 Found 278 hits with Last Name = 'kawanishi' and Initial = 'm'
Found 278 hits with Last Name = 'kawanishi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50444438
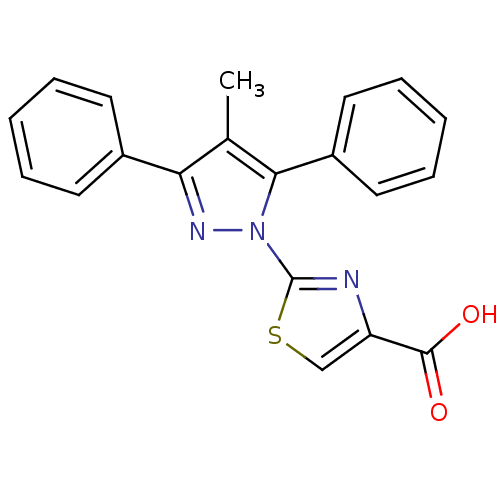
(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493969
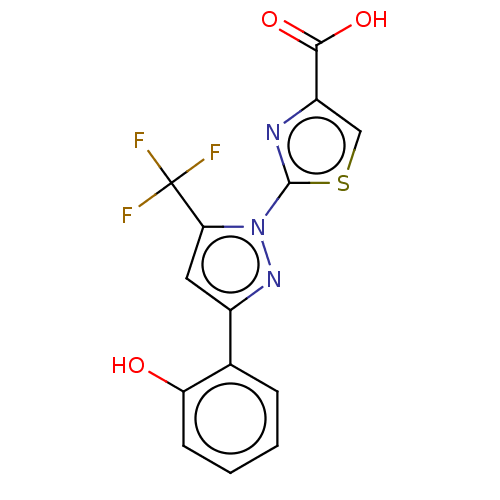
(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to EP4 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP4 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to EP3 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP3 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50444438

(CHEMBL2442495)Show SMILES Cc1c(nn(-c2nc(cs2)C(O)=O)c1-c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C20H15N3O2S/c1-13-17(14-8-4-2-5-9-14)22-23(18(13)15-10-6-3-7-11-15)20-21-16(12-26-20)19(24)25/h2-12H,1H3,(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50493969

(CHEMBL2442490)Show SMILES OC(=O)c1csc(n1)-n1nc(cc1C(F)(F)F)-c1ccccc1O Show InChI InChI=1S/C14H8F3N3O3S/c15-14(16,17)11-5-8(7-3-1-2-4-10(7)21)19-20(11)13-18-9(6-24-13)12(22)23/h1-6,21H,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP2 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50493968

(CHEMBL2442485)Show SMILES COc1ccc(cc1)-c1cc(nn1-c1nc(cs1)C(O)=O)C(F)(F)F Show InChI InChI=1S/C15H10F3N3O3S/c1-24-9-4-2-8(3-5-9)11-6-12(15(16,17)18)20-21(11)14-19-10(7-25-14)13(22)23/h2-7H,1H3,(H,22,23) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to human EP1 receptor |
Bioorg Med Chem Lett 23: 6064-7 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.032
BindingDB Entry DOI: 10.7270/Q2959MJ5 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Binding affinity to EP2 receptor (unknown origin) |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542127
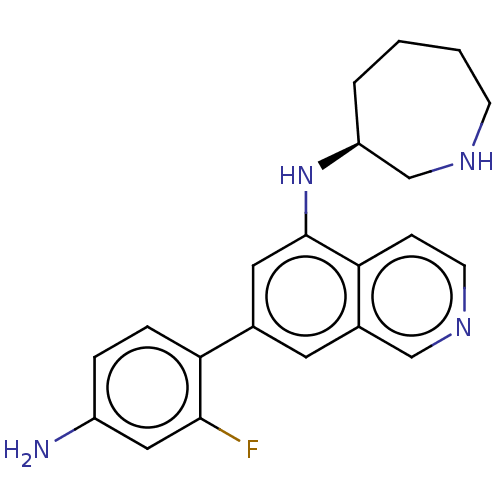
(CHEMBL4635482)Show SMILES Nc1ccc(c(F)c1)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C21H23FN4/c22-20-11-16(23)4-5-18(20)14-9-15-12-25-8-6-19(15)21(10-14)26-17-3-1-2-7-24-13-17/h4-6,8-12,17,24,26H,1-3,7,13,23H2/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542123

