

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50370067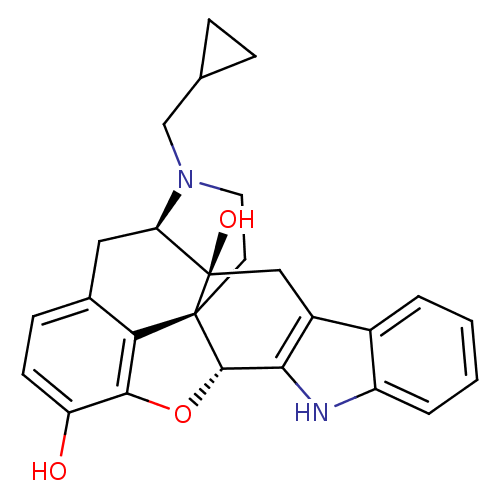 (CHEMBL1237164) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 from rat brain membranes using [3H]DADLE | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582411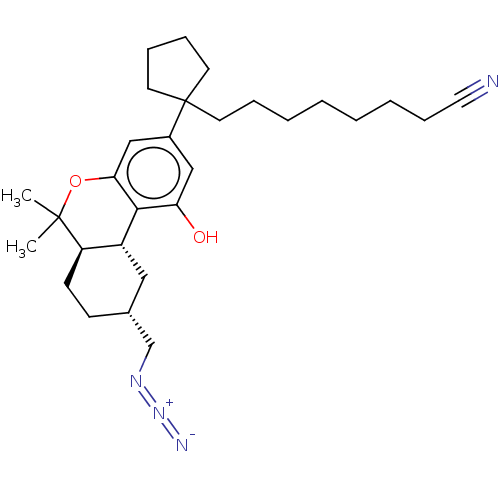 (CHEMBL5084325) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582411 (CHEMBL5084325) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002109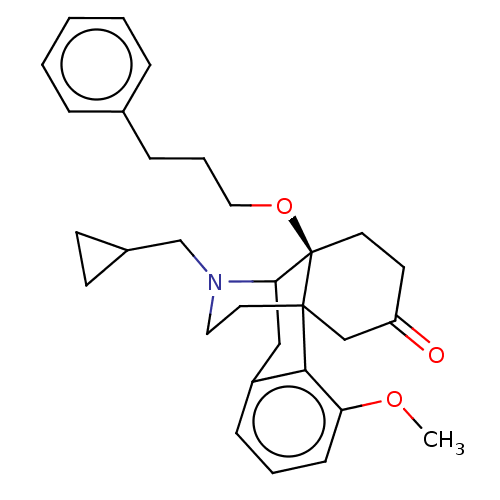 (CHEMBL370735) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 from rat brain membranes using [3H]DAMGO | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582403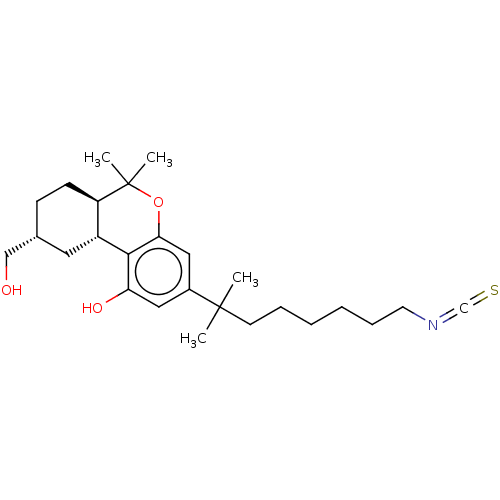 (CHEMBL5085420) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582406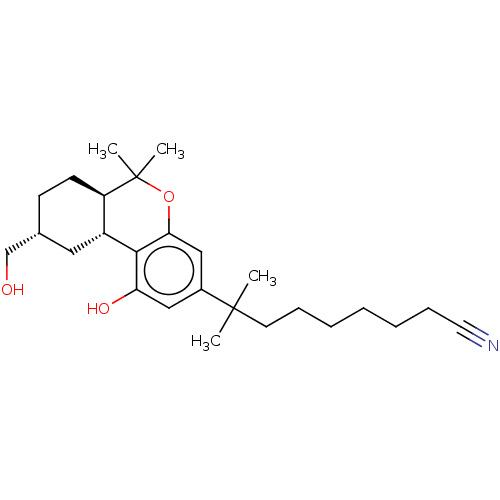 (CHEMBL5091754) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002106 (CHEMBL370966) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 from rat brain membranes using [3H]DAMGO | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582408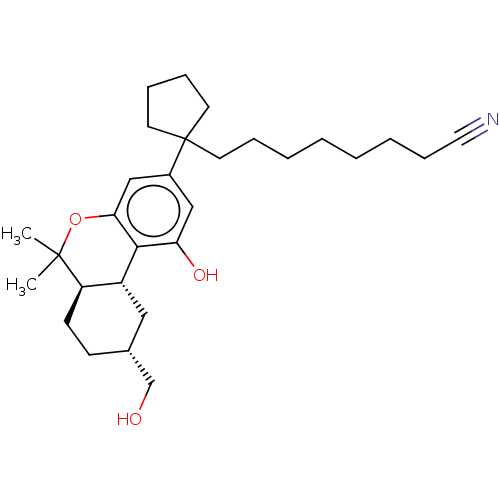 (CHEMBL5080279) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582412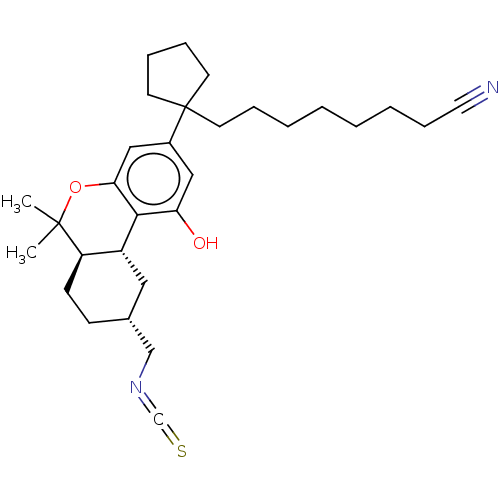 (CHEMBL5087407) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582405 (CHEMBL5074603) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582400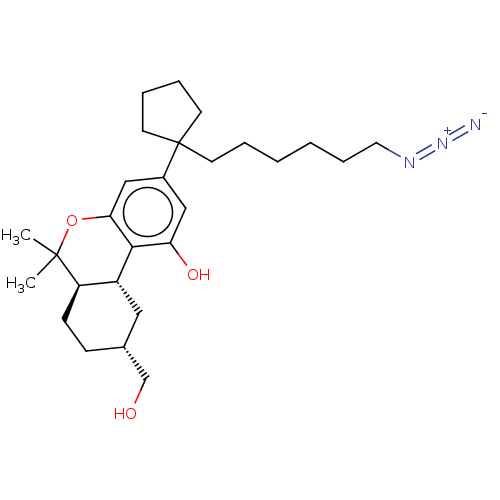 (CHEMBL5077315) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582406 (CHEMBL5091754) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582401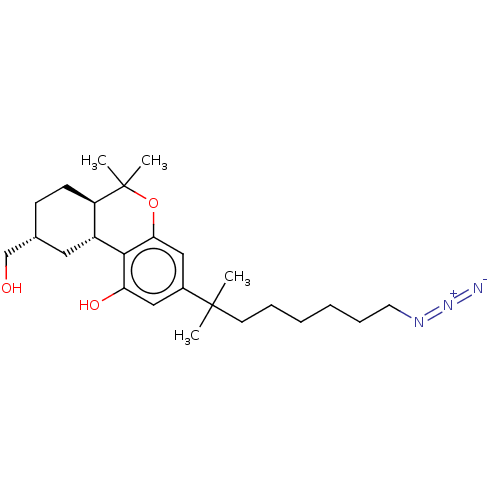 (CHEMBL5081770) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582408 (CHEMBL5080279) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582402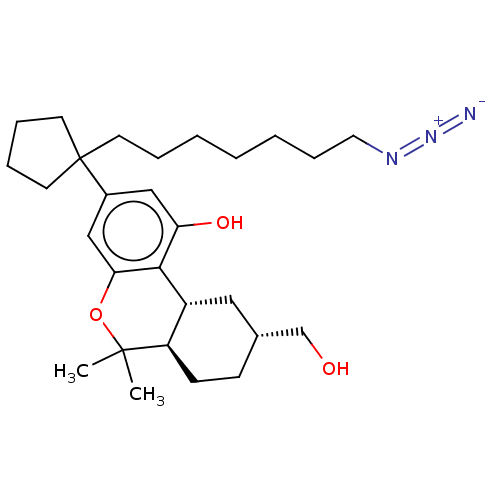 (CHEMBL5080450) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582412 (CHEMBL5087407) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582409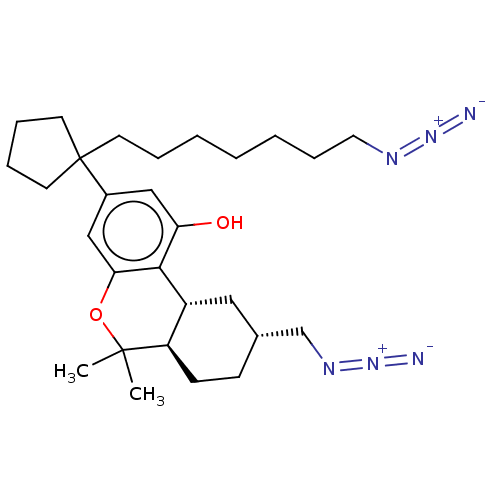 (CHEMBL5081699) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002059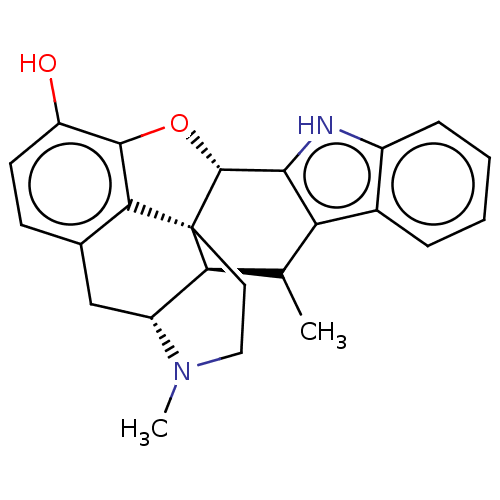 (CHEMBL606795) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 from rat brain membranes using [3H]DADLE | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002069 (CHEMBL611398) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 from rat brain membranes using [3H]DADLE | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002041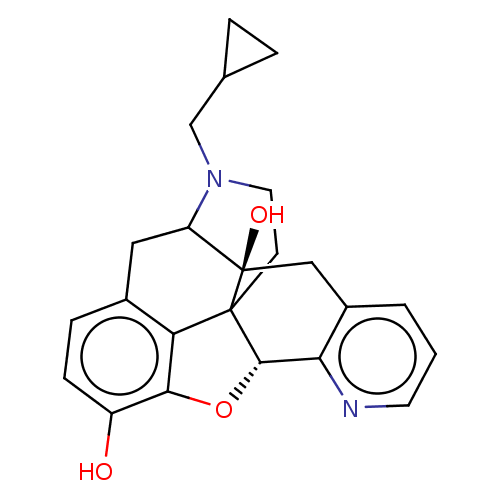 (CHEMBL195784) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 from rat brain membranes using [3H]DADLE | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002081 (CHEMBL610798) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 