 Found 1207 hits with Last Name = 'lorenz' and Initial = 'm'
Found 1207 hits with Last Name = 'lorenz' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human p110alpha/p85alpha using PIP2 as substrate in presence of ATP measured by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50570237

(CHEMBL4864407)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCc1cncc(c1)-c1cnc3ccc-2cn13 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human p110delta/p85alpha using PIP2 as substrate in presence of ATP measured by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12396
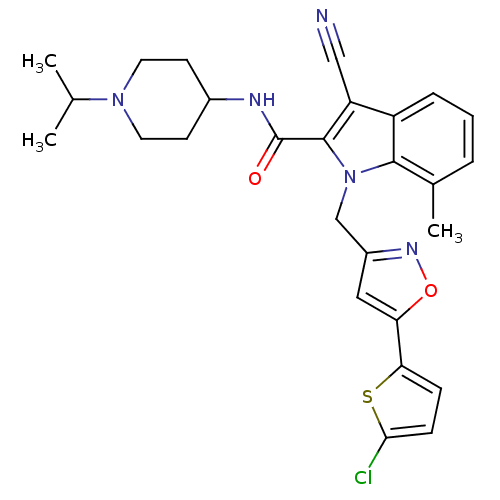
(1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-3...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1c(C#N)c2cccc(C)c2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C27H28ClN5O2S/c1-16(2)32-11-9-18(10-12-32)30-27(34)26-21(14-29)20-6-4-5-17(3)25(20)33(26)15-19-13-22(35-31-19)23-7-8-24(28)36-23/h4-8,13,16,18H,9-12,15H2,1-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human p110delta/p85alpha using PIP2 as substrate in presence of ATP measured by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50570237

(CHEMBL4864407)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCc1cncc(c1)-c1cnc3ccc-2cn13 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p110alpha H1047R mutant/p85alpha (unknown origin) using PIP2:3PS lipid kinase as substrate in presence of ATP measured by ADP-Glo lipid... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12389
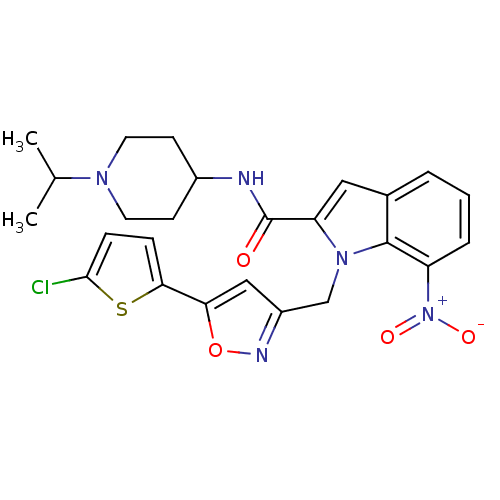
(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2cccc([N+]([O-])=O)c2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26ClN5O4S/c1-15(2)29-10-8-17(9-11-29)27-25(32)20-12-16-4-3-5-19(31(33)34)24(16)30(20)14-18-13-21(35-28-18)22-6-7-23(26)36-22/h3-7,12-13,15,17H,8-11,14H2,1-2H3,(H,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50570237

(CHEMBL4864407)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCc1cncc(c1)-c1cnc3ccc-2cn13 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human p110alpha/p85alpha using PIP2 as substrate in presence of ATP measured by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12387

(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2cccc(C)c2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C26H29ClN4O2S/c1-16(2)30-11-9-19(10-12-30)28-26(32)21-13-18-6-4-5-17(3)25(18)31(21)15-20-14-22(33-29-20)23-7-8-24(27)34-23/h4-8,13-14,16,19H,9-12,15H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50570237

(CHEMBL4864407)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCc1cncc(c1)-c1cnc3ccc-2cn13 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of p110alpha E542K mutant/p85alpha (unknown origin) using PIP2:3PS lipid kinase as substrate in presence of ATP measured by ADP-Glo lipid ... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12395
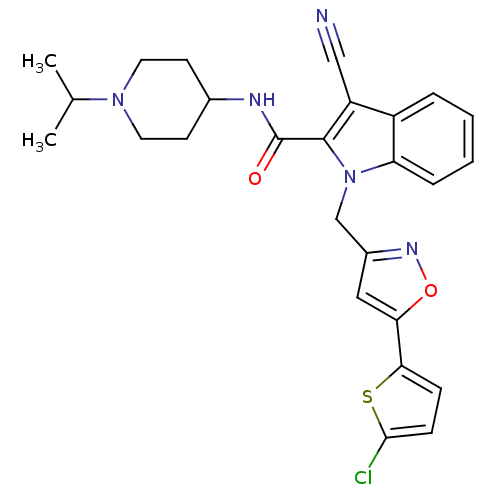
(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1c(C#N)c2ccccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C26H26ClN5O2S/c1-16(2)31-11-9-17(10-12-31)29-26(33)25-20(14-28)19-5-3-4-6-21(19)32(25)15-18-13-22(34-30-18)23-7-8-24(27)35-23/h3-8,13,16-17H,9-12,15H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human p110gamma using PIP2 as substrate in presence of ATP measured by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50145416

