

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288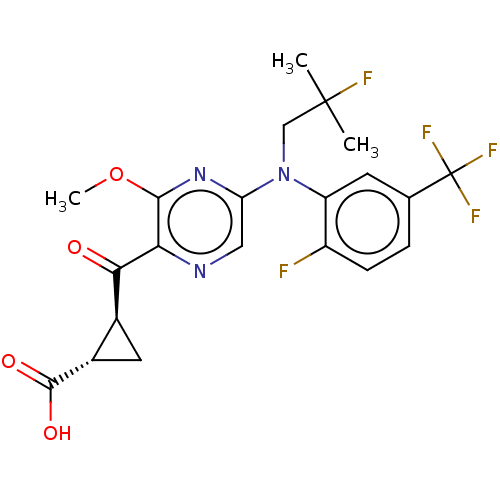 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287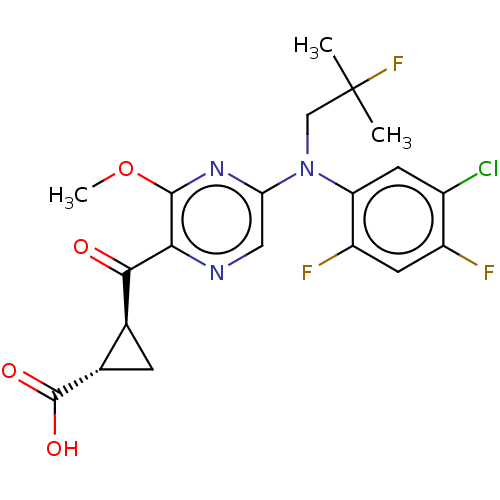 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519568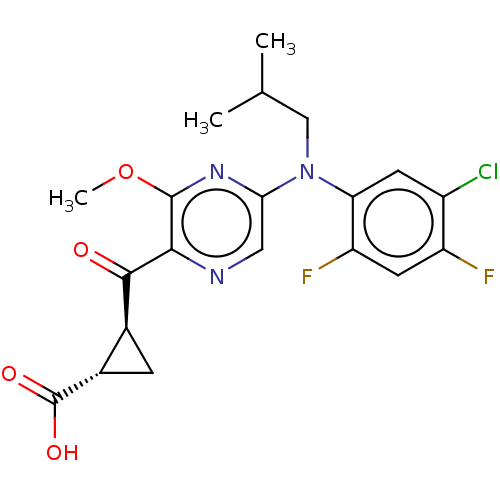 (CHEMBL4549822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519570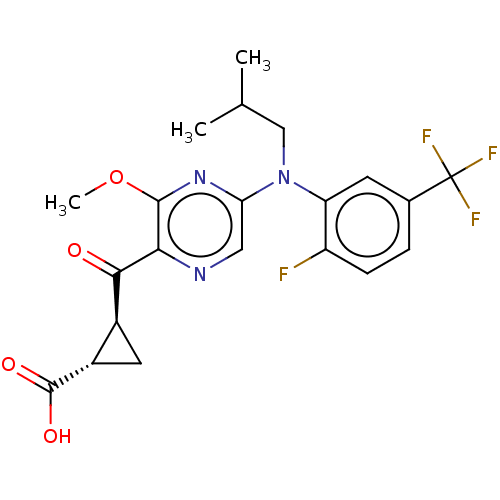 (CHEMBL4567083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519585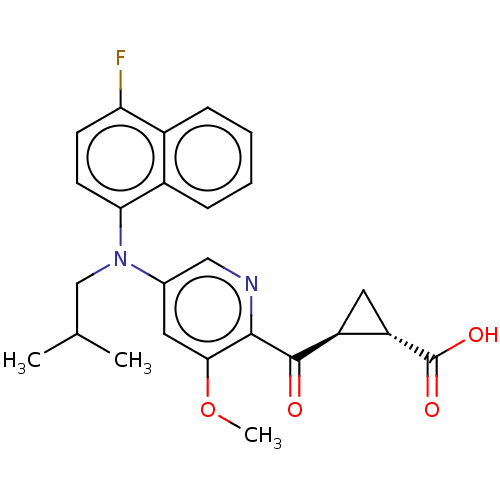 (CHEMBL4464773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.381 