from rat brain membranes using [3H]DADLE | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582400 (CHEMBL5077315) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582406 (CHEMBL5091754) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582403 (CHEMBL5085420) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582400 (CHEMBL5077315) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582407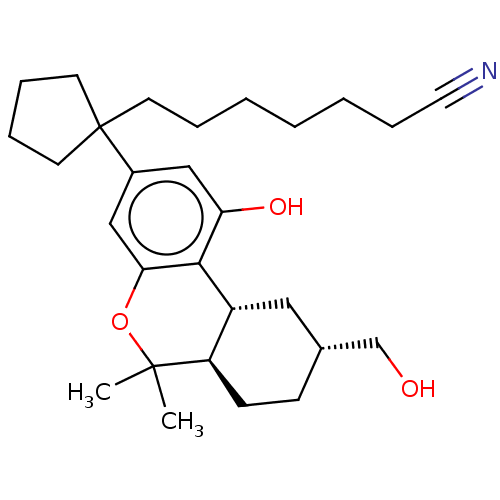 (CHEMBL5071417) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582401 (CHEMBL5081770) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002111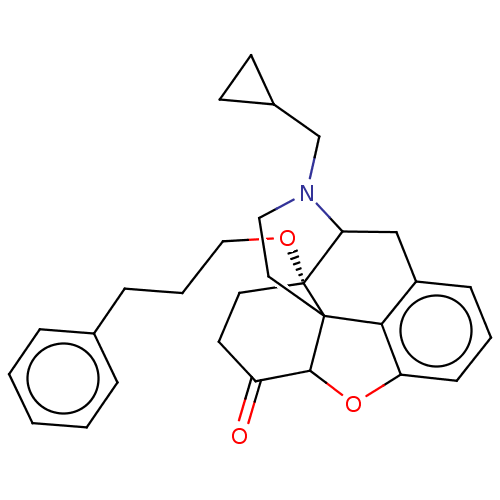 (CHEMBL371845) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 from rat brain membranes using [3H]DAMGO | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002105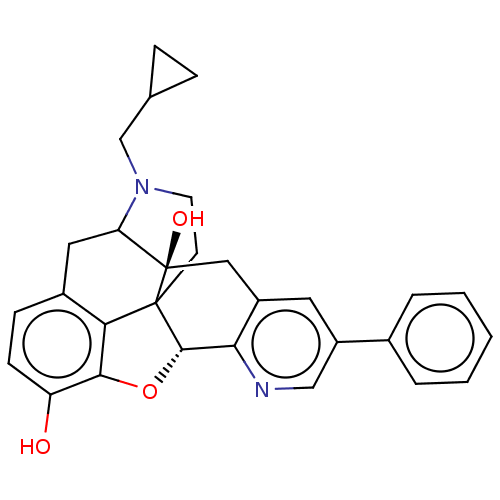 (CHEMBL192443) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 from rat brain membranes using [3H]DADLE | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083761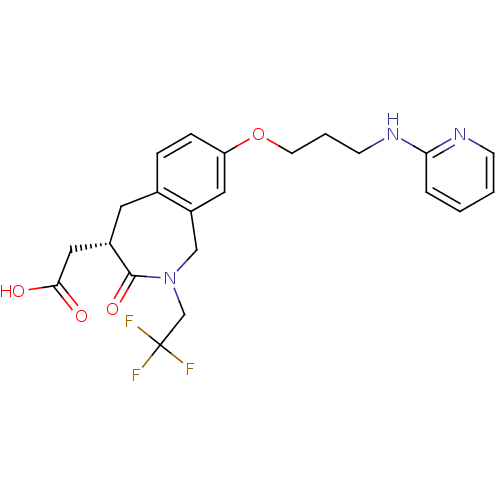 (CHEMBL87429 | [3-Oxo-8-[3-(pyridin-2-ylamino)-prop...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582408 (CHEMBL5080279) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582404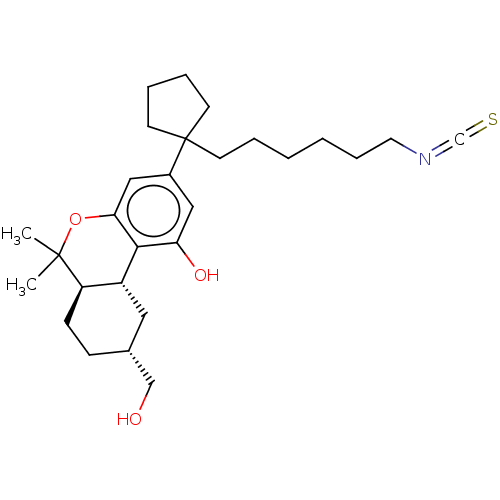 (CHEMBL5092703) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582405 (CHEMBL5074603) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582407 (CHEMBL5071417) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288109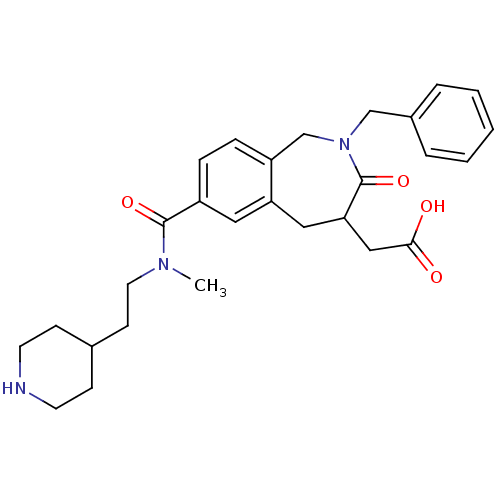 (CHEMBL82123 | {2-Benzyl-7-[methyl-(2-piperidin-4-y...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582405 (CHEMBL5074603) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50002116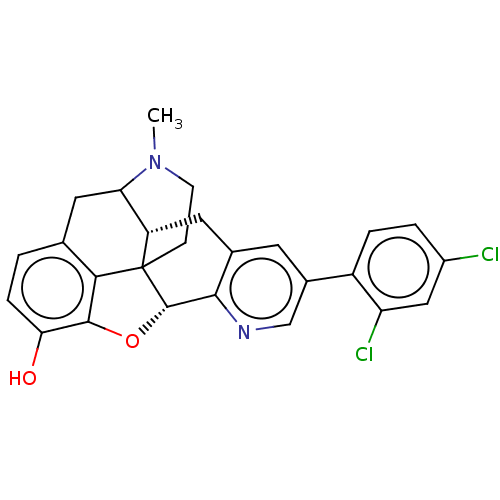 (CHEMBL363777) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Medicine& Dentistry of New Jersey-Robert Wood Johnson Medical School (UMDNJ-RWJMS) Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor delta 1 from rat brain membranes using [3H]DADLE | J Med Chem 48: 1620-9 (2005) Checked by Author Article DOI: 10.1021/jm049117e BindingDB Entry DOI: 10.7270/Q2ZP47MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50582404 (CHEMBL5092703) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from rat brain membrane CB1 receptor assessed as inhibition constant incubated for 1 hr by TopCount scintillation countin... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50173945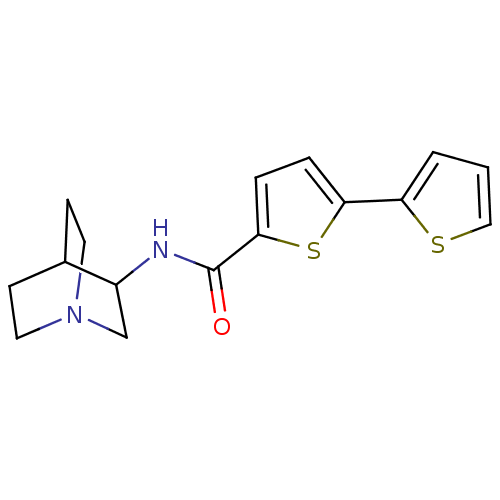 (CHEMBL195190 | [2,2']Bithiophenyl-5-carboxylic aci...