(GSK2126458 | Omipalisib)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccnnc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)15-2-4-22-20(10-15)19(7-8-28-22)16-6-9-30-31-14-16/h2-14,32H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human p110beta/p85alpha using PIP2 as substrate in presence of ATP measured by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12384
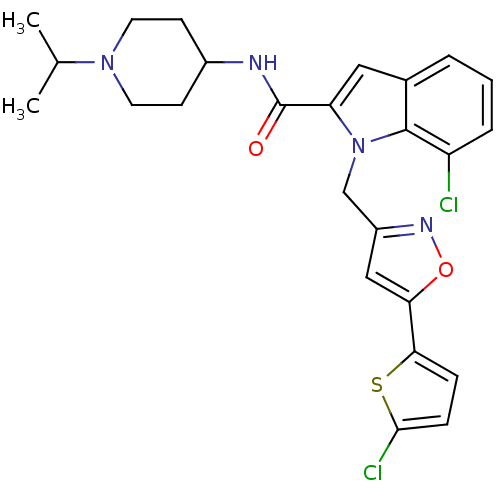
(2-Carboxyindole Scaffold 35 | 7-Chloro-1-[5-(5-chl...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2cccc(Cl)c2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26Cl2N4O2S/c1-15(2)30-10-8-17(9-11-30)28-25(32)20-12-16-4-3-5-19(26)24(16)31(20)14-18-13-21(33-29-18)22-6-7-23(27)34-22/h3-7,12-13,15,17H,8-11,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50570237

(CHEMBL4864407)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCc1cncc(c1)-c1cnc3ccc-2cn13 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human p110beta/p85alpha using PIP2 as substrate in presence of ATP measured by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50570237

(CHEMBL4864407)Show SMILES COc1ncc-2cc1NS(=O)(=O)c1cccc(c1)C(=O)NCc1cncc(c1)-c1cnc3ccc-2cn13 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human p110gamma using PIP2 as substrate in presence of ATP measured by HTRF assay |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113109
BindingDB Entry DOI: 10.7270/Q2JH3QZ4 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12381
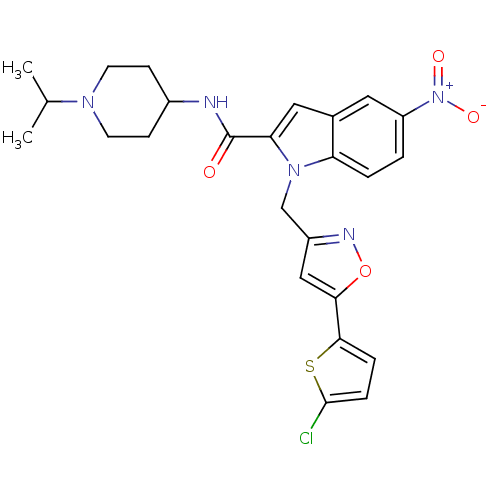
(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2cc(ccc2n1Cc1cc(on1)-c1ccc(Cl)s1)[N+]([O-])=O Show InChI InChI=1S/C25H26ClN5O4S/c1-15(2)29-9-7-17(8-10-29)27-25(32)21-12-16-11-19(31(33)34)3-4-20(16)30(21)14-18-13-22(35-28-18)23-5-6-24(26)36-23/h3-6,11-13,15,17H,7-10,14H2,1-2H3,(H,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12388
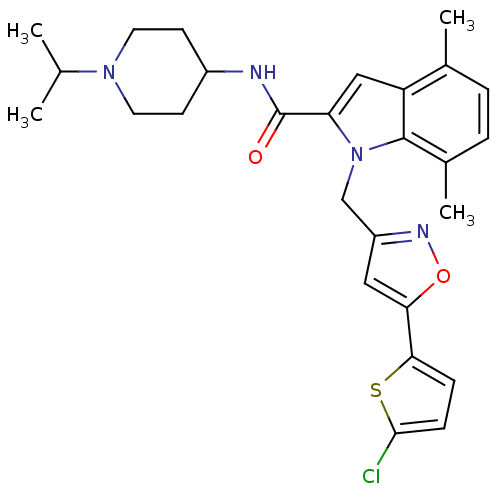
(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2c(C)ccc(C)c2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C27H31ClN4O2S/c1-16(2)31-11-9-19(10-12-31)29-27(33)22-14-21-17(3)5-6-18(4)26(21)32(22)15-20-13-23(34-30-20)24-7-8-25(28)35-24/h5-8,13-14,16,19H,9-12,15H2,1-4H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM15867

