 Found 51 hits with Last Name = 'nophsker' and Initial = 'm'
Found 51 hits with Last Name = 'nophsker' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198665
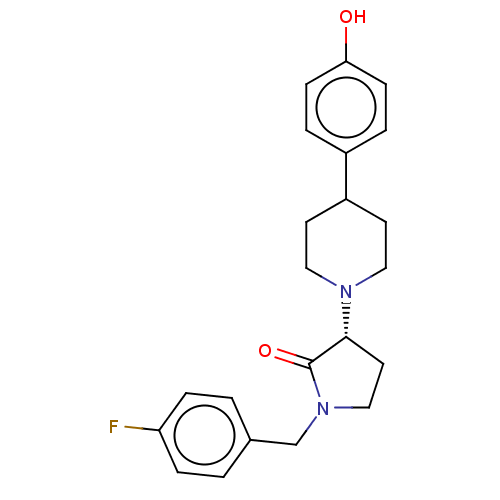
(US9221796, 2b)Show SMILES Oc1ccc(cc1)C1CCN(CC1)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H25FN2O2/c23-19-5-1-16(2-6-19)15-25-14-11-21(22(25)27)24-12-9-18(10-13-24)17-3-7-20(26)8-4-17/h1-8,18,21,26H,9-15H2/t21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198694
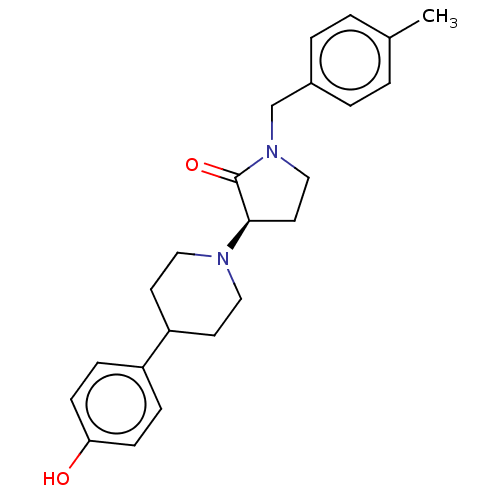
(US9221796, 23b)Show SMILES Cc1ccc(CN2CC[C@@H](N3CCC(CC3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c1-17-2-4-18(5-3-17)16-25-15-12-22(23(25)27)24-13-10-20(11-14-24)19-6-8-21(26)9-7-19/h2-9,20,22,26H,10-16H2,1H3/t22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198726
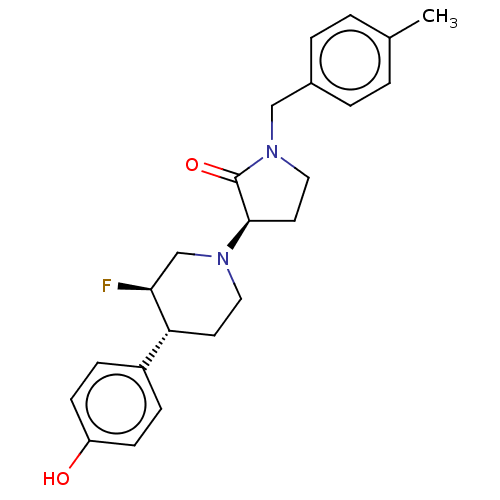
(US9221796, 46, P-2)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@@H]([C@@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50330324

(CHEMBL4170867)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to GluN2B receptor in human cortex |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50330409

(CHEMBL4168402)Show SMILES Cc1ccc(CN2CC[C@@H](N3CCC(c4ccc(O)cc4)C(F)(F)C3)C2=O)cc1 |r| Show InChI InChI=1S/C23H26F2N2O2/c1-16-2-4-17(5-3-16)14-26-13-11-21(22(26)29)27-12-10-20(23(24,25)15-27)18-6-8-19(28)9-7-18/h2-9,20-21,28H,10-15H2,1H3/t20?,21-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM50330410