(CHEMBL4636488)Show SMILES C1CNC[C@H](C1)Nc1cc(cc2cnccc12)-c1ccccc1 |r| Show InChI InChI=1S/C20H21N3/c1-2-5-15(6-3-1)16-11-17-13-22-10-8-19(17)20(12-16)23-18-7-4-9-21-14-18/h1-3,5-6,8,10-13,18,21,23H,4,7,9,14H2/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276262

(CHEMBL4129609)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(nc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)12-7-6-10-13(9-4-2-1-3-5-9)23-24(14(10)22-12)16-21-11(8-27-16)15(25)26/h1-8H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542116

(CHEMBL4646995)Show InChI InChI=1S/C20H22N4/c21-17-5-3-14(4-6-17)15-10-16-12-23-9-7-19(16)20(11-15)24-18-2-1-8-22-13-18/h3-7,9-12,18,22,24H,1-2,8,13,21H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50449137
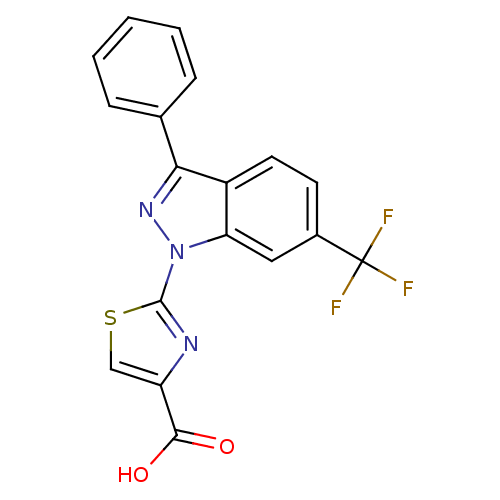
(CHEMBL3127163)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ccc(cc12)C(F)(F)F Show InChI InChI=1S/C18H10F3N3O2S/c19-18(20,21)11-6-7-12-14(8-11)24(17-22-13(9-27-17)16(25)26)23-15(12)10-4-2-1-3-5-10/h1-9H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542119
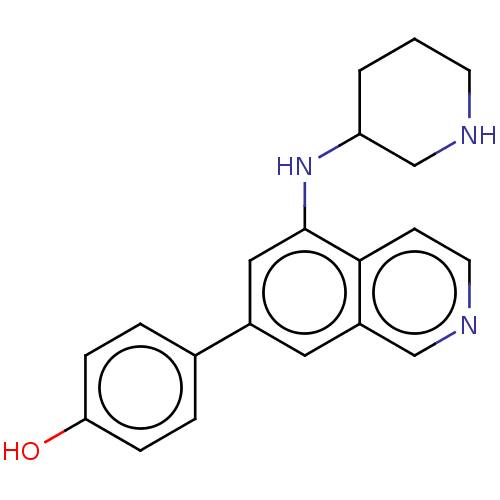
(CHEMBL4644939)Show InChI InChI=1S/C20H21N3O/c24-18-5-3-14(4-6-18)15-10-16-12-22-9-7-19(16)20(11-15)23-17-2-1-8-21-13-17/h3-7,9-12,17,21,23-24H,1-2,8,13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276250
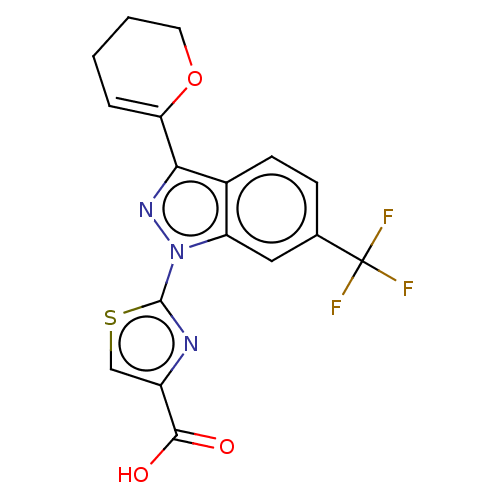
(CHEMBL4126319)Show SMILES OC(=O)c1csc(n1)-n1nc(C2=CCCCO2)c2ccc(cc12)C(F)(F)F |t:12| Show InChI InChI=1S/C17H12F3N3O3S/c18-17(19,20)9-4-5-10-12(7-9)23(16-21-11(8-27-16)15(24)25)22-14(10)13-3-1-2-6-26-13/h3-5,7-8H,1-2,6H2,(H,24,25) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542128
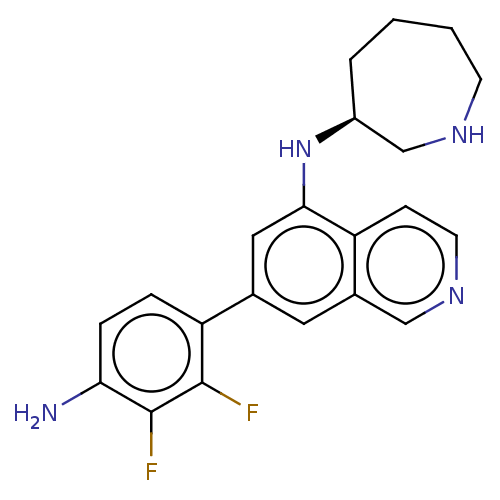
(CHEMBL4636337)Show SMILES Nc1ccc(c(F)c1F)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C21H22F2N4/c22-20-17(4-5-18(24)21(20)23)13-9-14-11-26-8-6-16(14)19(10-13)27-15-3-1-2-7-25-12-15/h4-6,8-11,15,25,27H,1-3,7,12,24H2/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542132