(1-{[(5-chloropyridin-2-yl)carbamoyl]methyl}-N-[1-(...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2ccccc2n1CC(=O)Nc1ccc(Cl)cn1 Show InChI InChI=1S/C24H28ClN5O2/c1-16(2)29-11-9-19(10-12-29)27-24(32)21-13-17-5-3-4-6-20(17)30(21)15-23(31)28-22-8-7-18(25)14-26-22/h3-8,13-14,16,19H,9-12,15H2,1-2H3,(H,27,32)(H,26,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
Bioorg Med Chem Lett 14: 4191-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.020
BindingDB Entry DOI: 10.7270/Q22805WQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12391
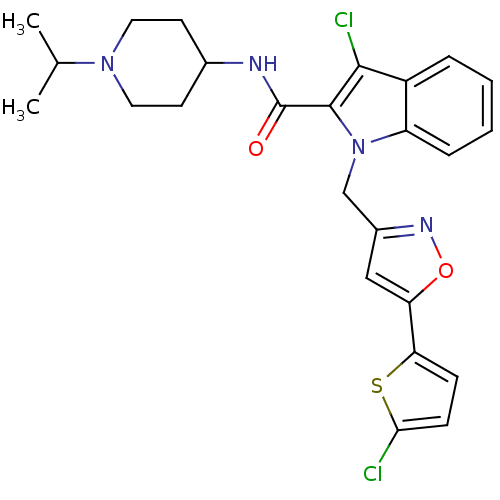
(2-Carboxyindole Scaffold 23 | 3-chloro-1-{[5-(5-ch...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1c(Cl)c2ccccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26Cl2N4O2S/c1-15(2)30-11-9-16(10-12-30)28-25(32)24-23(27)18-5-3-4-6-19(18)31(24)14-17-13-20(33-29-17)21-7-8-22(26)34-21/h3-8,13,15-16H,9-12,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12379
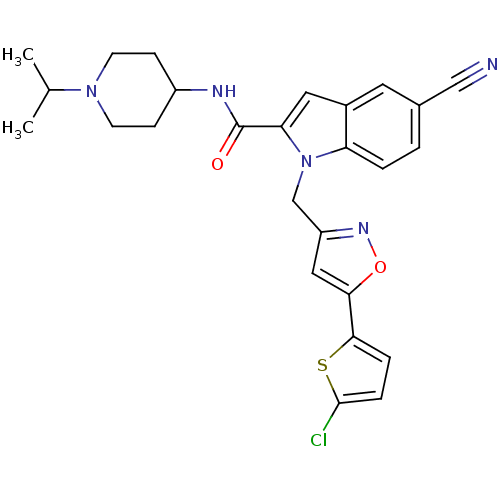
(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2cc(ccc2n1Cc1cc(on1)-c1ccc(Cl)s1)C#N Show InChI InChI=1S/C26H26ClN5O2S/c1-16(2)31-9-7-19(8-10-31)29-26(33)22-12-18-11-17(14-28)3-4-21(18)32(22)15-20-13-23(34-30-20)24-5-6-25(27)35-24/h3-6,11-13,16,19H,7-10,15H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12390
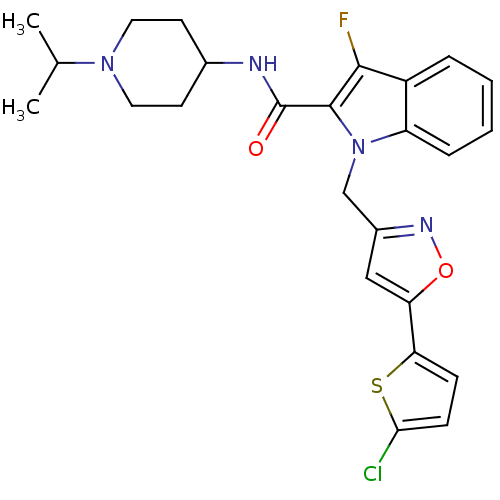
(1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-3...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1c(F)c2ccccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26ClFN4O2S/c1-15(2)30-11-9-16(10-12-30)28-25(32)24-23(27)18-5-3-4-6-19(18)31(24)14-17-13-20(33-29-17)21-7-8-22(26)34-21/h3-8,13,15-16H,9-12,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12392
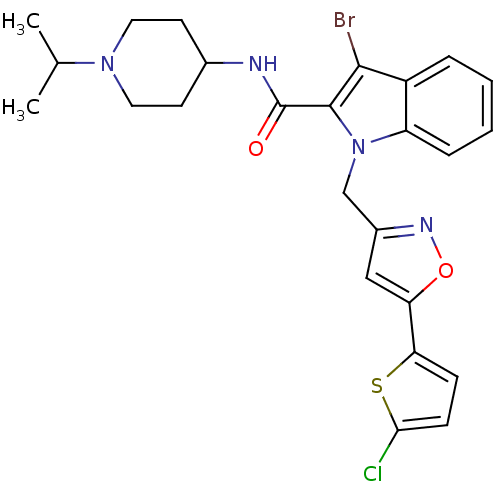
(2-Carboxyindole Scaffold 24 | 3-Bromo-1-[5-(5-chlo...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1c(Br)c2ccccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26BrClN4O2S/c1-15(2)30-11-9-16(10-12-30)28-25(32)24-23(26)18-5-3-4-6-19(18)31(24)14-17-13-20(33-29-17)21-7-8-22(27)34-21/h3-8,13,15-16H,9-12,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50420714
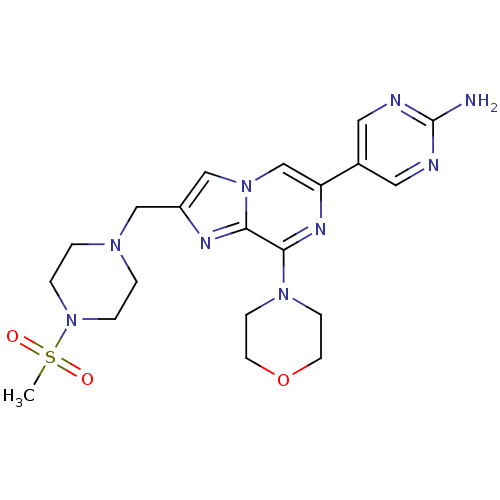
(CHEMBL2087474)Show SMILES CS(=O)(=O)N1CCN(Cc2cn3cc(nc(N4CCOCC4)c3n2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H27N9O3S/c1-33(30,31)29-4-2-26(3-5-29)12-16-13-28-14-17(15-10-22-20(21)23-11-15)25-19(18(28)24-16)27-6-8-32-9-7-27/h10-11,13-14H,2-9,12H2,1H3,(H2,21,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO)
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha (p110alpha/p85alpha) by ADP accumulation based HTRF assay |
Bioorg Med Chem Lett 22: 3460-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.090
BindingDB Entry DOI: 10.7270/Q26W9CCB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50420730
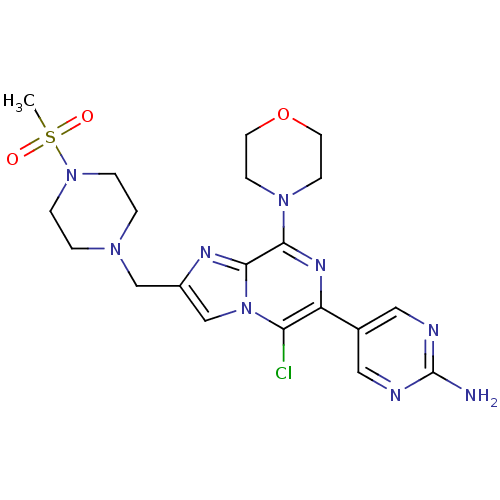
(CHEMBL2087493)Show SMILES CS(=O)(=O)N1CCN(Cc2cn3c(Cl)c(nc(N4CCOCC4)c3n2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26ClN9O3S/c1-34(31,32)29-4-2-27(3-5-29)12-15-13-30-17(21)16(14-10-23-20(22)24-11-14)26-18(19(30)25-15)28-6-8-33-9-7-28/h10-11,13H,2-9,12H2,1H3,(H2,22,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO)
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha (p110alpha/p85alpha) by ADP accumulation based HTRF assay |
Bioorg Med Chem Lett 22: 3460-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.090
BindingDB Entry DOI: 10.7270/Q26W9CCB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50388102
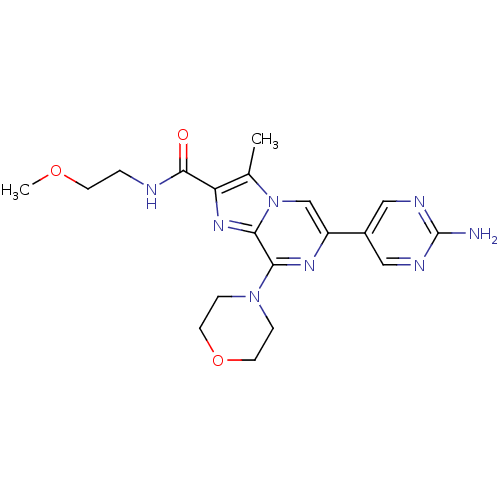
(CHEMBL2058172)Show SMILES COCCNC(=O)c1nc2c(nc(cn2c1C)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C19H24N8O3/c1-12-15(18(28)21-3-6-29-2)25-17-16(26-4-7-30-8-5-26)24-14(11-27(12)17)13-9-22-19(20)23-10-13/h9-11H,3-8H2,1-2H3,(H,21,28)(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha by HTRF assay |
Bioorg Med Chem Lett 22: 5208-14 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.093
BindingDB Entry DOI: 10.7270/Q24Q7W1Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50365517