(CHEMBL4161899)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Rattus norvegicus (Rat)) | BDBM198735
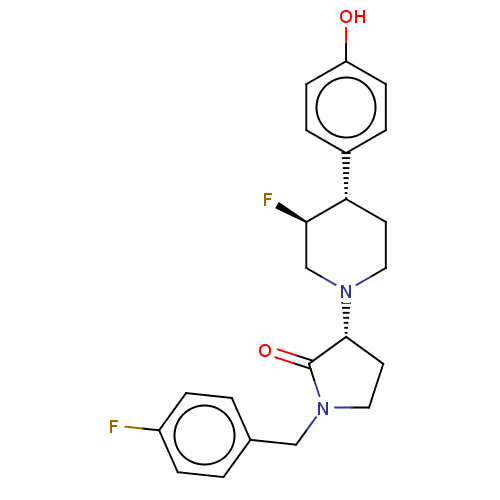
(US9221796, 48, P-3)Show SMILES Oc1ccc(cc1)[C@@H]1CCN(C[C@H]1F)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H24F2N2O2/c23-17-5-1-15(2-6-17)13-26-12-10-21(22(26)28)25-11-9-19(20(24)14-25)16-3-7-18(27)8-4-16/h1-8,19-21,27H,9-14H2/t19-,20+,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]Ro 25-6981 from GluN2B receptor in Sprague-Dawley rat forebrain membranes incubated for 1 hr by topcount micro scintillation coun... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074676
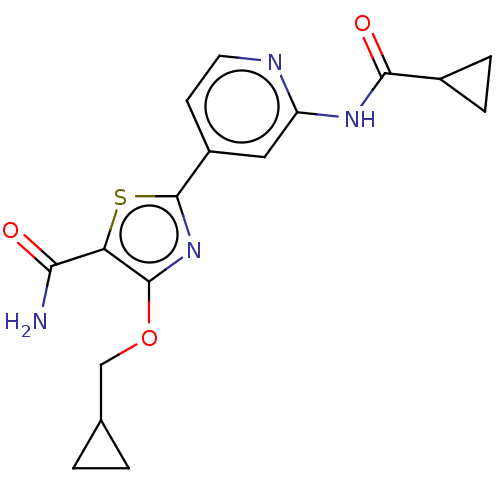
(CHEMBL3410091)Show SMILES NC(=O)c1sc(nc1OCC1CC1)-c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C17H18N4O3S/c18-14(22)13-16(24-8-9-1-2-9)21-17(25-13)11-5-6-19-12(7-11)20-15(23)10-3-4-10/h5-7,9-10H,1-4,8H2,(H2,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074675
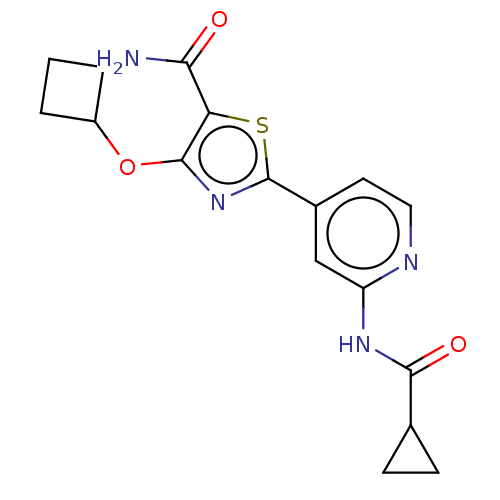
(CHEMBL3410092)Show SMILES NC(=O)c1sc(nc1OC1CCC1)-c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C17H18N4O3S/c18-14(22)13-16(24-11-2-1-3-11)21-17(25-13)10-6-7-19-12(8-10)20-15(23)9-4-5-9/h6-9,11H,1-5H2,(H2,18,22)(H,19,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074677
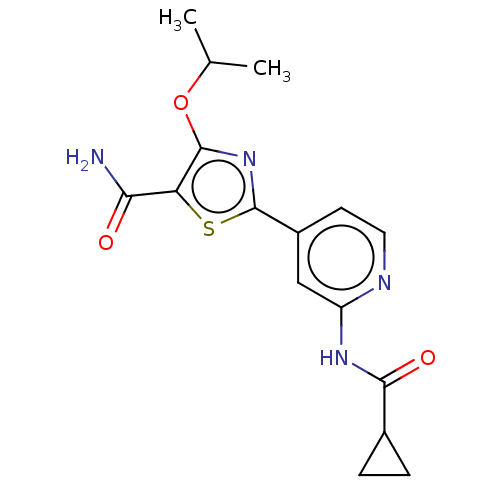
(CHEMBL3410090)Show SMILES CC(C)Oc1nc(sc1C(N)=O)-c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C16H18N4O3S/c1-8(2)23-15-12(13(17)21)24-16(20-15)10-5-6-18-11(7-10)19-14(22)9-3-4-9/h5-9H,3-4H2,1-2H3,(H2,17,21)(H,18,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074576

