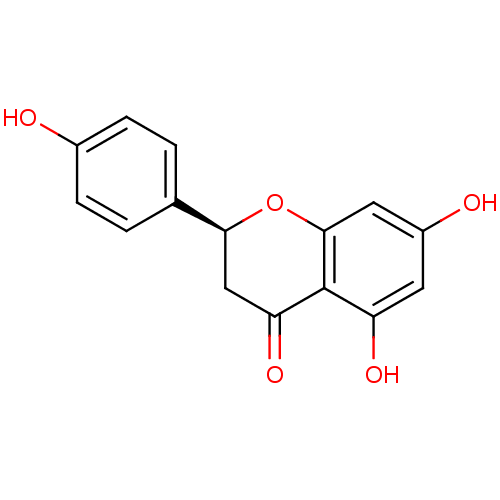 ((2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188389 (CHEMBL210465 | N-(3,4-dimethoxyphenethyl)-4,7,7-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11-beta-HSD1 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM26664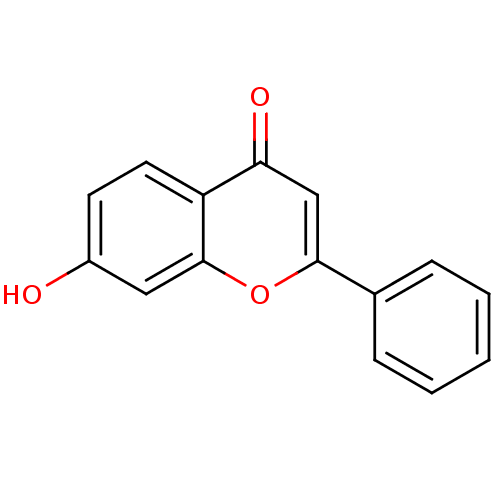 (7-Hydroxy-flavone, 5a | 7-Hydroxyflavone, 11 | 7-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50244255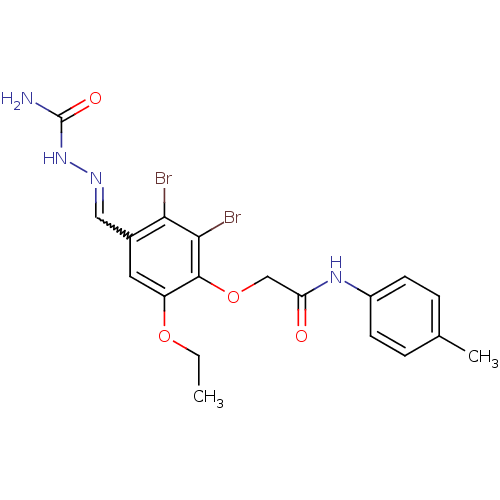 (1-(4-(2-(p-toluidino)-2-oxoethoxy)-2,3-dibromo-5-e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188387 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-amino-2...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50244290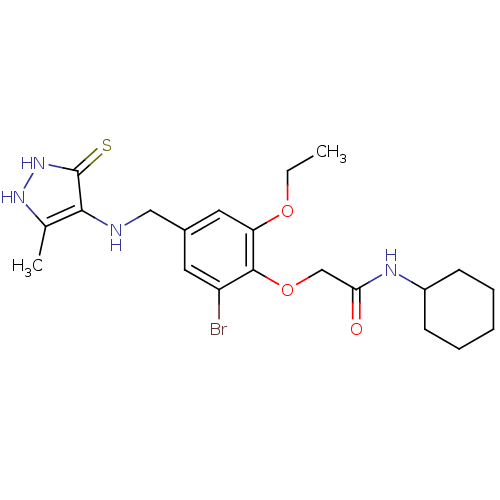 (2-(2-bromo-6-ethoxy-4-((3-mercapto-5-methyl-4H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188385 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-acetoxy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD2 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188391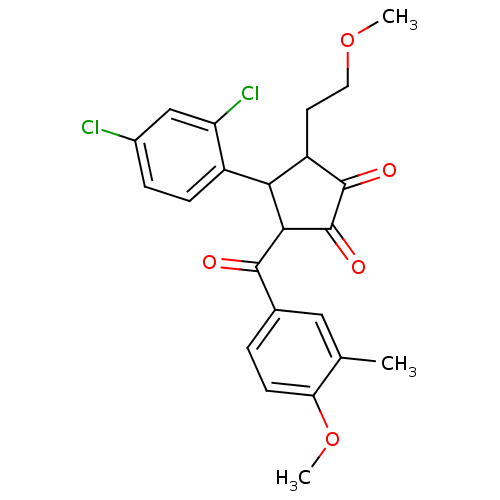 (4-(2,4-dichlorophenyl)-2-hydroxy-3-(4-methoxy-3-me...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17-beta-HSD2 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188391 (4-(2,4-dichlorophenyl)-2-hydroxy-3-(4-methoxy-3-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM9461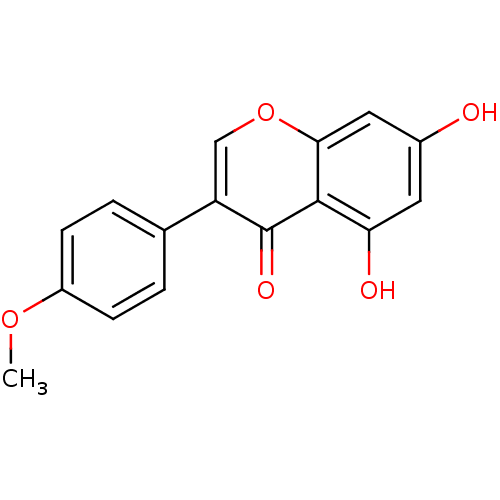 (5,7-dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD2 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM50188386 (2-((3S,4aR,6aR,6bS,8aS,11S,12aR,14aR,14bS)-11-(met...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17-beta-HSD2 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50188390 (4-((5R,10S,13R,14R)-3-acetoxy-4,4,10,13,14-pentame...