(CHEMBL1957508)Show SMILES COCCCNC(=O)c1cn2cc(nc(N3CCOCC3)c2n1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C22H25N7O3/c1-31-9-3-6-23-22(30)19-14-29-13-18(15-4-2-5-17-16(15)12-24-27-17)25-20(21(29)26-19)28-7-10-32-11-8-28/h2,4-5,12-14H,3,6-11H2,1H3,(H,23,30)(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO). C/Melchor Fern£ndez Almagro 3
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110delta/p85alpha |
Bioorg Med Chem Lett 22: 1874-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.074
BindingDB Entry DOI: 10.7270/Q29Z95DX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12372
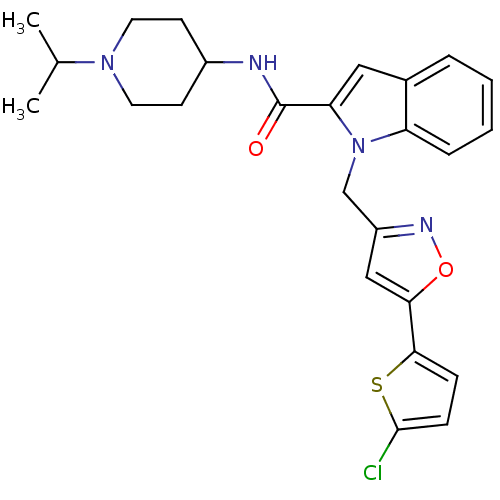
(1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2ccccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H27ClN4O2S/c1-16(2)29-11-9-18(10-12-29)27-25(31)21-13-17-5-3-4-6-20(17)30(21)15-19-14-22(32-28-19)23-7-8-24(26)33-23/h3-8,13-14,16,18H,9-12,15H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
Bioorg Med Chem Lett 14: 4197-201 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.019
BindingDB Entry DOI: 10.7270/Q2610XKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12400
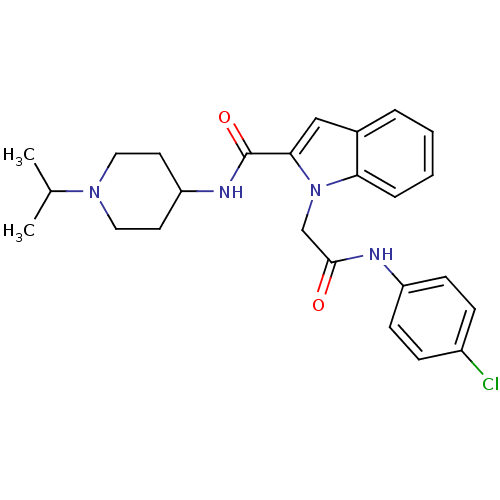
(1-[(4-Chloro-phenylcarbamoyl)-methyl]-1H-indole-2-...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2ccccc2n1CC(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C25H29ClN4O2/c1-17(2)29-13-11-21(12-14-29)28-25(32)23-15-18-5-3-4-6-22(18)30(23)16-24(31)27-20-9-7-19(26)8-10-20/h3-10,15,17,21H,11-14,16H2,1-2H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12393
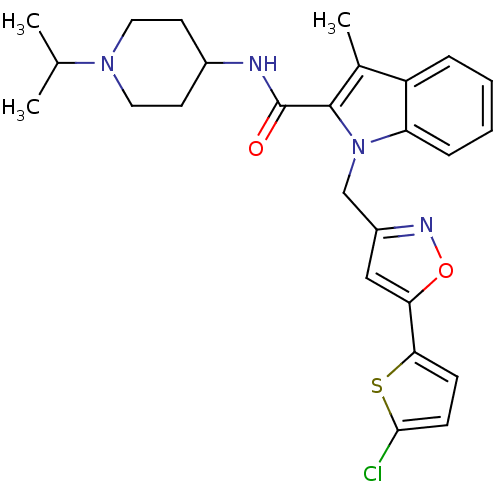
(1-[5-(5-Chlorothiophen-2-yl)isoxazol-3-ylmethyl]-3...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1c(C)c2ccccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C26H29ClN4O2S/c1-16(2)30-12-10-18(11-13-30)28-26(32)25-17(3)20-6-4-5-7-21(20)31(25)15-19-14-22(33-29-19)23-8-9-24(27)34-23/h4-9,14,16,18H,10-13,15H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12385
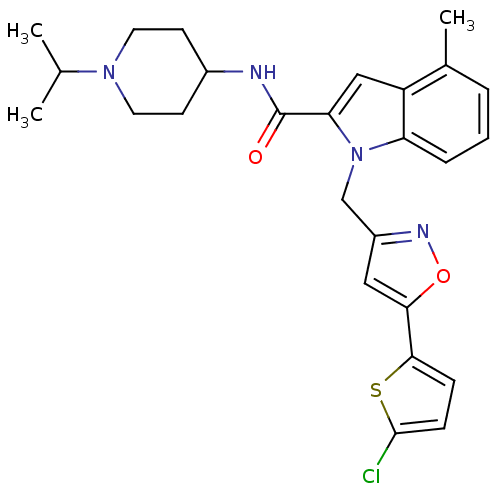
(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2c(C)cccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C26H29ClN4O2S/c1-16(2)30-11-9-18(10-12-30)28-26(32)22-14-20-17(3)5-4-6-21(20)31(22)15-19-13-23(33-29-19)24-7-8-25(27)34-24/h4-8,13-14,16,18H,9-12,15H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12374
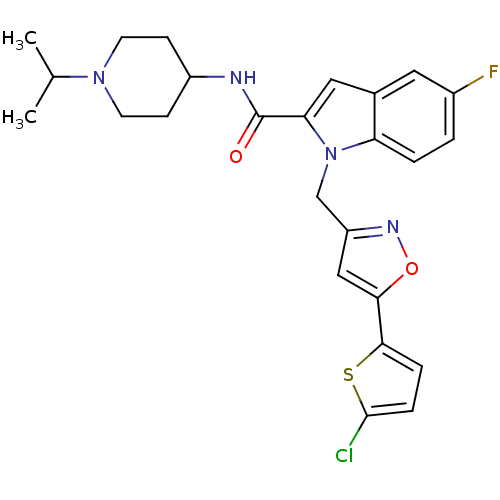
(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2cc(F)ccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26ClFN4O2S/c1-15(2)30-9-7-18(8-10-30)28-25(32)21-12-16-11-17(27)3-4-20(16)31(21)14-19-13-22(33-29-19)23-5-6-24(26)34-23/h3-6,11-13,15,18H,7-10,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12372