(CHEMBL3410111)Show SMILES CC(C)Oc1nc(ncc1C(N)=O)-c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C17H19N5O3/c1-9(2)25-17-12(14(18)23)8-20-15(22-17)11-5-6-19-13(7-11)21-16(24)10-3-4-10/h5-10H,3-4H2,1-2H3,(H2,18,23)(H,19,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074681

(CHEMBL3410089)Show InChI InChI=1S/C14H14N4O3S/c1-21-13-10(11(15)19)22-14(18-13)8-4-5-16-9(6-8)17-12(20)7-2-3-7/h4-7H,2-3H2,1H3,(H2,15,19)(H,16,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074663

(CHEMBL3410094)Show InChI InChI=1S/C18H17N3O2/c22-17(11-1-2-11)21-16-10-13(5-7-19-16)12-3-4-15-14(9-12)6-8-20-18(15)23/h3-5,7,9-11H,1-2,6,8H2,(H,20,23)(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074595

(CHEMBL3410096)Show InChI InChI=1S/C19H19N3O2/c23-18(12-3-4-12)22-17-11-14(7-9-20-17)13-5-6-16-15(10-13)2-1-8-21-19(16)24/h5-7,9-12H,1-4,8H2,(H,21,24)(H,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM198665

(US9221796, 2b)Show SMILES Oc1ccc(cc1)C1CCN(CC1)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H25FN2O2/c23-19-5-1-16(2-6-19)15-25-14-11-21(22(25)27)24-12-9-18(10-13-24)17-3-7-20(26)8-4-17/h1-8,18,21,26H,9-15H2/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to GluN2B receptor in human cortex |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074581
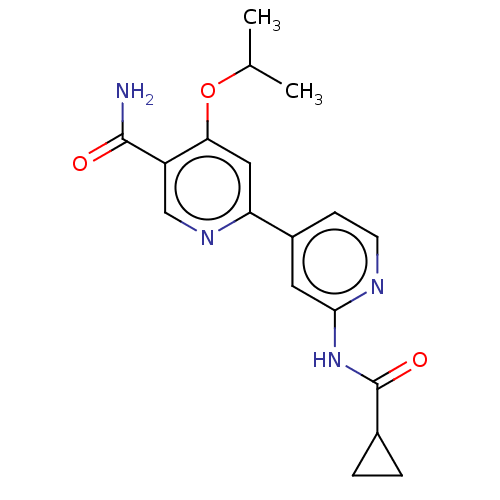
(CHEMBL3410107)Show SMILES CC(C)Oc1cc(ncc1C(N)=O)-c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C18H20N4O3/c1-10(2)25-15-8-14(21-9-13(15)17(19)23)12-5-6-20-16(7-12)22-18(24)11-3-4-11/h5-11H,3-4H2,1-2H3,(H2,19,23)(H,20,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074593
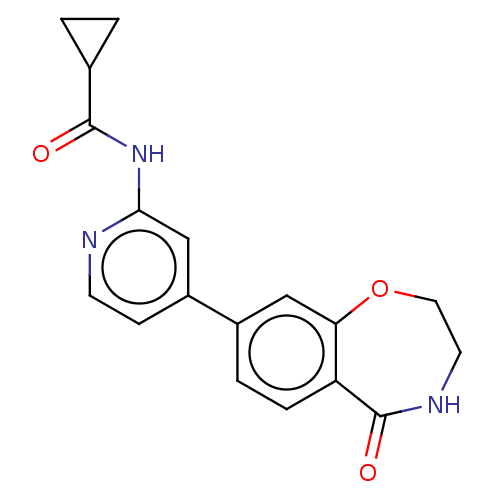