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human nicotinic acetylcholine receptor alpha 7 expressed in GH4C1 cell using [3H]methyllycaconitine | Bioorg Med Chem Lett 15: 4727-30 (2005) Article DOI: 10.1016/j.bmcl.2005.07.070 BindingDB Entry DOI: 10.7270/Q28K79W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582409 (CHEMBL5081699) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083763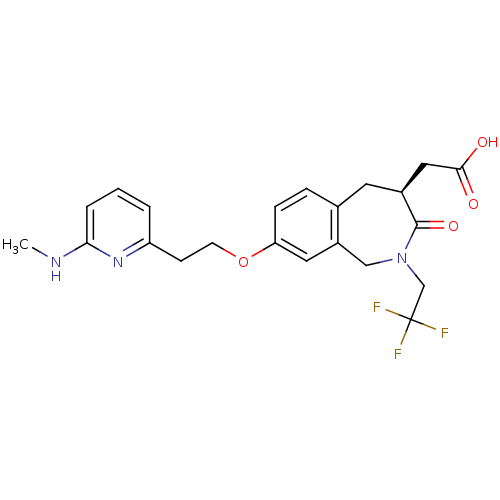 (CHEMBL86992 | [(S)-8-[2-(6-Methylamino-pyridin-2-y...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50083764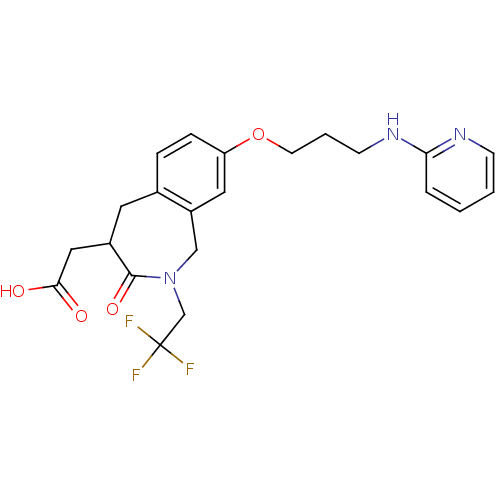 (CHEMBL421533 | [3-Oxo-8-[3-(pyridin-2-ylamino)-pro...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for Vitronectin receptor (alpha V beta 3) | J Med Chem 43: 22-6 (2000) BindingDB Entry DOI: 10.7270/Q290230D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-5 (Homo sapiens (Human)) | BDBM50078714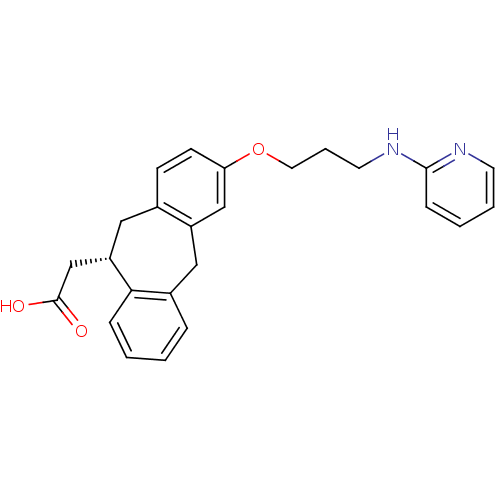 (CHEMBL288493 | SB-265123 | {(S)-3-[3-(Pyridin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity for non-peptide Vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 9: 1807-12 (1999) BindingDB Entry DOI: 10.7270/Q2Q23ZFC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Homo sapiens (Human)) | BDBM50173953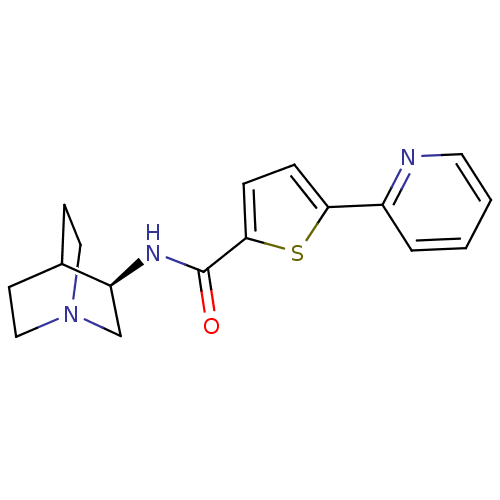 (CHEMBL364069 | N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company Ltd. Curated by ChEMBL | Assay Description Binding affinity towards human nicotinic acetylcholine