(1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2ccccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H27ClN4O2S/c1-16(2)29-11-9-18(10-12-29)27-25(31)21-13-17-5-3-4-6-20(17)30(21)15-19-14-22(32-28-19)23-7-8-24(26)33-23/h3-8,13-14,16,18H,9-12,15H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12400

(1-[(4-Chloro-phenylcarbamoyl)-methyl]-1H-indole-2-...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2ccccc2n1CC(=O)Nc1ccc(Cl)cc1 Show InChI InChI=1S/C25H29ClN4O2/c1-17(2)29-13-11-21(12-14-29)28-25(32)23-15-18-5-3-4-6-22(18)30(23)16-24(31)27-20-9-7-19(26)8-10-20/h3-10,15,17,21H,11-14,16H2,1-2H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
Bioorg Med Chem Lett 14: 4191-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.020
BindingDB Entry DOI: 10.7270/Q22805WQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12372

(1-{[5-(5-chlorothiophen-2-yl)-1,2-oxazol-3-yl]meth...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2ccccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H27ClN4O2S/c1-16(2)29-11-9-18(10-12-29)27-25(31)21-13-17-5-3-4-6-20(17)30(21)15-19-14-22(32-28-19)23-7-8-24(26)33-23/h3-8,13-14,16,18H,9-12,15H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
Bioorg Med Chem Lett 14: 4191-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.020
BindingDB Entry DOI: 10.7270/Q22805WQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM15862