(CHEMBL3410097)Show InChI InChI=1S/C18H17N3O3/c22-17(11-1-2-11)21-16-10-13(5-6-19-16)12-3-4-14-15(9-12)24-8-7-20-18(14)23/h3-6,9-11H,1-2,7-8H2,(H,20,23)(H,19,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074578
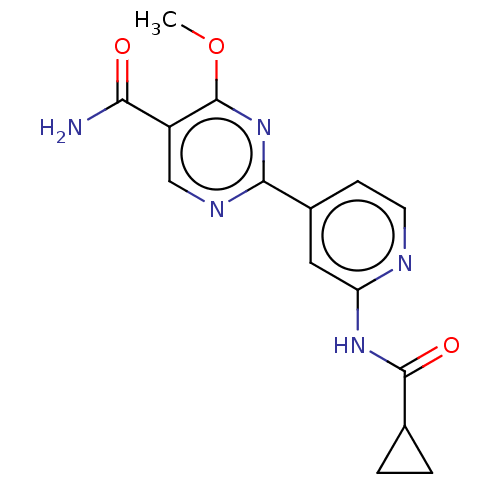
(CHEMBL3410110)Show InChI InChI=1S/C15H15N5O3/c1-23-15-10(12(16)21)7-18-13(20-15)9-4-5-17-11(6-9)19-14(22)8-2-3-8/h4-8H,2-3H2,1H3,(H2,16,21)(H,17,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074582
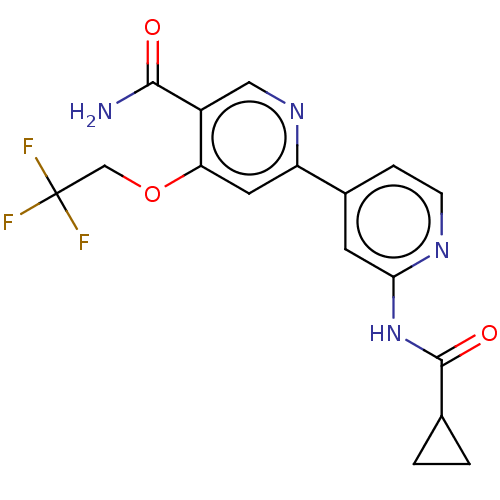
(CHEMBL3410106)Show SMILES NC(=O)c1cnc(cc1OCC(F)(F)F)-c1ccnc(NC(=O)C2CC2)c1 Show InChI InChI=1S/C17H15F3N4O3/c18-17(19,20)8-27-13-6-12(23-7-11(13)15(21)25)10-3-4-22-14(5-10)24-16(26)9-1-2-9/h3-7,9H,1-2,8H2,(H2,21,25)(H,22,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074584
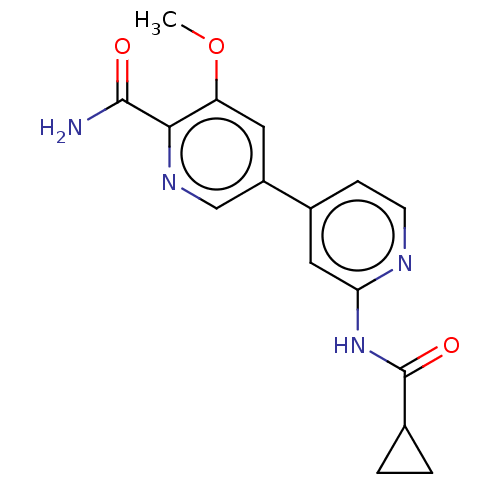
(CHEMBL3410104)Show InChI InChI=1S/C16H16N4O3/c1-23-12-6-11(8-19-14(12)15(17)21)10-4-5-18-13(7-10)20-16(22)9-2-3-9/h4-9H,2-3H2,1H3,(H2,17,21)(H,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074587
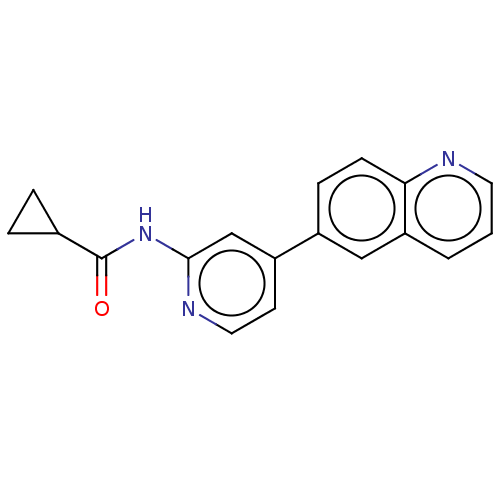
(CHEMBL3410101)Show InChI InChI=1S/C18H15N3O/c22-18(12-3-4-12)21-17-11-14(7-9-20-17)13-5-6-16-15(10-13)2-1-8-19-16/h1-2,5-12H,3-4H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074640
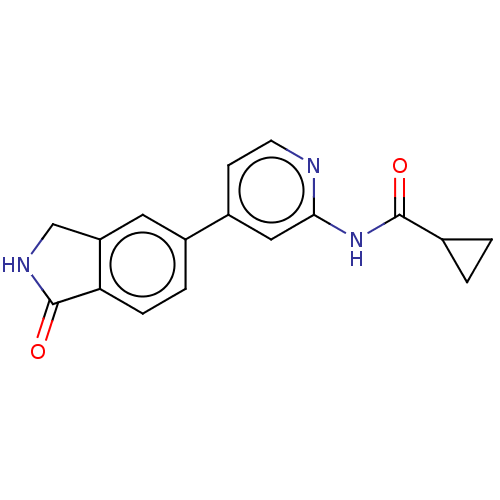
(CHEMBL3410095)Show InChI InChI=1S/C17H15N3O2/c21-16(10-1-2-10)20-15-8-12(5-6-18-15)11-3-4-14-13(7-11)9-19-17(14)22/h3-8,10H,1-2,9H2,(H,19,22)(H,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074692
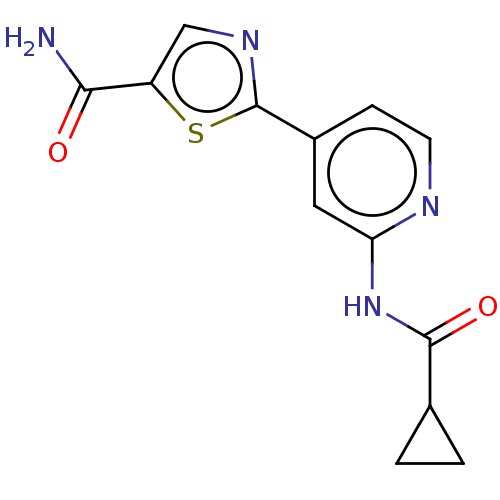
(CHEMBL3410087)Show InChI InChI=1S/C13H12N4O2S/c14-11(18)9-6-16-13(20-9)8-3-4-15-10(5-8)17-12(19)7-1-2-7/h3-7H,1-2H2,(H2,14,18)(H,15,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074583