receptor alpha 7 expressed in GH4C1 cell using [3H]methyllycaconitine | Bioorg Med Chem Lett 15: 4727-30 (2005) Article DOI: 10.1016/j.bmcl.2005.07.070 BindingDB Entry DOI: 10.7270/Q28K79W0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582404 (CHEMBL5092703) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288120 (CHEMBL313768 | {2-Cyclohexyl-7-[methyl-(2-piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582401 (CHEMBL5081770) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50582407 (CHEMBL5071417) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from human CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (MOUSE) | BDBM50582411 (CHEMBL5084325) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]CP55940 from mouse CB2 expressed in HEK293 cell membrane assessed as inhibition constant incubated for 1 hr by TopCount scintilla... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02053 BindingDB Entry DOI: 10.7270/Q2TB1BSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50288118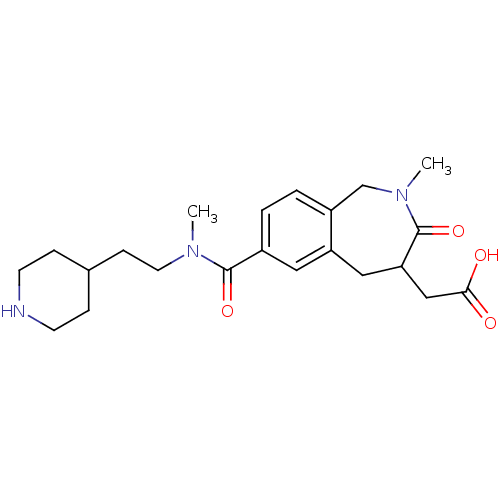 (CHEMBL312122 | {2-Methyl-7-[methyl-(2-piperidin-4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 6: 2481-2486 (1996) Article DOI: 10.1016/0960-894X(96)00432-5 BindingDB Entry DOI: 10.7270/Q25M65Q4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1886 total ) | Next | Last >> |