

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.224 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50282204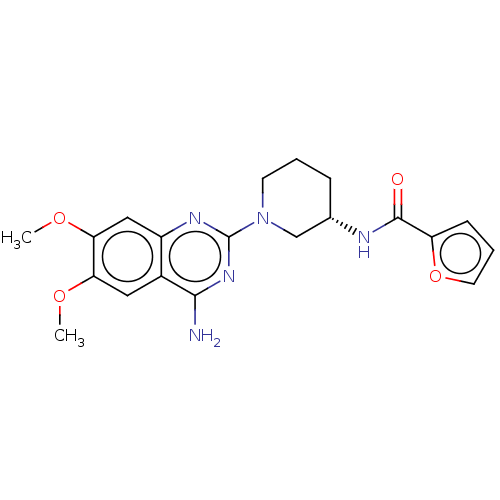 (CHEMBL4161165) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.282 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1B adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Rattus norvegicus (Rat)) | BDBM21280 (5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 284: 644-50 (1998) BindingDB Entry DOI: 10.7270/Q2D50KHT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002630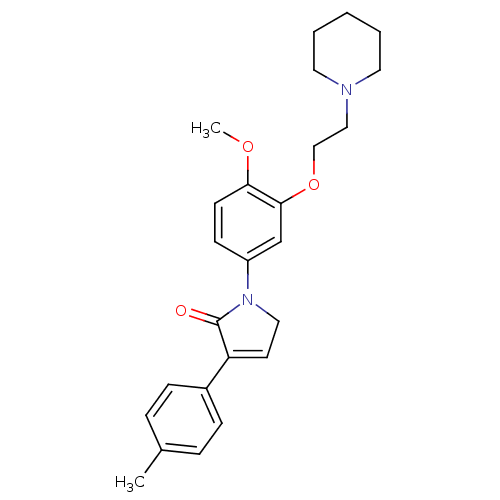 (CHEMBL377174) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM86731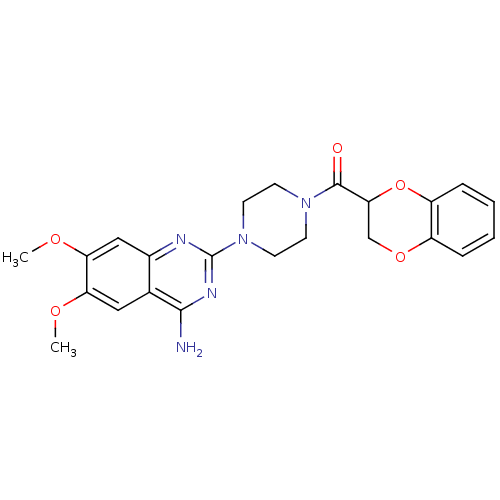 (CAS_74191-85-8 | Doxazosin | UK 33,274) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.324 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50282204 (CHEMBL4161165) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.331 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1B adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50282202 (CHEMBL4163587) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 