(CHEMBL3410105)Show InChI InChI=1S/C16H16N4O3/c1-23-13-7-12(19-8-11(13)15(17)21)10-4-5-18-14(6-10)20-16(22)9-2-3-9/h4-9H,2-3H2,1H3,(H2,17,21)(H,18,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 2B
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Negative allosteric modulation of human GluN2B receptor expressed in Xenopus laevis oocytes assessed as inhibition of glutamate/glycine-induced chann... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074580
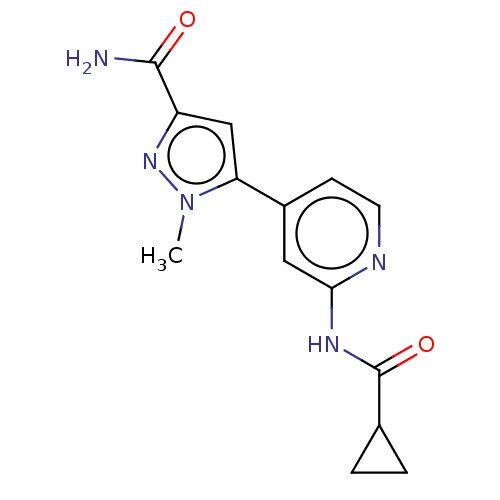
(CHEMBL3410108)Show InChI InChI=1S/C14H15N5O2/c1-19-11(7-10(18-19)13(15)20)9-4-5-16-12(6-9)17-14(21)8-2-3-8/h4-8H,2-3H2,1H3,(H2,15,20)(H,16,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074671
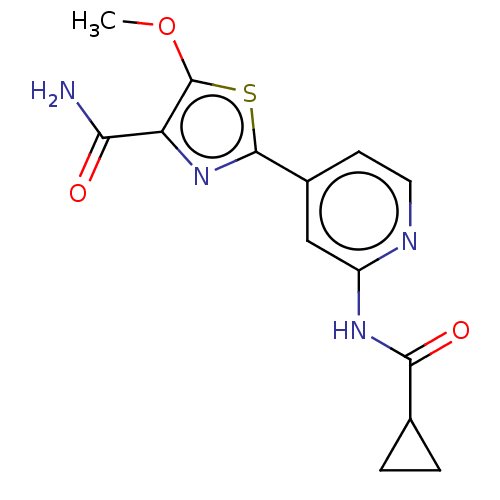
(CHEMBL3410093)Show InChI InChI=1S/C14H14N4O3S/c1-21-14-10(11(15)19)18-13(22-14)8-4-5-16-9(6-8)17-12(20)7-2-3-7/h4-7H,2-3H2,1H3,(H2,15,19)(H,16,17,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074579
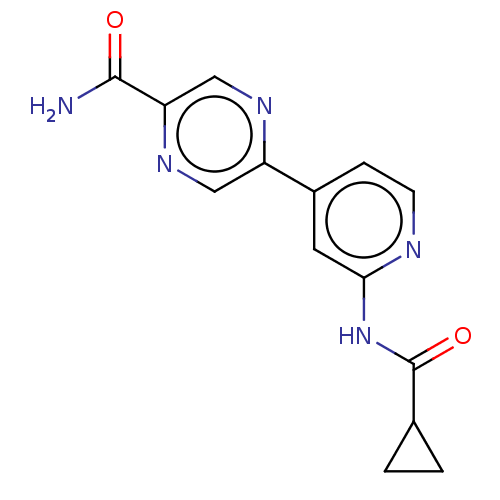
(CHEMBL3410109)Show InChI InChI=1S/C14H13N5O2/c15-13(20)11-7-17-10(6-18-11)9-3-4-16-12(5-9)19-14(21)8-1-2-8/h3-8H,1-2H2,(H2,15,20)(H,16,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074691
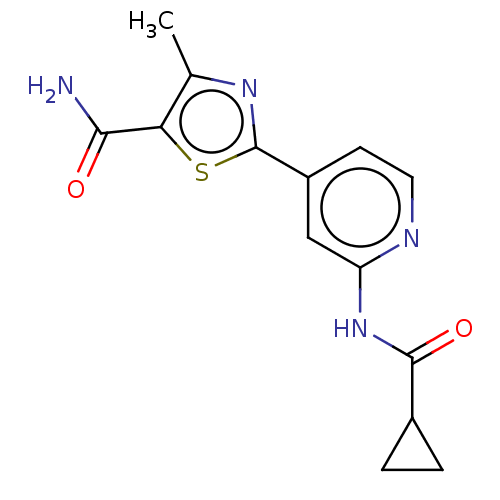
(CHEMBL3410088)Show InChI InChI=1S/C14H14N4O2S/c1-7-11(12(15)19)21-14(17-7)9-4-5-16-10(6-9)18-13(20)8-2-3-8/h4-6,8H,2-3H2,1H3,(H2,15,19)(H,16,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074588
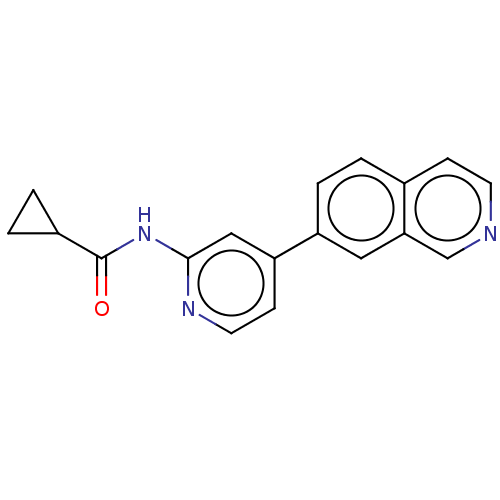
(CHEMBL3410100)Show InChI InChI=1S/C18H15N3O/c22-18(13-2-3-13)21-17-10-15(6-8-20-17)14-4-1-12-5-7-19-11-16(12)9-14/h1,4-11,13H,2-3H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074585
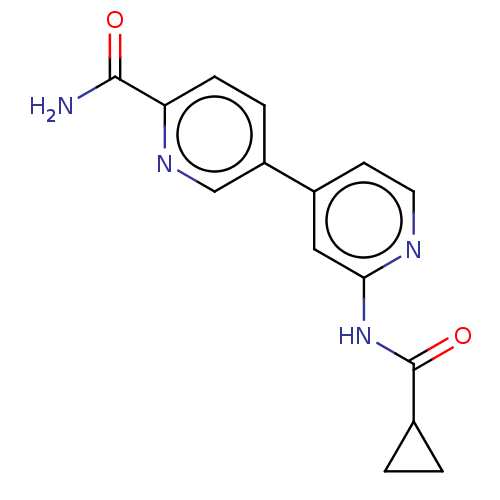
(CHEMBL3410103)Show InChI InChI=1S/C15H14N4O2/c16-14(20)12-4-3-11(8-18-12)10-5-6-17-13(7-10)19-15(21)9-1-2-9/h3-9H,1-2H2,(H2,16,20)(H,17,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074575