(CHEMBL4632523)Show SMILES Nc1ccc(cn1)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C20H23N5/c21-20-5-4-14(12-24-20)15-9-16-11-23-8-6-18(16)19(10-15)25-17-3-1-2-7-22-13-17/h4-6,8-12,17,22,25H,1-3,7,13H2,(H2,21,24)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542133
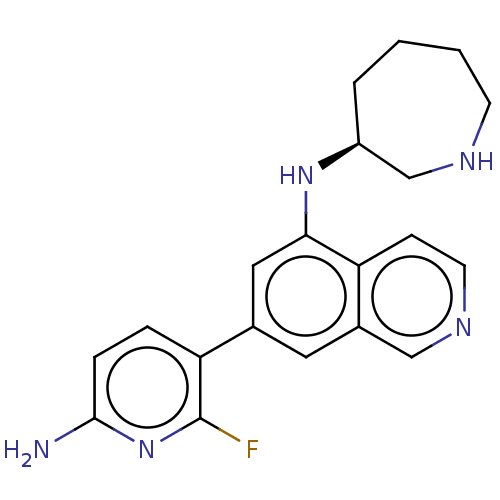
(CHEMBL4643553)Show SMILES Nc1ccc(c(F)n1)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C20H22FN5/c21-20-17(4-5-19(22)26-20)13-9-14-11-24-8-6-16(14)18(10-13)25-15-3-1-2-7-23-12-15/h4-6,8-11,15,23,25H,1-3,7,12H2,(H2,22,26)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542126

(CHEMBL4642166)Show SMILES Nc1ccc(cc1)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C21H24N4/c22-18-6-4-15(5-7-18)16-11-17-13-24-10-8-20(17)21(12-16)25-19-3-1-2-9-23-14-19/h4-8,10-13,19,23,25H,1-3,9,14,22H2/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542129
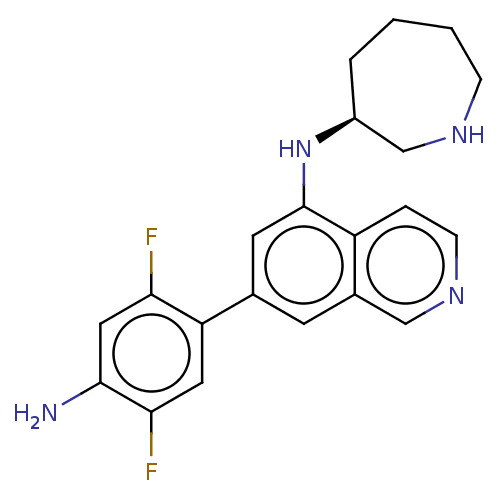
(CHEMBL4637452)Show SMILES Nc1cc(F)c(cc1F)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C21H22F2N4/c22-18-10-20(24)19(23)9-17(18)13-7-14-11-26-6-4-16(14)21(8-13)27-15-3-1-2-5-25-12-15/h4,6-11,15,25,27H,1-3,5,12,24H2/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542134
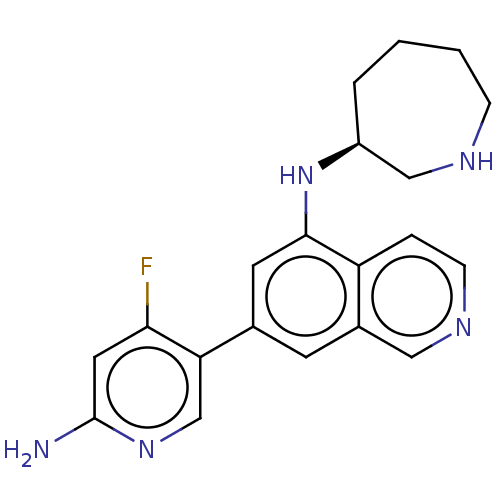
(CHEMBL4647780)Show SMILES Nc1cc(F)c(cn1)-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C20H22FN5/c21-18-9-20(22)25-12-17(18)13-7-14-10-24-6-4-16(14)19(8-13)26-15-3-1-2-5-23-11-15/h4,6-10,12,15,23,26H,1-3,5,11H2,(H2,22,25)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542125