(1-{[3-(5-chlorothiophen-2-yl)-1,2-oxazol-5-yl]meth...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2ccccc2n1Cc1cc(no1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H27ClN4O2S/c1-16(2)29-11-9-18(10-12-29)27-25(31)22-13-17-5-3-4-6-21(17)30(22)15-19-14-20(28-32-19)23-7-8-24(26)33-23/h3-8,13-14,16,18H,9-12,15H2,1-2H3,(H,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
Bioorg Med Chem Lett 14: 4191-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.020
BindingDB Entry DOI: 10.7270/Q22805WQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50365514
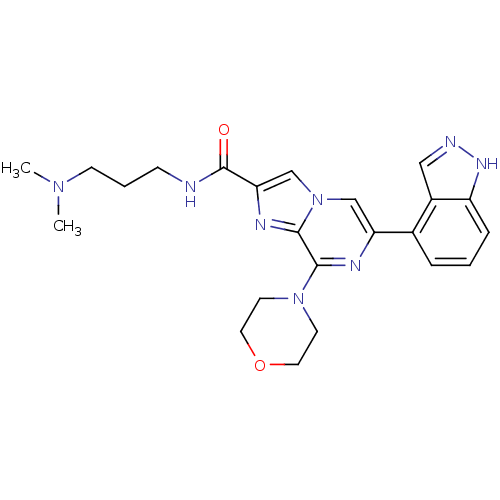
(CHEMBL1957505)Show SMILES CN(C)CCCNC(=O)c1cn2cc(nc(N3CCOCC3)c2n1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C23H28N8O2/c1-29(2)8-4-7-24-23(32)20-15-31-14-19(16-5-3-6-18-17(16)13-25-28-18)26-21(22(31)27-20)30-9-11-33-12-10-30/h3,5-6,13-15H,4,7-12H2,1-2H3,(H,24,32)(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO). C/Melchor Fern£ndez Almagro 3
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110delta/p85alpha |
Bioorg Med Chem Lett 22: 1874-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.074
BindingDB Entry DOI: 10.7270/Q29Z95DX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM15863
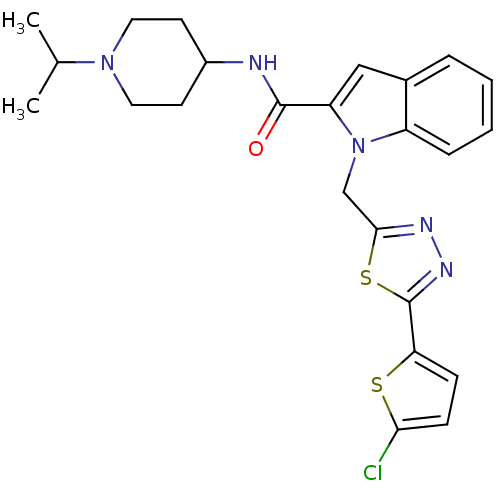
(1-{[5-(5-chlorothiophen-2-yl)-1,3,4-thiadiazol-2-y...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2ccccc2n1Cc1nnc(s1)-c1ccc(Cl)s1 Show InChI InChI=1S/C24H26ClN5OS2/c1-15(2)29-11-9-17(10-12-29)26-23(31)19-13-16-5-3-4-6-18(16)30(19)14-22-27-28-24(33-22)20-7-8-21(25)32-20/h3-8,13,15,17H,9-12,14H2,1-2H3,(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | -47.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma Deutschland GmbH
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
Bioorg Med Chem Lett 14: 4191-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.020
BindingDB Entry DOI: 10.7270/Q22805WQ |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12375
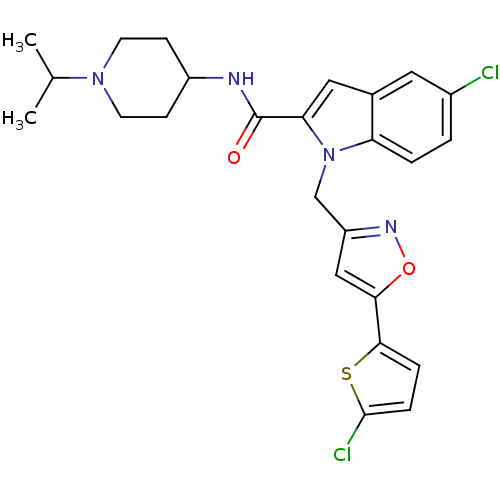
(2-Carboxyindole Scaffold 26 | 5-Chloro-1-[5-(5-chl...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2cc(Cl)ccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26Cl2N4O2S/c1-15(2)30-9-7-18(8-10-30)28-25(32)21-12-16-11-17(26)3-4-20(16)31(21)14-19-13-22(33-29-19)23-5-6-24(27)34-23/h3-6,11-13,15,18H,7-10,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50420719

(CHEMBL2087479)Show SMILES CS(=O)(=O)N1CCN(Cc2cn3cc(nc(N4CCOCC4)c3n2)-c2cnc(N)nc2C(F)(F)F)CC1 Show InChI InChI=1S/C21H26F3N9O3S/c1-37(34,35)33-4-2-30(3-5-33)11-14-12-32-13-16(15-10-26-20(25)29-17(15)21(22,23)24)28-19(18(32)27-14)31-6-8-36-9-7-31/h10,12-13H,2-9,11H2,1H3,(H2,25,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO)
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta (p110delta/p85alpha) by ADP accumulation based HTRF assay |
Bioorg Med Chem Lett 22: 3460-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.090
BindingDB Entry DOI: 10.7270/Q26W9CCB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50420736

(CHEMBL2087483)Show SMILES C[C@H]1CN(Cc2cn3cc(nc(N4CCOCC4)c3n2)-c2cnc(N)nc2)CCN1S(C)(=O)=O |r| Show InChI InChI=1S/C21H29N9O3S/c1-15-11-27(3-4-30(15)34(2,31)32)12-17-13-29-14-18(16-9-23-21(22)24-10-16)26-20(19(29)25-17)28-5-7-33-8-6-28/h9-10,13-15H,3-8,11-12H2,1-2H3,(H2,22,23,24)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO)
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha (p110alpha/p85alpha) by ADP accumulation based HTRF assay |
Bioorg Med Chem Lett 22: 3460-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.090
BindingDB Entry DOI: 10.7270/Q26W9CCB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50365513