(CHEMBL3410112)Show InChI InChI=1S/C14H13N5O2/c15-13(20)11-4-3-10(18-19-11)9-5-6-16-12(7-9)17-14(21)8-1-2-8/h3-8H,1-2H2,(H2,15,20)(H,16,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074589
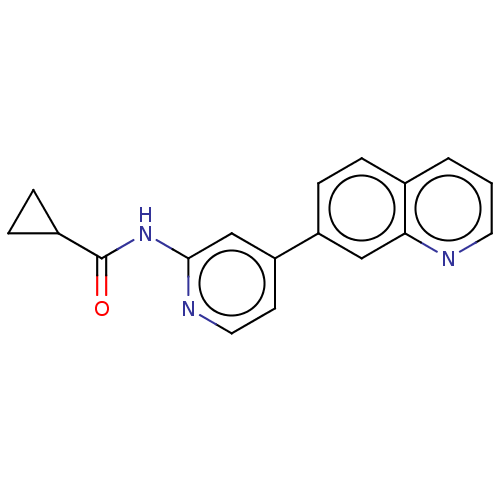
(CHEMBL3410099)Show InChI InChI=1S/C18H15N3O/c22-18(13-4-5-13)21-17-11-15(7-9-20-17)14-6-3-12-2-1-8-19-16(12)10-14/h1-3,6-11,13H,4-5H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM198694