(CHEMBL4643470)Show InChI InChI=1S/C17H17N3/c18-7-9-20-17-11-14(13-4-2-1-3-5-13)10-15-12-19-8-6-16(15)17/h1-6,8,10-12,20H,7,9,18H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542121
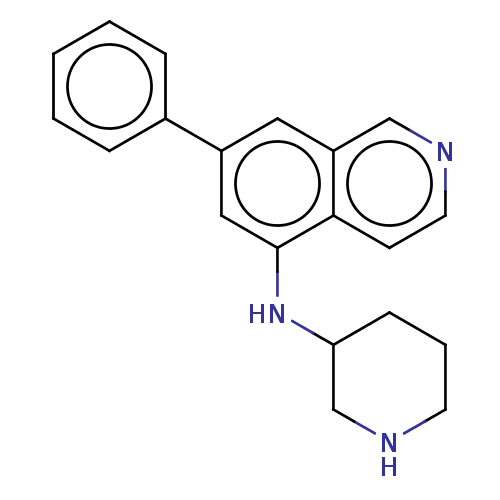
(CHEMBL4641918)Show InChI InChI=1S/C20H21N3/c1-2-5-15(6-3-1)16-11-17-13-22-10-8-19(17)20(12-16)23-18-7-4-9-21-14-18/h1-3,5-6,8,10-13,18,21,23H,4,7,9,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346122

