

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279520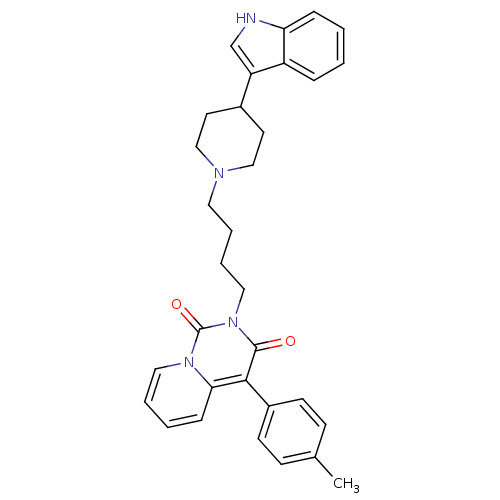 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279541 (2-(4-(4-(1H-indol-3-yl)piperidin-1-yl)butyl)-4-(2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279541 (2-(4-(4-(1H-indol-3-yl)piperidin-1-yl)butyl)-4-(2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279520 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279540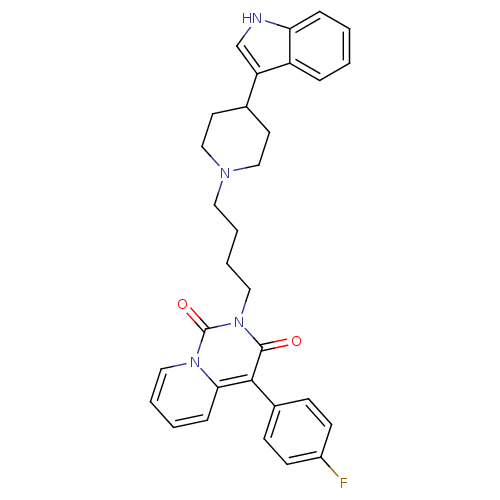 (4-(4-Fluoro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301431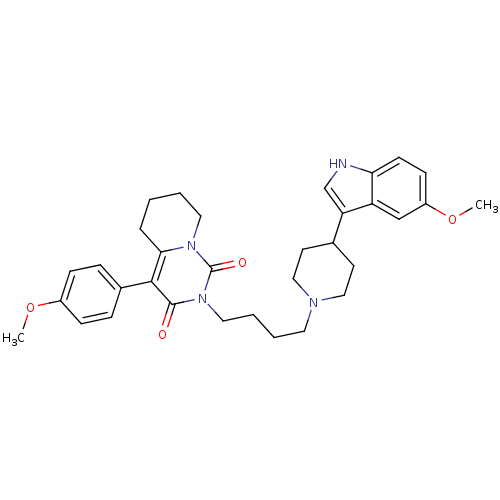 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279561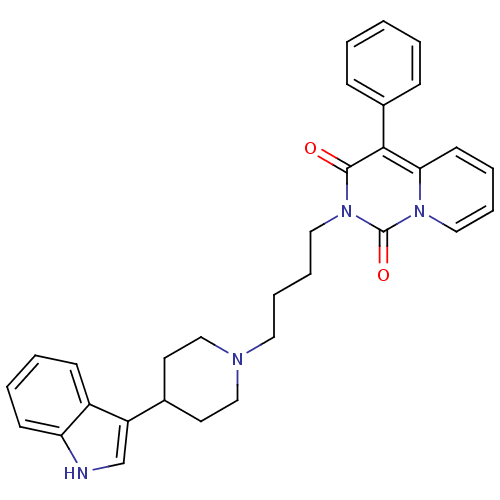 (2-(4-(4-(1H-indol-3-yl)piperidin-1-yl)butyl)-4-phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301442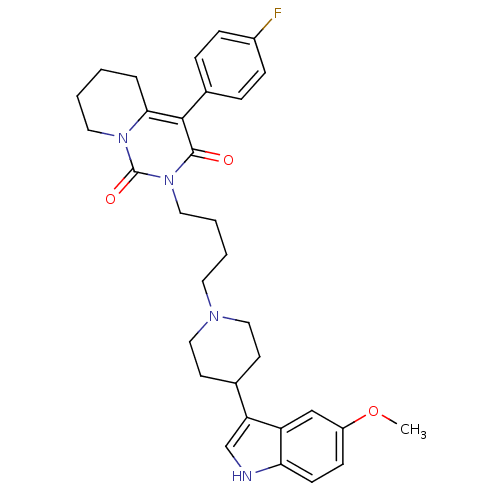 (4-(4-Fluoro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301429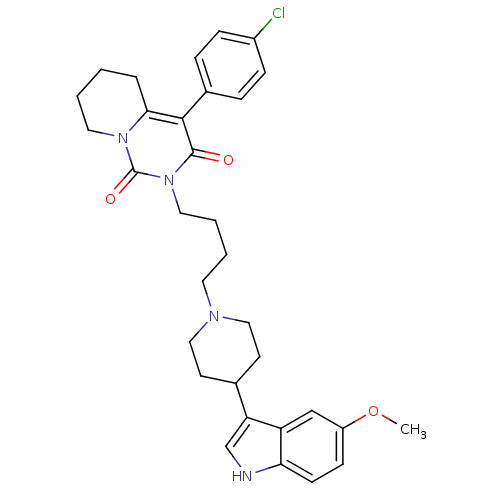 (4-(4-Chloro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279586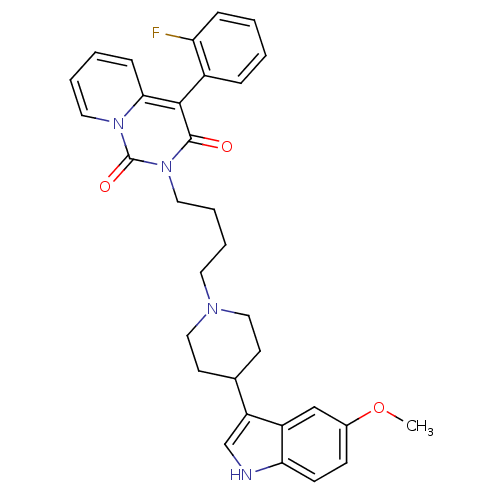 (4-(2-fluorophenyl)-2-(4-(4-(5-methoxy-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301432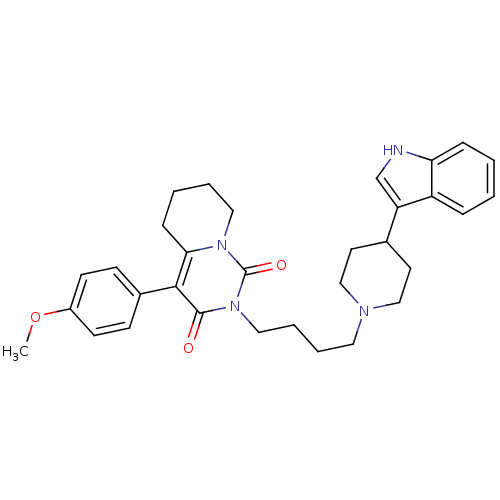 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301446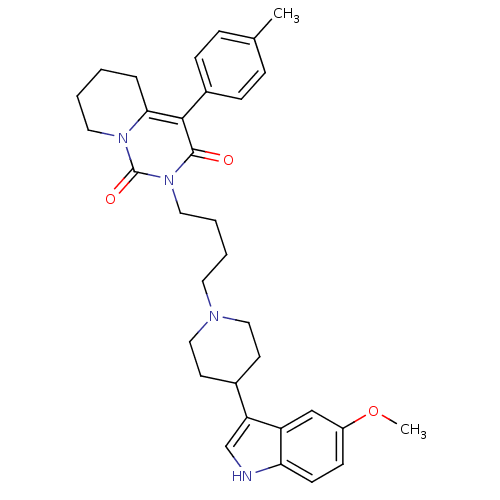 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301445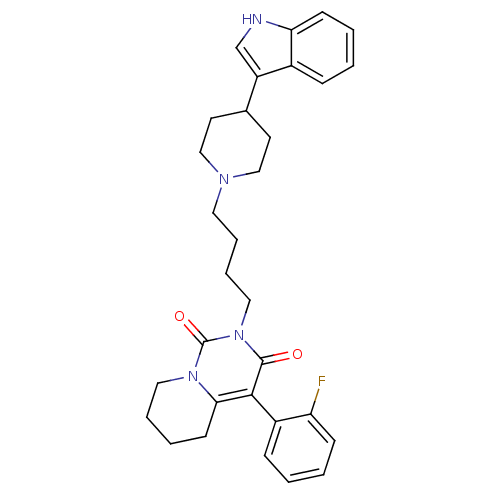 (4-(2-Fluoro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279585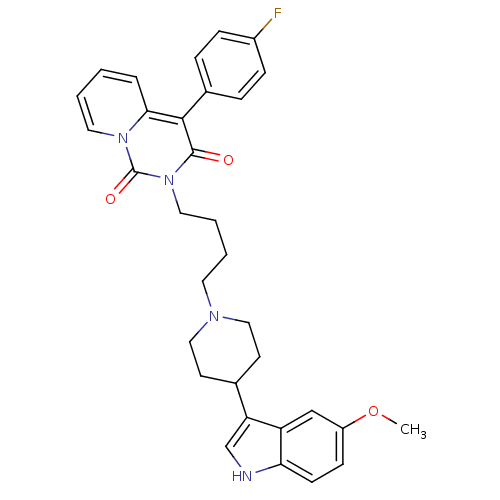 (4-(4-fluorophenyl)-2-(4-(4-(5-methoxy-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301441 (4-(2-Chloro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36.2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279539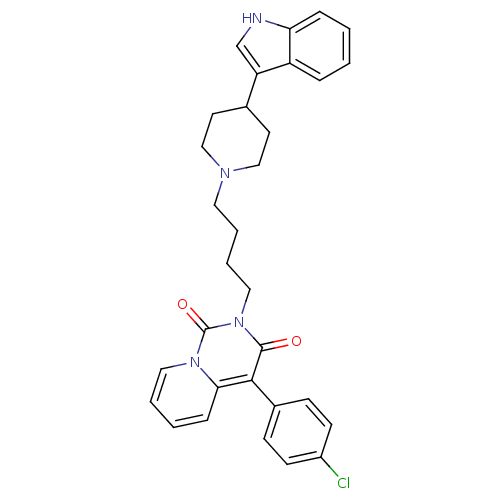 (4-(4-Chloro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279540 (4-(4-Fluoro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50088153 (4-Chloro-6-furan-3-yl-2-(4-methyl-piperazin-1-yl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Curated by ChEMBL | Assay Description Affinity 5-hydroxytryptamine 2A receptor of the rat brain cortex was assessed on the basis of their ability to displace [3H]-ketanserin | J Med Chem 43: 1901-9 (2000) BindingDB Entry DOI: 10.7270/Q2QF8S3H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279564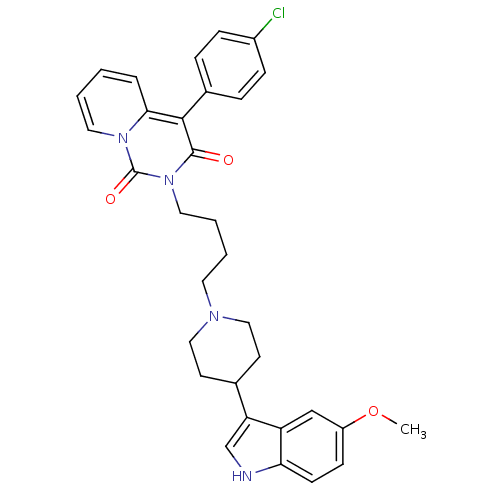 (4-(4-chlorophenyl)-2-(4-(4-(5-methoxy-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279519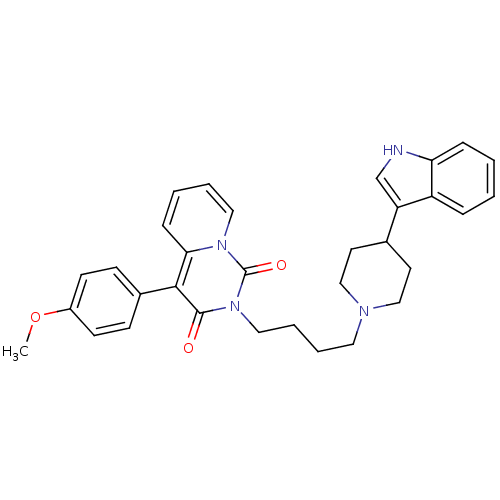 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301430 (4-(2-Fluoro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 45.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301428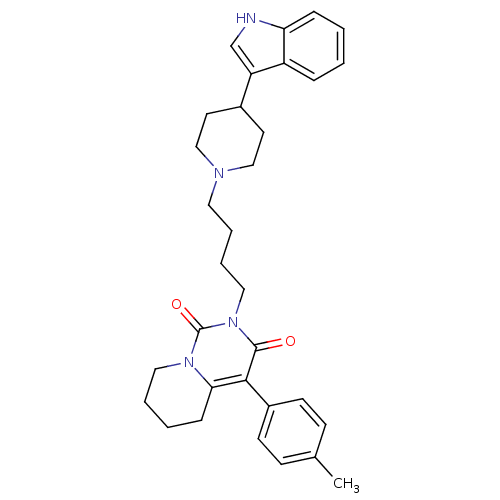 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301443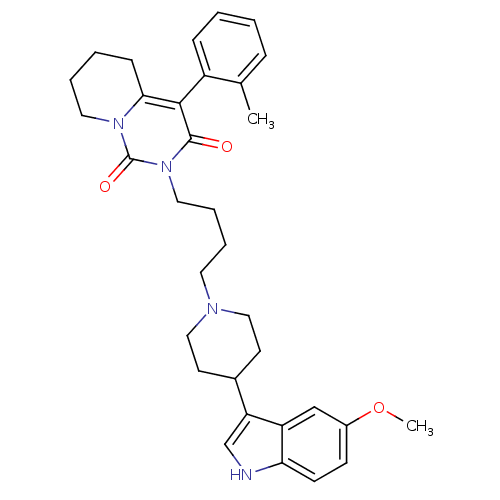 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279587 (2-(4-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50301444 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5-HT1A receptor in rat hippocampus | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279585 (4-(4-fluorophenyl)-2-(4-(4-(5-methoxy-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 54.