(US9221796, 23b)Show SMILES Cc1ccc(CN2CC[C@@H](N3CCC(CC3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c1-17-2-4-18(5-3-17)16-25-15-12-22(23(25)27)24-13-10-20(11-14-24)19-6-8-21(26)9-7-19/h2-9,20,22,26H,10-16H2,1H3/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074586
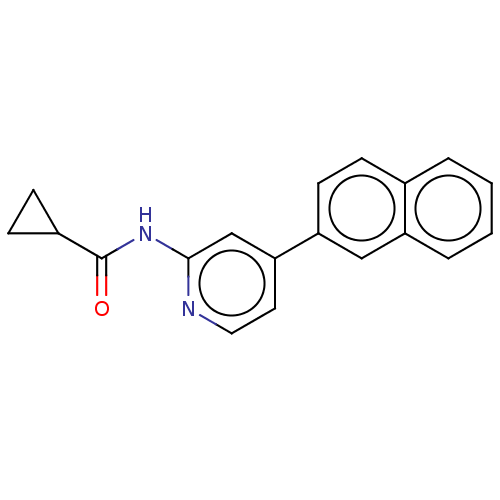
(CHEMBL3410102)Show InChI InChI=1S/C19H16N2O/c22-19(14-6-7-14)21-18-12-17(9-10-20-18)16-8-5-13-3-1-2-4-15(13)11-16/h1-5,8-12,14H,6-7H2,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM198665