(2-(4-(2-(4-bromo-5-methylthiophen-2-yl)-5-ethyl-6-...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1)-c1cc(Br)c(C)s1 Show InChI InChI=1S/C20H20BrN3O2S/c1-4-15-11(2)22-20(17-10-16(21)12(3)27-17)24-19(15)23-14-7-5-13(6-8-14)9-18(25)26/h5-8,10H,4,9H2,1-3H3,(H,25,26)(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542130
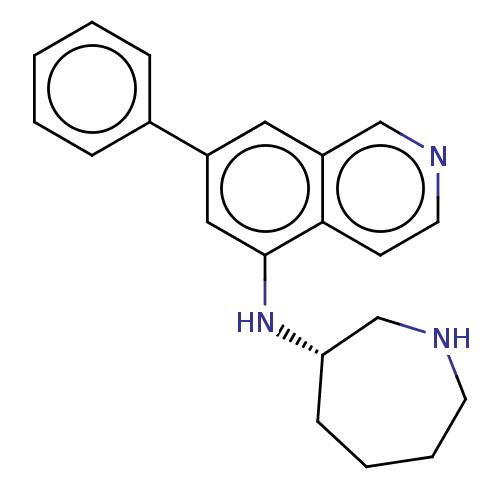
(CHEMBL4632384)Show SMILES C1CC[C@@H](CNC1)Nc1cc(cc2cnccc12)-c1ccccc1 |r| Show InChI InChI=1S/C21H23N3/c1-2-6-16(7-3-1)17-12-18-14-23-11-9-20(18)21(13-17)24-19-8-4-5-10-22-15-19/h1-3,6-7,9,11-14,19,22,24H,4-5,8,10,15H2/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276265
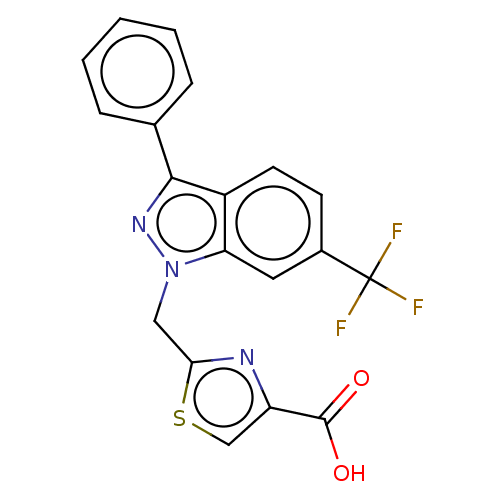
(CHEMBL4129545)Show SMILES OC(=O)c1csc(Cn2nc(-c3ccccc3)c3ccc(cc23)C(F)(F)F)n1 Show InChI InChI=1S/C19H12F3N3O2S/c20-19(21,22)12-6-7-13-15(8-12)25(9-16-23-14(10-28-16)18(26)27)24-17(13)11-4-2-1-3-5-11/h1-8,10H,9H2,(H,26,27) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276264
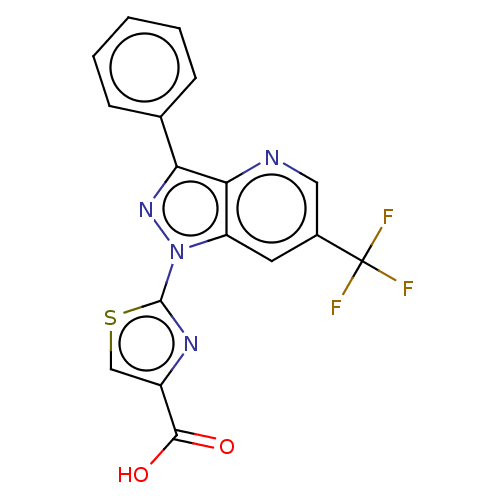
(CHEMBL4128163)Show SMILES OC(=O)c1csc(n1)-n1nc(-c2ccccc2)c2ncc(cc12)C(F)(F)F Show InChI InChI=1S/C17H9F3N4O2S/c18-17(19,20)10-6-12-14(21-7-10)13(9-4-2-1-3-5-9)23-24(12)16-22-11(8-27-16)15(25)26/h1-8H,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276249
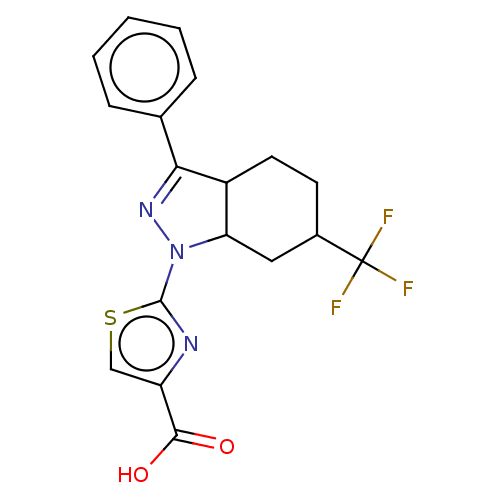
(CHEMBL4126167)Show SMILES OC(=O)c1csc(n1)N1N=C(C2CCC(CC12)C(F)(F)F)c1ccccc1 |c:10| Show InChI InChI=1S/C18H16F3N3O2S/c19-18(20,21)11-6-7-12-14(8-11)24(17-22-13(9-27-17)16(25)26)23-15(12)10-4-2-1-3-5-10/h1-5,9,11-12,14H,6-8H2,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM50276263
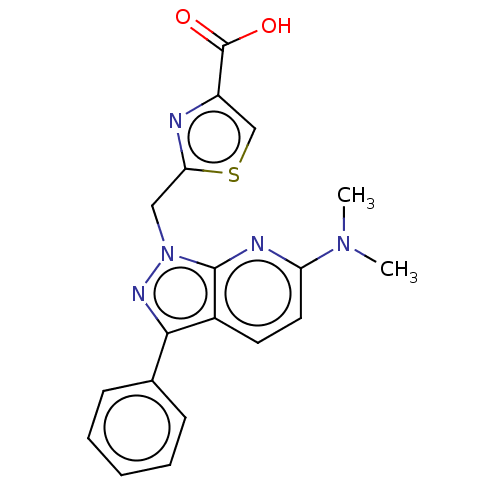
(CHEMBL4129401)Show SMILES CN(C)c1ccc2c(nn(Cc3nc(cs3)C(O)=O)c2n1)-c1ccccc1 Show InChI InChI=1S/C19H17N5O2S/c1-23(2)15-9-8-13-17(12-6-4-3-5-7-12)22-24(18(13)21-15)10-16-20-14(11-27-16)19(25)26/h3-9,11H,10H2,1-2H3,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Antagonist activity at EP1 receptor (unknown origin) by reporter gene assay |
Bioorg Med Chem Lett 28: 2408-2412 (2018)
Article DOI: 10.1016/j.bmcl.2018.06.022
BindingDB Entry DOI: 10.7270/Q2T72KZT |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542122