(CHEMBL1957504)Show SMILES O=C(NCCN1CCOCC1)c1cn2cc(nc(N3CCOCC3)c2n1)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C24H28N8O3/c33-24(25-4-5-30-6-10-34-11-7-30)21-16-32-15-20(17-2-1-3-19-18(17)14-26-29-19)27-22(23(32)28-21)31-8-12-35-13-9-31/h1-3,14-16H,4-13H2,(H,25,33)(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO). C/Melchor Fern£ndez Almagro 3
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110delta/p85alpha |
Bioorg Med Chem Lett 22: 1874-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.074
BindingDB Entry DOI: 10.7270/Q29Z95DX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12382
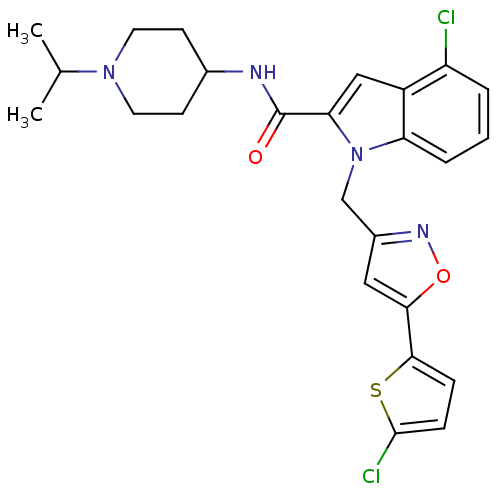
(2-Carboxyindole Scaffold 33 | 4-Chloro-1-[5-(5-chl...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2c(Cl)cccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26Cl2N4O2S/c1-15(2)30-10-8-16(9-11-30)28-25(32)21-13-18-19(26)4-3-5-20(18)31(21)14-17-12-22(33-29-17)23-6-7-24(27)34-23/h3-7,12-13,15-16H,8-11,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12383
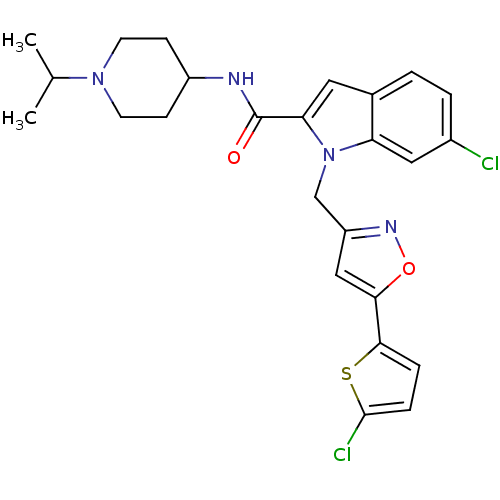
(2-Carboxyindole Scaffold 34 | 6-Chloro-1-[5-(5-chl...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2ccc(Cl)cc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26Cl2N4O2S/c1-15(2)30-9-7-18(8-10-30)28-25(32)21-11-16-3-4-17(26)12-20(16)31(21)14-19-13-22(33-29-19)23-5-6-24(27)34-23/h3-6,11-13,15,18H,7-10,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12397
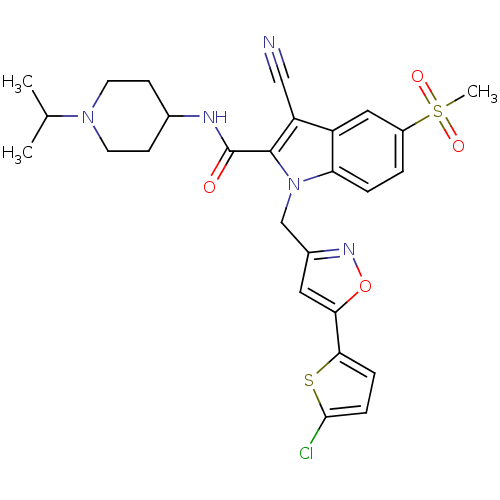
(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1c(C#N)c2cc(ccc2n1Cc1cc(on1)-c1ccc(Cl)s1)S(C)(=O)=O Show InChI InChI=1S/C27H28ClN5O4S2/c1-16(2)32-10-8-17(9-11-32)30-27(34)26-21(14-29)20-13-19(39(3,35)36)4-5-22(20)33(26)15-18-12-23(37-31-18)24-6-7-25(28)38-24/h4-7,12-13,16-17H,8-11,15H2,1-3H3,(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50420719