0.525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1B adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50171290 (5-(4-Bromo-phenyl)-1-(2,4-dichloro-phenyl)-4-ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 905-14 (2004) Article DOI: 10.1124/jpet.104.067884 BindingDB Entry DOI: 10.7270/Q2RR1WTX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21280 (5-(4-chloro-3-methylphenyl)-1-[(4-methylphenyl)met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 284: 644-50 (1998) BindingDB Entry DOI: 10.7270/Q2D50KHT | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM86731 (CAS_74191-85-8 | Doxazosin | UK 33,274) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Compound was measured for the apparent inhibition constant at pepsin | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50282204 (CHEMBL4161165) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM86731 (CAS_74191-85-8 | Doxazosin | UK 33,274) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.776 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1A adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002642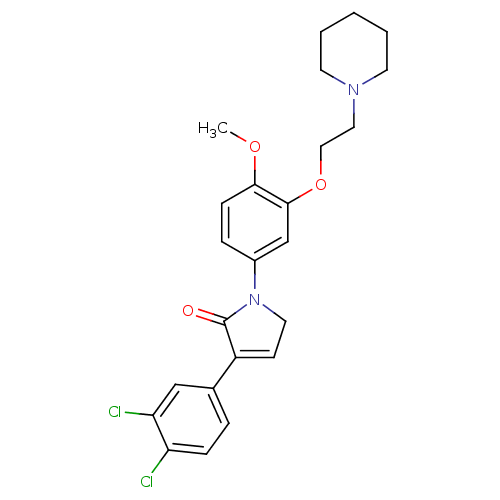 (CHEMBL213987) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002616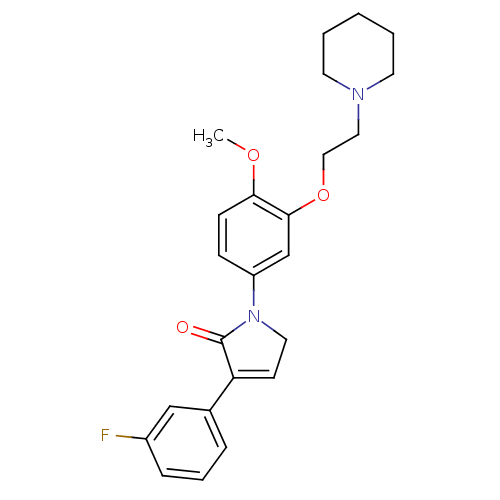 (CHEMBL213839) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515472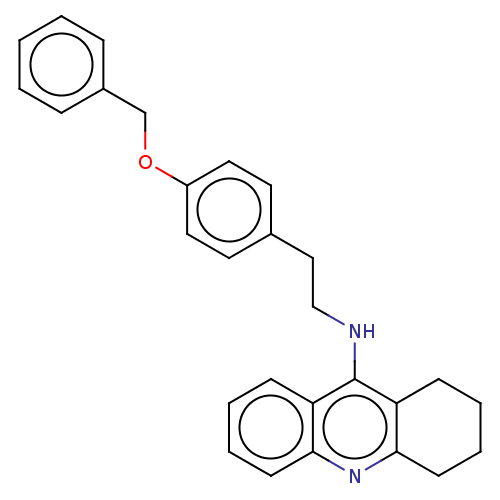 (CHEMBL4469822) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515454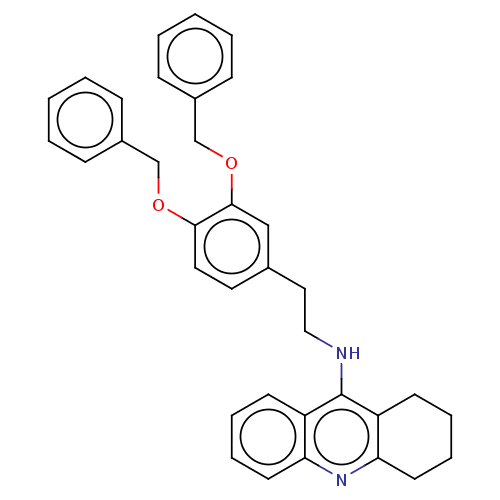 (CHEMBL4550977) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002678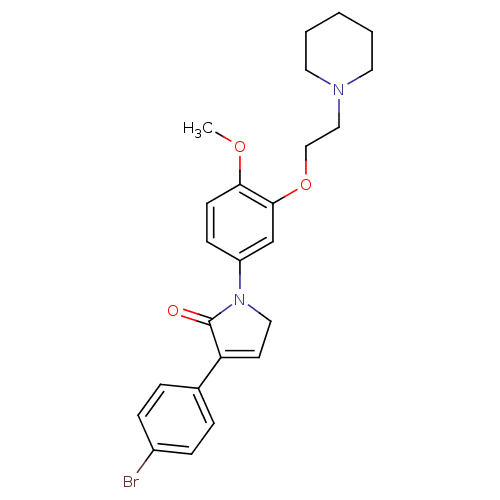 (CHEMBL210053) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515453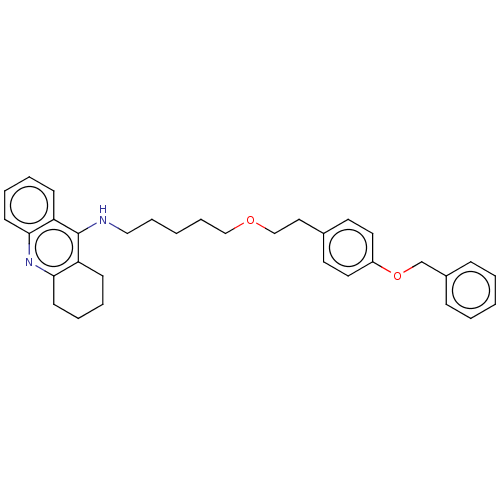 (CHEMBL4588525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002663 (CHEMBL386332) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515473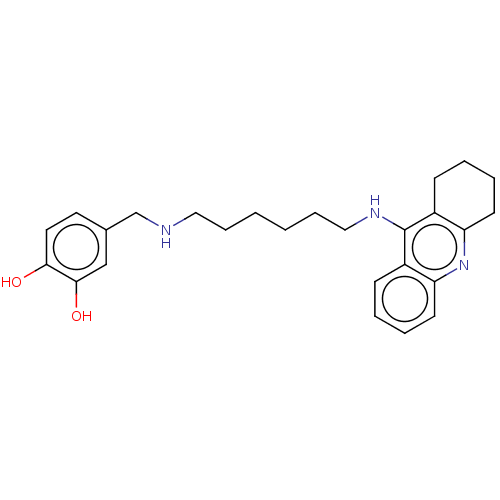 (CHEMBL4572757) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515470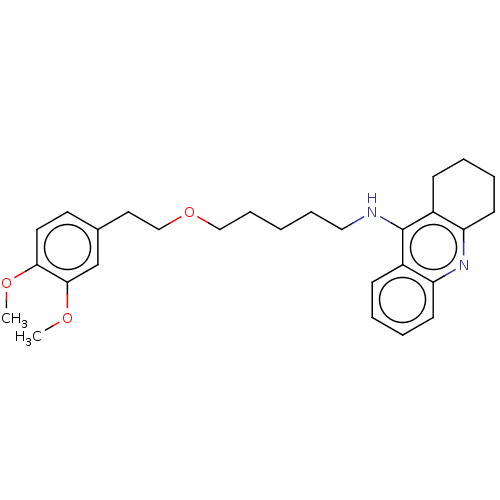 (CHEMBL4555120) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515453 (CHEMBL4588525) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515465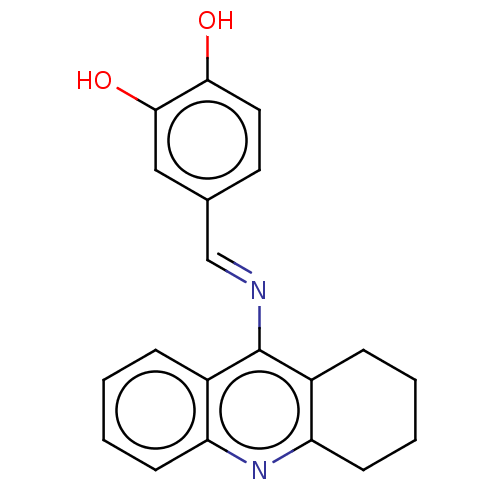 (CHEMBL4536715) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50282202 (CHEMBL4163587) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human