(CHEMBL4642061)Show SMILES CC(=O)Nc1ccc(cc1)-c1cc(NC2CCCNC2)c2ccncc2c1 Show InChI InChI=1S/C22H24N4O/c1-15(27)25-19-6-4-16(5-7-19)17-11-18-13-24-10-8-21(18)22(12-17)26-20-3-2-9-23-14-20/h4-8,10-13,20,23,26H,2-3,9,14H2,1H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542111

(CHEMBL4644763)Show SMILES C1C[C@@H](CN1)Oc1ccc2cncc(-c3cnc4[nH]ccc4c3)c2c1 |r| Show InChI InChI=1S/C20H18N4O/c1-2-16(25-17-4-5-21-11-17)8-18-14(1)9-22-12-19(18)15-7-13-3-6-23-20(13)24-10-15/h1-3,6-10,12,17,21H,4-5,11H2,(H,23,24)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542131

(CHEMBL4634517)Show SMILES Fc1cccc(F)c1-c1cc(N[C@H]2CCCCNC2)c2ccncc2c1 |r| Show InChI InChI=1S/C21H21F2N3/c22-18-5-3-6-19(23)21(18)14-10-15-12-25-9-7-17(15)20(11-14)26-16-4-1-2-8-24-13-16/h3,5-7,9-12,16,24,26H,1-2,4,8,13H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346121
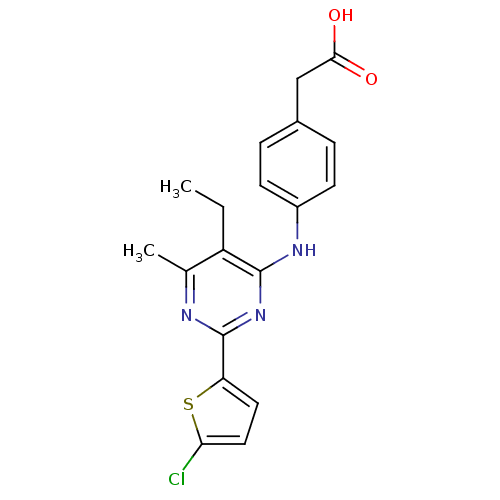
(2-(4-(2-(5-chlorothiophen-2-yl)-5-ethyl-6-methylpy...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1)-c1ccc(Cl)s1 Show InChI InChI=1S/C19H18ClN3O2S/c1-3-14-11(2)21-19(15-8-9-16(20)26-15)23-18(14)22-13-6-4-12(5-7-13)10-17(24)25/h4-9H,3,10H2,1-2H3,(H,24,25)(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346123

(2-(4-(5-ethyl-2-(5-fluorothiophen-2-yl)-6-methylpy...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1F)-c1ccc(F)s1 Show InChI InChI=1S/C19H17F2N3O2S/c1-3-12-10(2)22-19(15-6-7-16(21)27-15)24-18(12)23-14-5-4-11(8-13(14)20)9-17(25)26/h4-8H,3,9H2,1-2H3,(H,25,26)(H,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D2
(Homo sapiens (Human)) | BDBM50542119

(CHEMBL4644939)Show InChI InChI=1S/C20H21N3O/c24-18-5-3-14(4-6-18)15-10-16-12-22-9-7-19(16)20(11-15)23-17-2-1-8-21-13-17/h3-7,9-12,17,21,23-24H,1-2,8,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 (unknown origin) |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346120

(2-(4-(2-(5-bromothiophen-2-yl)-5-ethyl-6-methylpyr...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1)-c1ccc(Br)s1 Show InChI InChI=1S/C19H18BrN3O2S/c1-3-14-11(2)21-19(15-8-9-16(20)26-15)23-18(14)22-13-6-4-12(5-7-13)10-17(24)25/h4-9H,3,10H2,1-2H3,(H,24,25)(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4D
(Homo sapiens (Human)) | BDBM50346088