(US9221796, 2b)Show SMILES Oc1ccc(cc1)C1CCN(CC1)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H25FN2O2/c23-19-5-1-16(2-6-19)15-25-14-11-21(22(25)27)24-12-9-18(10-13-24)17-3-7-20(26)8-4-17/h1-8,18,21,26H,9-15H2/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50074591
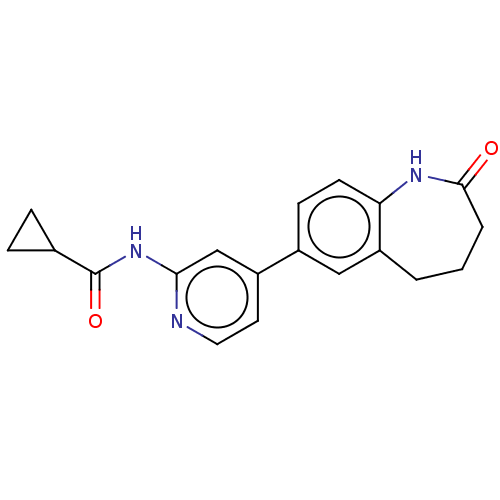
(CHEMBL3410098)Show InChI InChI=1S/C19H19N3O2/c23-18-3-1-2-15-10-13(6-7-16(15)21-18)14-8-9-20-17(11-14)22-19(24)12-4-5-12/h6-12H,1-5H2,(H,21,23)(H,20,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 730 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of GSK3beta (unknown origin) activity by competitive binding assay |
Bioorg Med Chem Lett 25: 1856-63 (2015)
Article DOI: 10.1016/j.bmcl.2015.03.046
BindingDB Entry DOI: 10.7270/Q2222WH5 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM198735

(US9221796, 48, P-3)Show SMILES Oc1ccc(cc1)[C@@H]1CCN(C[C@H]1F)[C@@H]1CCN(Cc2ccc(F)cc2)C1=O |r| Show InChI InChI=1S/C22H24F2N2O2/c23-17-5-1-15(2-6-17)13-26-12-10-21(22(26)28)25-11-9-19(20(24)14-25)16-3-7-18(27)8-4-16/h1-8,19-21,27H,9-14H2/t19-,20+,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50330324

(CHEMBL4170867)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 in human liver microsomes |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes using BFC/BZR as substrate |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 in human liver microsomes |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2B6
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of CYP2B6 in human liver microsomes |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM198728

(US9221796, 46, P-4)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@H]([C@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM198726

(US9221796, 46, P-2)Show SMILES Cc1ccc(CN2CC[C@@H](N3CC[C@@H]([C@@H](F)C3)c3ccc(O)cc3)C2=O)cc1 |r| Show InChI InChI=1S/C23H27FN2O2/c1-16-2-4-17(5-3-16)14-26-13-11-22(23(26)28)25-12-10-20(21(24)15-25)18-6-8-19(27)9-7-18/h2-9,20-22,27H,10-15H2,1H3/t20-,21+,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in HEK293 cells assessed as reduction in peak tail current after 2 to 5 mins by patch clamp based electrophysiology... |
ACS Med Chem Lett 9: 472-477 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00080
BindingDB Entry DOI: 10.7270/Q2PR7ZJT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data