alpha1D adrenoceptor expressed in CHO cell membranes after 30 mins | Eur J Med Chem 136: 259-269 (2017) Article DOI: 10.1016/j.ejmech.2017.05.003 BindingDB Entry DOI: 10.7270/Q2W95CQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002664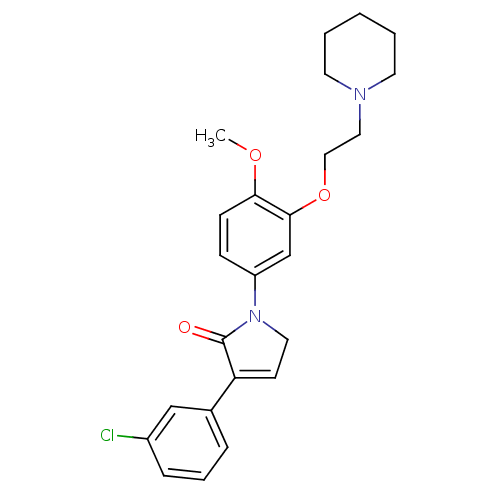 (CHEMBL212270) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515459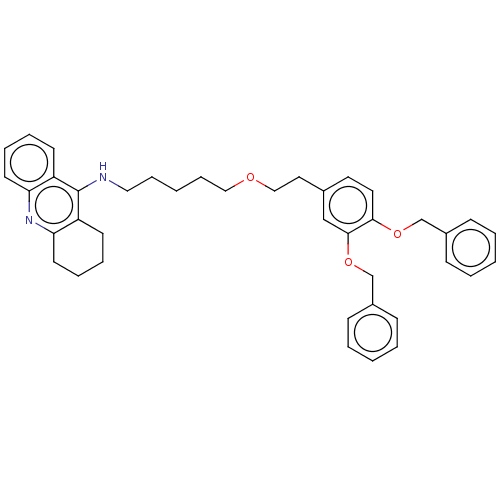 (CHEMBL4545701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50516288 (CHEMBL4530405) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Alma Mater Studiorum-University of Bologna Curated by ChEMBL | Assay Description Displacement of 3[H]-CP55940 from human recombinant CB2 receptor expressed in HEK293 cell membranes measured after 90 mins by Cheng-Prusoff equation ... | Eur J Med Chem 178: 243-258 (2019) Article DOI: 10.1016/j.ejmech.2019.05.080 BindingDB Entry DOI: 10.7270/Q2VX0KVX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515460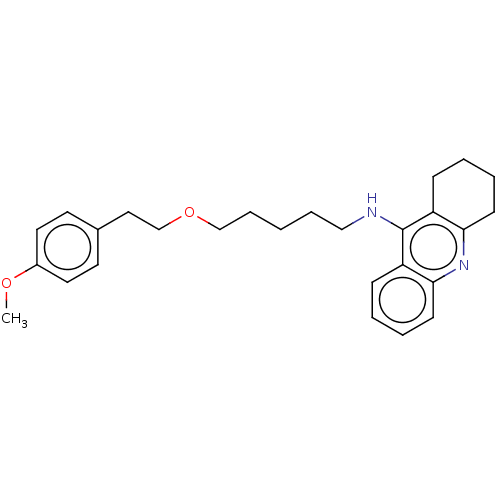 (CHEMBL4475228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515468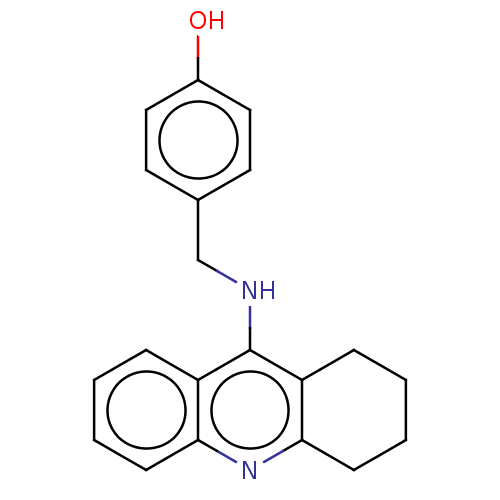 (CHEMBL4483710) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515461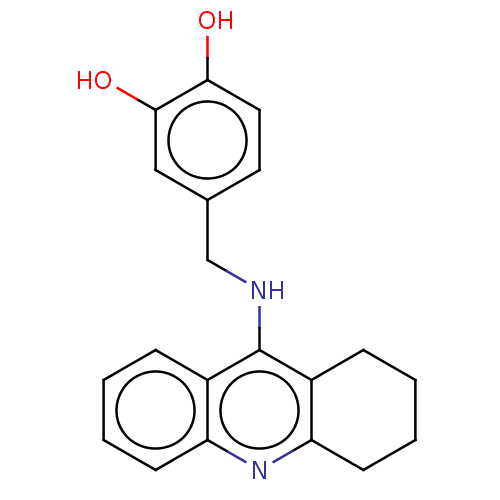 (CHEMBL4535585) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002689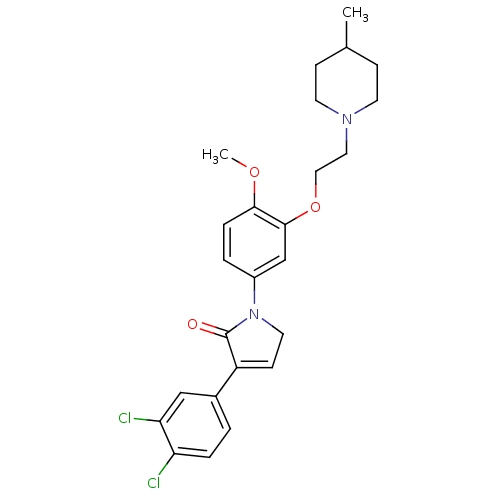 (CHEMBL209573) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002668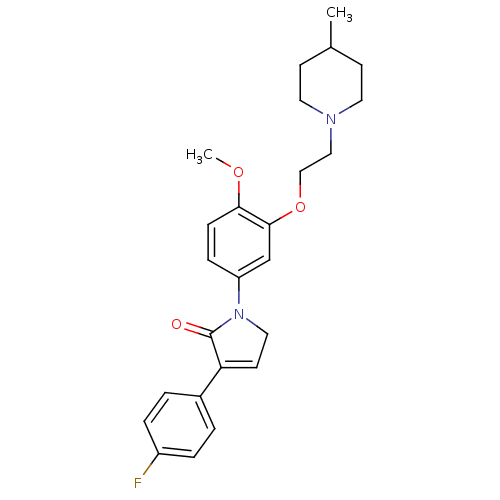 (CHEMBL215864) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50171290 (5-(4-Bromo-phenyl)-1-(2,4-dichloro-phenyl)-4-ethyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 310: 905-14 (2004) Article DOI: 10.1124/jpet.104.067884 BindingDB Entry DOI: 10.7270/Q2RR1WTX | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50514053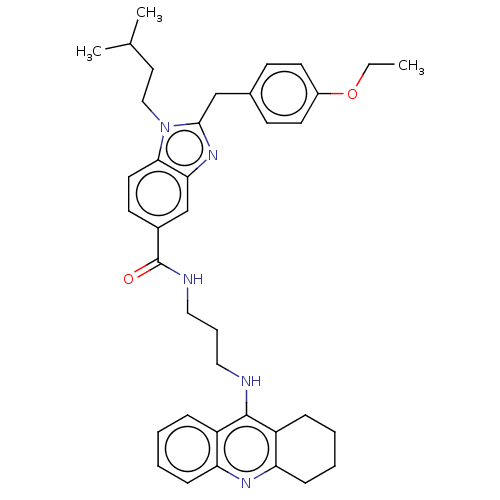 (CHEMBL4441666) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of recombinant human AChE assessed as inhibition constant for enzyme-inhibitor complex using acetylthiocholine iodide as substrate preincu... | J Med Chem 62: 9078-9102 (2019) Article DOI: 10.1021/acs.jmedchem.9b00623 BindingDB Entry DOI: 10.7270/Q20G3PHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002625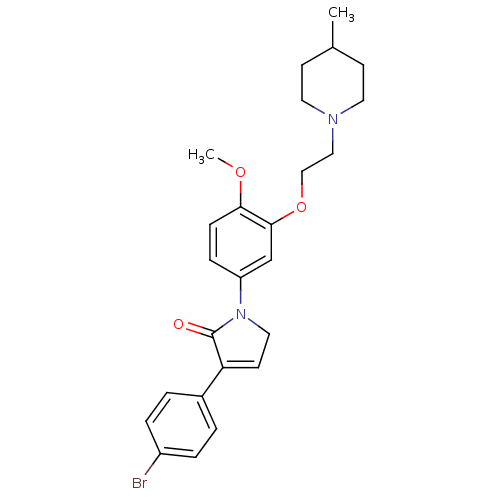 (CHEMBL209432) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002685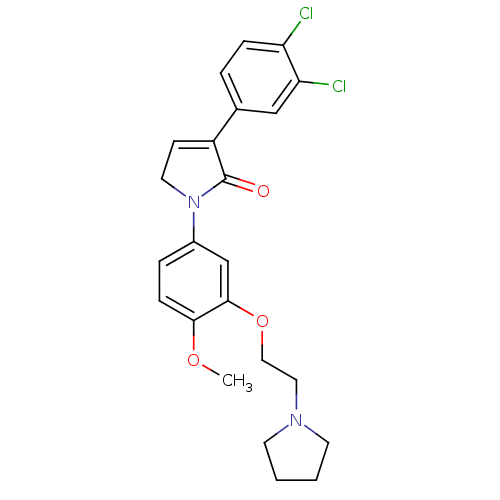 (CHEMBL211850) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002632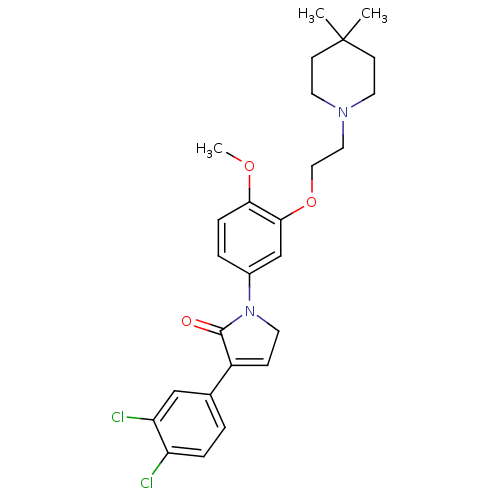 (CHEMBL376962) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515471 (CHEMBL4531167) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515459 (CHEMBL4545701) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-inhibitor complex using butyrylthiocholine iodide as substrate measured... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002676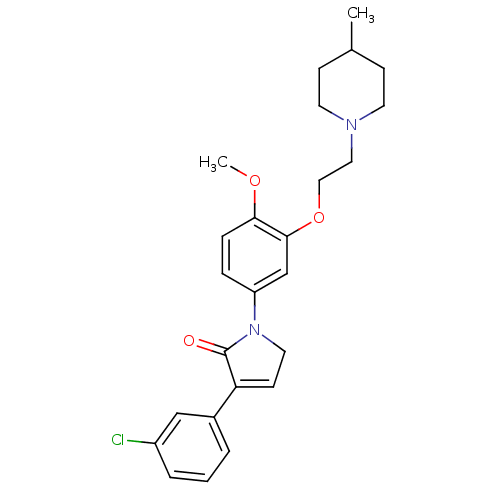 (CHEMBL212886) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002687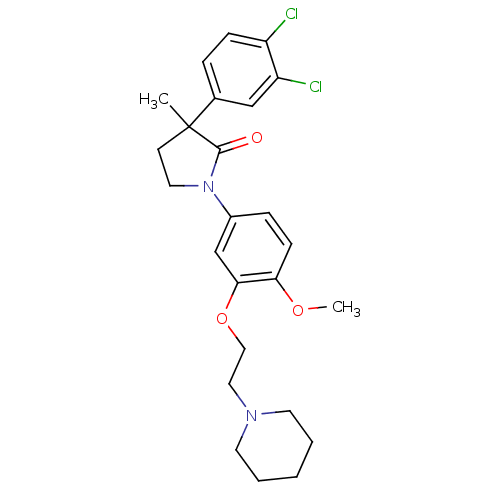 (CHEMBL214398) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002646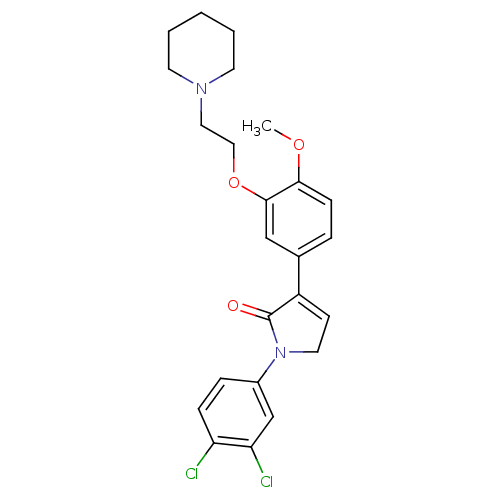 (CHEMBL210007) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002680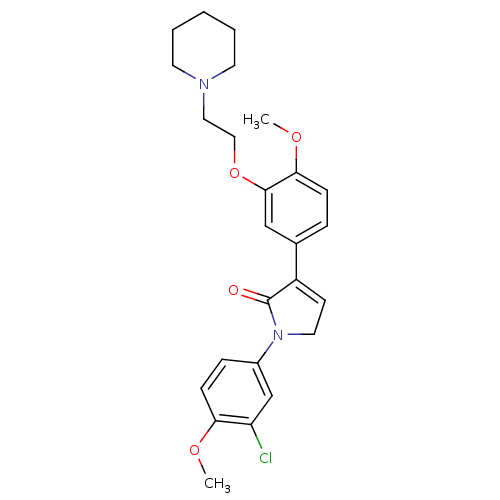 (CHEMBL387022) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002647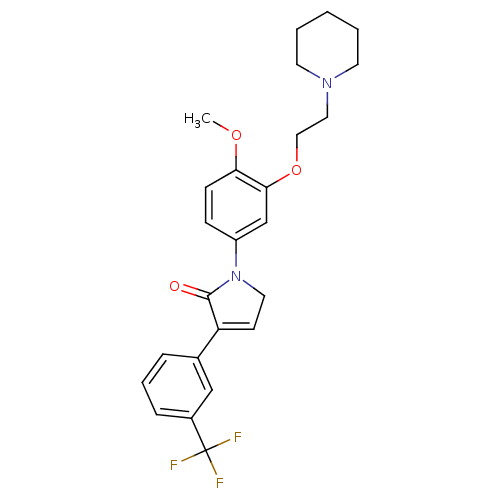 (CHEMBL212064) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002669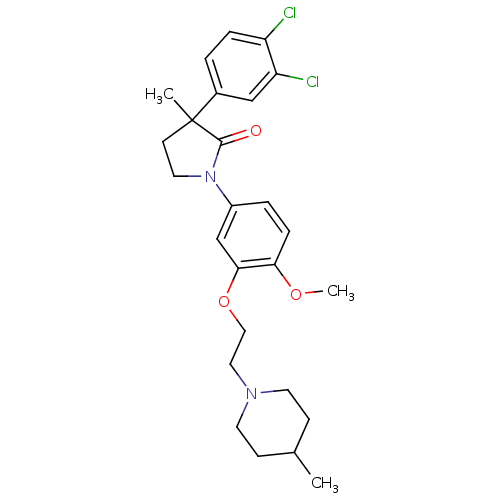 (CHEMBL212769) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM81780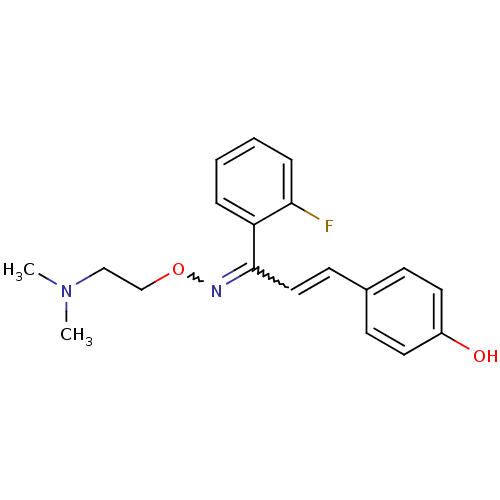 (CAS_6438382 | NSC_6438382 | SR 46349B) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 262: 759-68 (1992) BindingDB Entry DOI: 10.7270/Q27S7M7Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50515469 (CHEMBL4521755) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad de Sevilla Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE assessed as dissociation constant for enzyme-substrate-inhibitor complex using butyrylthiocholine iodide as substrat... | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.07.053 BindingDB Entry DOI: 10.7270/Q23R0X60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50002670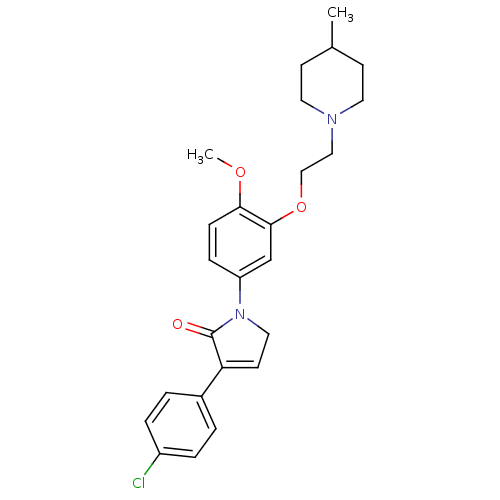 (CHEMBL386431) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Psychiatry Centre of Excellence for Drug Discovery Curated by ChEMBL | Assay Description Binding affinity to human 5HT2C receptor | Bioorg Med Chem Lett 16: 3906-12 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.05.034 BindingDB Entry DOI: 10.7270/Q2FN17PF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 916 total ) | Next | Last >> |