((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@]1(CC[C@@H](CC1)C(O)=O)C#N |r,wU:17.22,14.25,(4.79,2.97,;3.46,3.74,;2.12,2.97,;.79,3.74,;-.54,2.97,;-.54,1.43,;.79,.66,;2.12,1.43,;3.46,.66,;3.46,-.88,;4.7,-1.79,;4.23,-3.25,;2.69,-3.25,;2.21,-1.79,;-1.88,.66,;-3.42,.71,;-4.23,-.6,;-3.51,-1.96,;-1.97,-2.01,;-1.16,-.7,;-4.33,-3.26,;-3.6,-4.62,;-5.87,-3.21,;-1.77,2.19,;-1.66,3.73,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23)/t14-,20- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4D3 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542100

(CHEMBL4645728)Show SMILES Nc1ccc(cc1)-c1cncc2ccc(O[C@H]3CCNC3)cc12 |r| Show InChI InChI=1S/C19H19N3O/c20-15-4-1-13(2-5-15)19-12-22-10-14-3-6-16(9-18(14)19)23-17-7-8-21-11-17/h1-6,9-10,12,17,21H,7-8,11,20H2/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346100

(4-(5-allyl-6-ethyl-2-phenylpyrimidin-4-ylamino)ben...)Show SMILES CCc1nc(nc(Nc2ccc(cc2)C(O)=O)c1CC=C)-c1ccccc1 Show InChI InChI=1S/C22H21N3O2/c1-3-8-18-19(4-2)24-20(15-9-6-5-7-10-15)25-21(18)23-17-13-11-16(12-14-17)22(26)27/h3,5-7,9-14H,1,4,8H2,2H3,(H,26,27)(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346119

(2-(4-(5-ethyl-6-methyl-2-(5-(methylthio)thiophen-2...)Show SMILES CCc1c(C)nc(nc1Nc1ccc(CC(O)=O)cc1)-c1ccc(SC)s1 Show InChI InChI=1S/C20H21N3O2S2/c1-4-15-12(2)21-20(16-9-10-18(26-3)27-16)23-19(15)22-14-7-5-13(6-8-14)11-17(24)25/h5-10H,4,11H2,1-3H3,(H,24,25)(H,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | |
Protein kinase C zeta type
(Homo sapiens (Human)) | BDBM50542124

(CHEMBL4641607)Show SMILES C1CNC[C@@H](C1)Nc1cc(cc2cnccc12)-c1ccccc1 |r| Show InChI InChI=1S/C20H21N3/c1-2-5-15(6-3-1)16-11-17-13-22-10-8-19(17)20(12-16)23-18-7-4-9-21-14-18/h1-3,5-6,8,10-13,18,21,23H,4,7,9,14H2/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCzeta using biotin-KKKKRFSFKKSFK substrate and ATP incubated for 30 mins by TR-FRET method |
J Med Chem 63: 7143-7162 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00449
BindingDB Entry DOI: 10.7270/Q24T6NWX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50346088

((1r,4r)-4-cyano-4-(3-(cyclopentyloxy)-4-methoxyphe...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@]1(CC[C@@H](CC1)C(O)=O)C#N |r,wU:17.22,14.25,(4.79,2.97,;3.46,3.74,;2.12,2.97,;.79,3.74,;-.54,2.97,;-.54,1.43,;.79,.66,;2.12,1.43,;3.46,.66,;3.46,-.88,;4.7,-1.79,;4.23,-3.25,;2.69,-3.25,;2.21,-1.79,;-1.88,.66,;-3.42,.71,;-4.23,-.6,;-3.51,-1.96,;-1.97,-2.01,;-1.16,-.7,;-4.33,-3.26,;-3.6,-4.62,;-5.87,-3.21,;-1.77,2.19,;-1.66,3.73,)| Show InChI InChI=1S/C20H25NO4/c1-24-17-7-6-15(12-18(17)25-16-4-2-3-5-16)20(13-21)10-8-14(9-11-20)19(22)23/h6-7,12,14,16H,2-5,8-11H2,1H3,(H,22,23)/t14-,20- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Asahi Kasei Pharma Corporation
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4B1 incubated for 10 mins using cAMP and [3H]cAMP substrates |
Bioorg Med Chem Lett 19: 3174-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.121
BindingDB Entry DOI: 10.7270/Q26110NQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data