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of GR-mediated transactivation of galactosidase reporter gene in HEK293 cells in absence of 11betaHSD1 | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50188388 ((R)-2-((2S,4aR,6S,6aS,6bR,8aR,10S,12aS,12bR,14bR)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11-beta-HSD1 transfected in intact HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM23419 ((2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD2 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM19459 (5,7-dihydroxy-3-(4-hydroxyphenyl)-4H-chromen-4-one...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD2 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50188385 ((2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10-acetoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50244365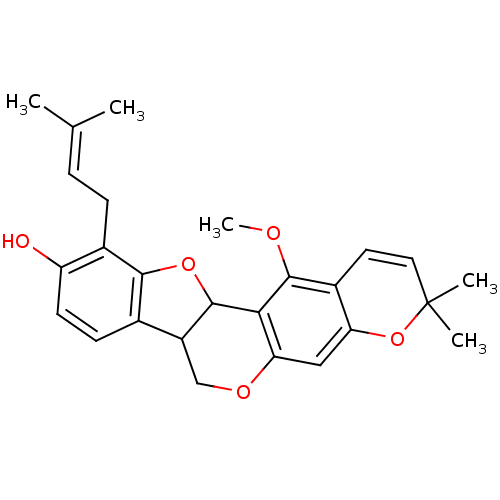 (13-Methoxy-3,3-dimethyl-11-(3-methyl-but-2-enyl)-7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50244327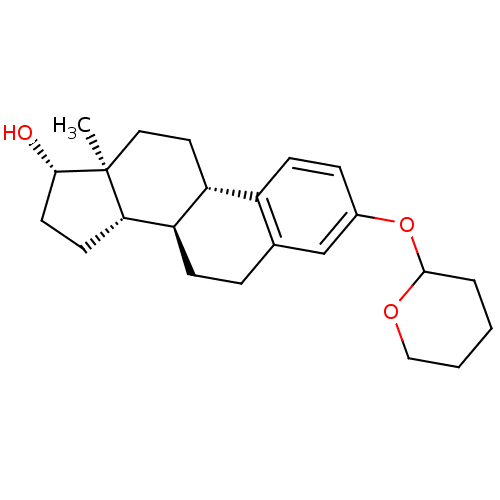 ((8R,9S,13S,14S,17S)-13-methyl-3-(tetrahydro-2H-pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 11beta-HSD1 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM26664 (7-Hydroxy-flavone, 5a | 7-Hydroxyflavone, 11 | 7-h...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD2 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 2 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17beta-HSD2 expressed in HEK293 cell lysate assessed as conversion of radiolabeled estrone to estradiol | J Med Chem 51: 4188-99 (2008) Article DOI: 10.1021/jm800054h BindingDB Entry DOI: 10.7270/Q2Q81F0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 17-beta-hydroxysteroid dehydrogenase type 1 (Homo sapiens (Human)) | BDBM50188389 (CHEMBL210465 | N-(3,4-dimethoxyphenethyl)-4,7,7-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 17betaHSD1 expressed in HEK293 cells | J Med Chem 49: 3454-66 (2006) Article DOI: 10.1021/jm0600794 BindingDB Entry DOI: 10.7270/Q2PN958F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 63 total ) | Next | Last >> |