(CHEMBL2087479)Show SMILES CS(=O)(=O)N1CCN(Cc2cn3cc(nc(N4CCOCC4)c3n2)-c2cnc(N)nc2C(F)(F)F)CC1 Show InChI InChI=1S/C21H26F3N9O3S/c1-37(34,35)33-4-2-30(3-5-33)11-14-12-32-13-16(15-10-26-20(25)29-17(15)21(22,23)24)28-19(18(32)27-14)31-6-8-36-9-7-31/h10,12-13H,2-9,11H2,1H3,(H2,25,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO)
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha (p110alpha/p85alpha) by ADP accumulation based HTRF assay |
Bioorg Med Chem Lett 22: 3460-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.090
BindingDB Entry DOI: 10.7270/Q26W9CCB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
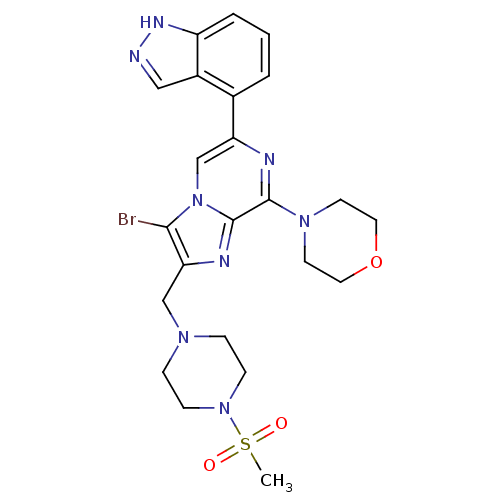
(Homo sapiens (Human)) | BDBM50365507
(CHEMBL1957498)Show SMILES CS(=O)(=O)N1CCN(Cc2nc3c(nc(cn3c2Br)-c2cccc3[nH]ncc23)N2CCOCC2)CC1 Show InChI InChI=1S/C23H27BrN8O3S/c1-36(33,34)31-7-5-29(6-8-31)14-20-21(24)32-15-19(16-3-2-4-18-17(16)13-25-28-18)26-22(23(32)27-20)30-9-11-35-12-10-30/h2-4,13,15H,5-12,14H2,1H3,(H,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO). C/Melchor Fern£ndez Almagro 3
Curated by ChEMBL
| Assay Description
Inhibition of human PI3K p110delta/p85alpha |
Bioorg Med Chem Lett 22: 1874-8 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.074
BindingDB Entry DOI: 10.7270/Q29Z95DX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50388102

(CHEMBL2058172)Show SMILES COCCNC(=O)c1nc2c(nc(cn2c1C)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C19H24N8O3/c1-12-15(18(28)21-3-6-29-2)25-17-16(26-4-7-30-8-5-26)24-14(11-27(12)17)13-9-22-19(20)23-10-13/h9-11H,3-8H2,1-2H3,(H,21,28)(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta by HTRF assay |
Bioorg Med Chem Lett 22: 5208-14 (2012)
Article DOI: 10.1016/j.bmcl.2012.06.093
BindingDB Entry DOI: 10.7270/Q24Q7W1Z |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM12376
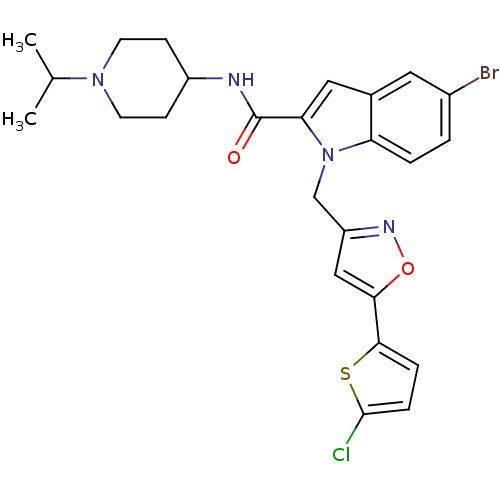
(2-Carboxyindole Scaffold 27 | 5-Bromo-1-[5-(5-chlo...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2cc(Br)ccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C25H26BrClN4O2S/c1-15(2)30-9-7-18(8-10-30)28-25(32)21-12-16-11-17(26)3-4-20(16)31(21)14-19-13-22(33-29-19)23-5-6-24(27)34-23/h3-6,11-13,15,18H,7-10,14H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
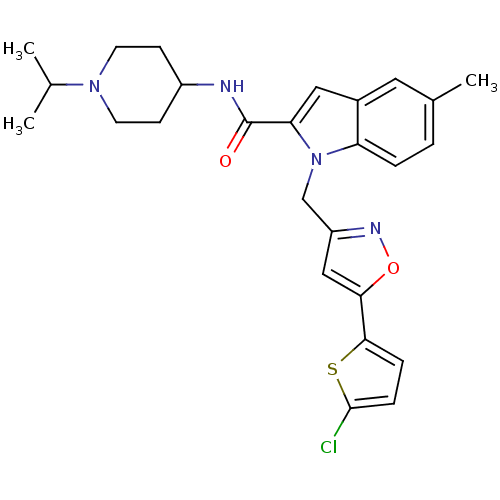
(Homo sapiens (Human)) | BDBM12378
(1-[5-(5-Chloro-thiophen-2-yl)-isoxazol-3-ylmethyl]...)Show SMILES CC(C)N1CCC(CC1)NC(=O)c1cc2cc(C)ccc2n1Cc1cc(on1)-c1ccc(Cl)s1 Show InChI InChI=1S/C26H29ClN4O2S/c1-16(2)30-10-8-19(9-11-30)28-26(32)22-13-18-12-17(3)4-5-21(18)31(22)15-20-14-23(33-29-20)24-6-7-25(27)34-24/h4-7,12-14,16,19H,8-11,15H2,1-3H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9 | -45.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
Aventis Pharma
| Assay Description
The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrates S-2765. The hydrolysis rates of chromogenic s... |
J Med Chem 48: 4511-25 (2005)
Article DOI: 10.1021/jm0490540
BindingDB Entry DOI: 10.7270/Q21834RB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase regulatory subunit alpha/4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50420730

(CHEMBL2087493)Show SMILES CS(=O)(=O)N1CCN(Cc2cn3c(Cl)c(nc(N4CCOCC4)c3n2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C20H26ClN9O3S/c1-34(31,32)29-4-2-27(3-5-29)12-15-13-30-17(21)16(14-10-23-20(22)24-11-14)26-18(19(30)25-15)28-6-8-33-9-7-28/h10-11,13H,2-9,12H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Spanish National Cancer Research Centre (CNIO)
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta (p110delta/p85alpha) by ADP accumulation based HTRF assay |
Bioorg Med Chem Lett 22: 3460-6 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.090
BindingDB Entry DOI: 10.7270/Q26W9CCB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data