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279561 (2-(4-(4-(1H-indol-3-yl)piperidin-1-yl)butyl)-4-phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 58.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279563 (2-(4-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279586 (4-(2-fluorophenyl)-2-(4-(4-(5-methoxy-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 59.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301432 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 71.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301442 (4-(4-Fluoro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301428 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-p...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50279562 (2-(4-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from 5HT1A receptor in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301431 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301441 (4-(2-Chloro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301445 (4-(2-Fluoro-phenyl)-2-{4-[4-(1H-indol-3-yl)-piperi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301437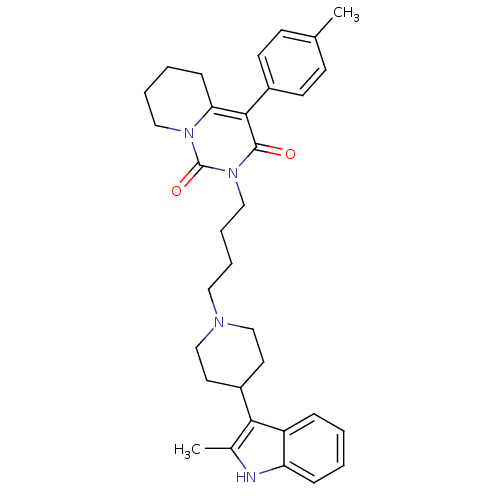 (2-{4-[4-(2-Methyl-1H-indol-3-yl)-piperidin-1-yl]-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301433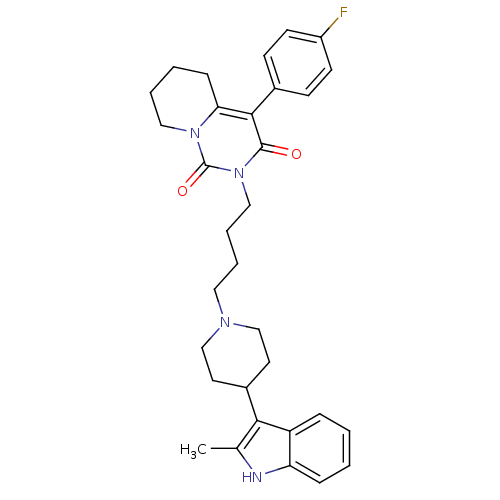 (4-(4-Fluoro-phenyl)-2-{4-[4-(2-methyl-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301444 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301443 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 124 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279587 (2-(4-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279562 (2-(4-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301446 (2-{4-[4-(5-Methoxy-1H-indol-3-yl)-piperidin-1-yl]-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279519 (2-{4-[4-(1H-Indol-3-yl)-piperidin-1-yl]-butyl}-4-(...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301430 (4-(2-Fluoro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301436 (4-(4-Methoxy-phenyl)-2-{4-[4-(2-methyl-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301429 (4-(4-Chloro-phenyl)-2-{4-[4-(5-methoxy-1H-indol-3-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 222 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279563 (2-(4-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50279564 (4-(4-chlorophenyl)-2-(4-(4-(5-methoxy-1H-indol-3-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from 5HTT in rat brain membranes | Eur J Med Chem 44: 1710-7 (2009) Article DOI: 10.1016/j.ejmech.2008.09.021 BindingDB Entry DOI: 10.7270/Q22N5249 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50301439 (2-{4-[4-(2-Methyl-1H-indol-3-yl)-piperidin-1-yl]-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 274 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Warsaw Curated by ChEMBL | Assay Description Displacement of [3H]citalopram from SERT in rat cerebral cortex by liquid scintillation counting | Eur J Med Chem 44: 4702-15 (2009) Article DOI: 10.1016/j.ejmech.2009.07.007 BindingDB Entry DOI: 10.7270/Q28C9W93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 70 total ) | Next | Last >> |