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519591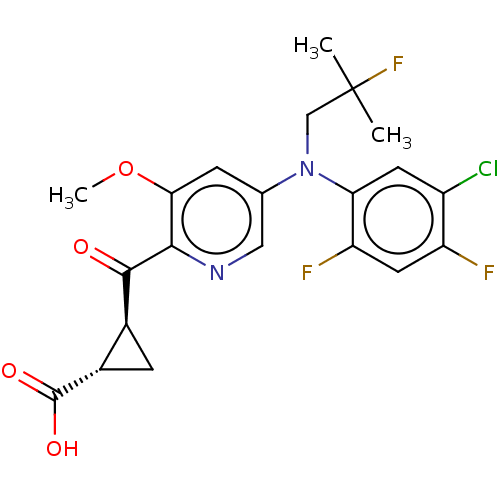 (CHEMBL4562583) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519574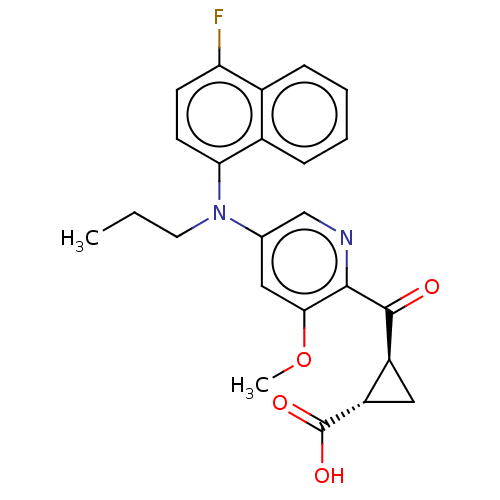 (CHEMBL4563433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.626 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519588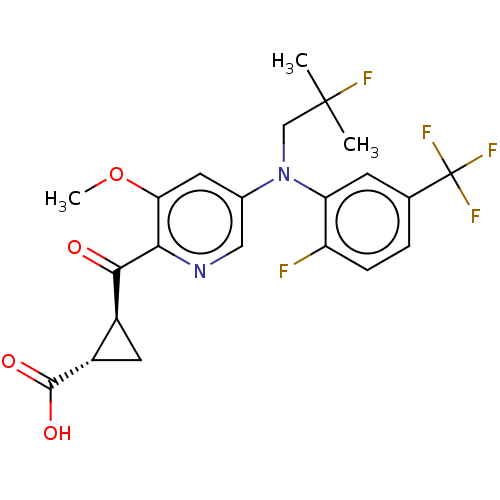 (CHEMBL4584584) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290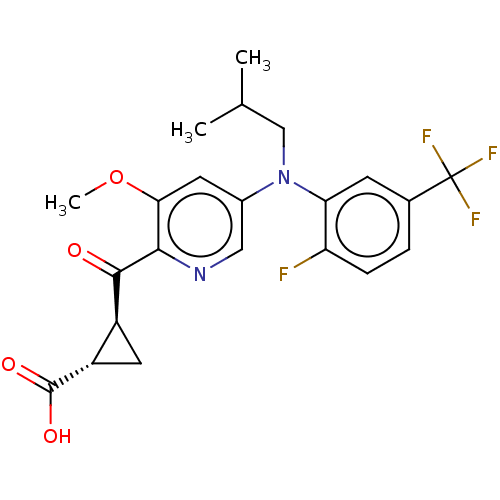 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519580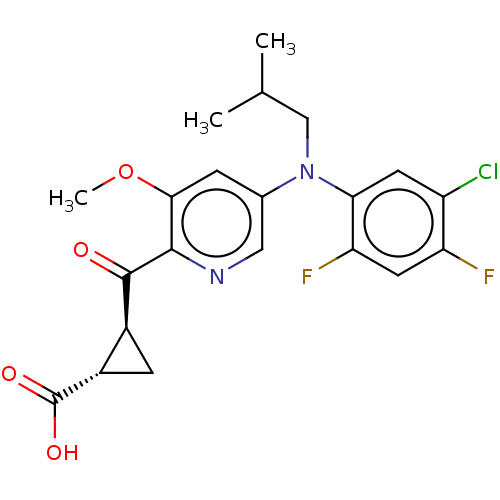 (CHEMBL4574439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519565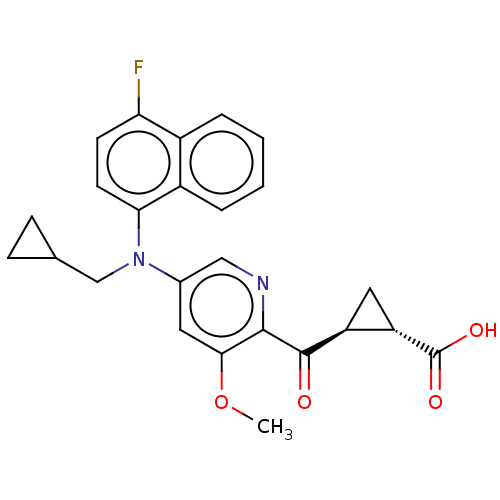 (CHEMBL4441362) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.758 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519567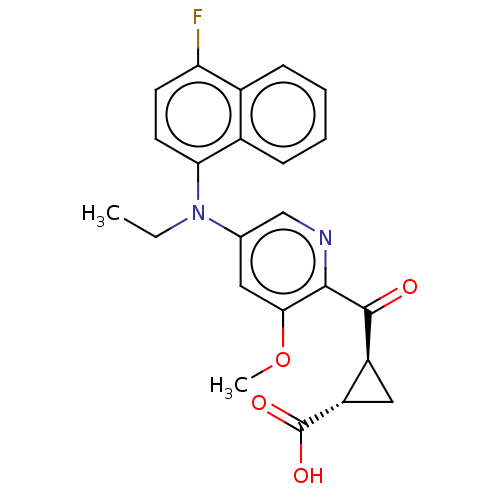 (CHEMBL4476024) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519571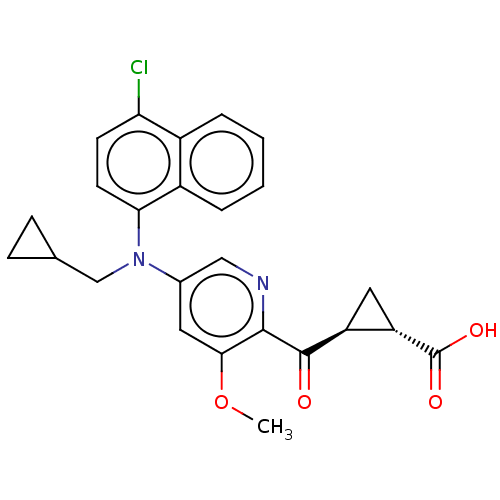 (CHEMBL4454609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519595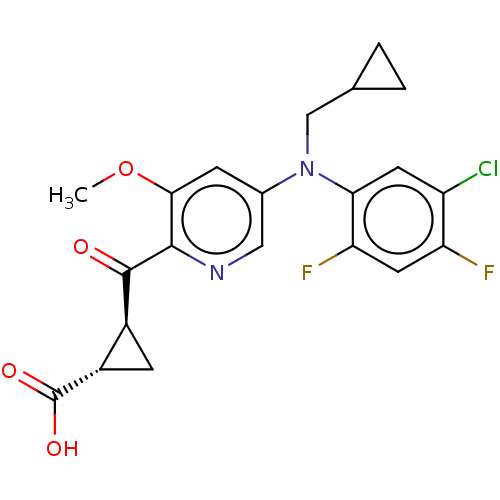 (CHEMBL4545014) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519569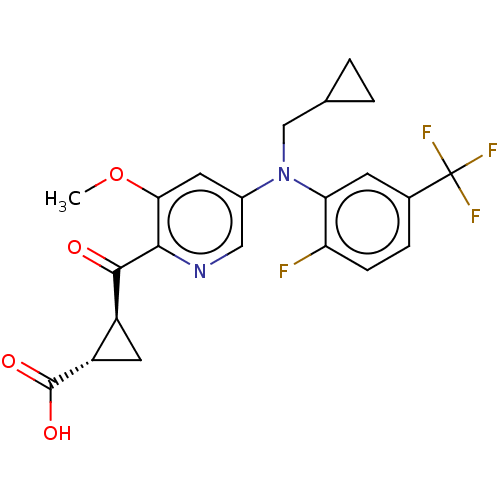 (CHEMBL4545950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519585 (CHEMBL4464773) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519582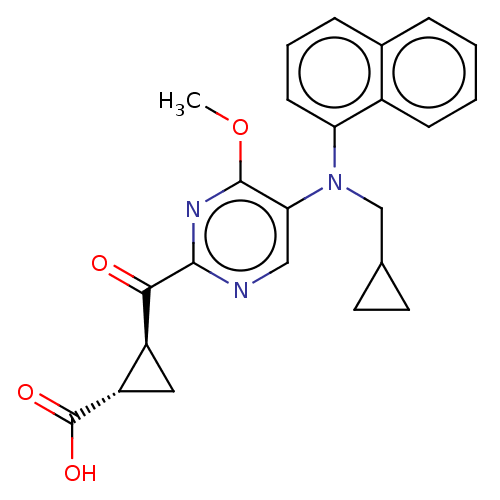 (CHEMBL4525972) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519570 (CHEMBL4567083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519587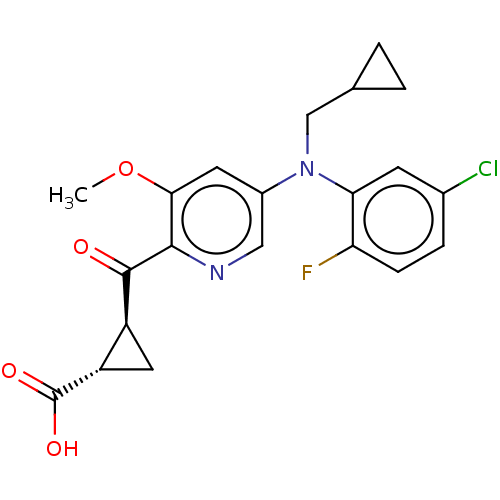 (CHEMBL4467942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519568 (CHEMBL4549822) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519590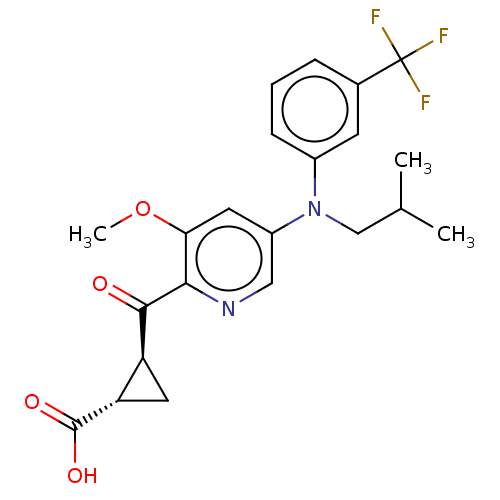 (CHEMBL4574370) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519592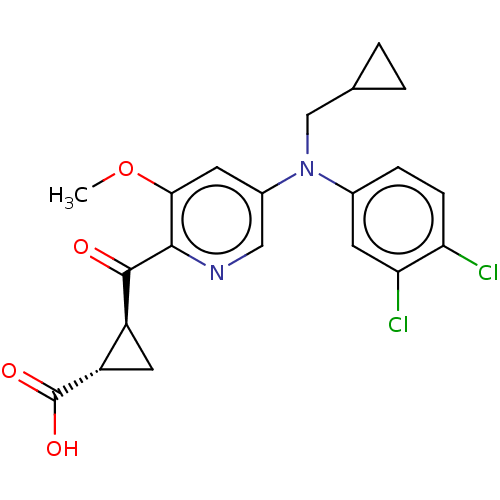 (CHEMBL4442047) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519579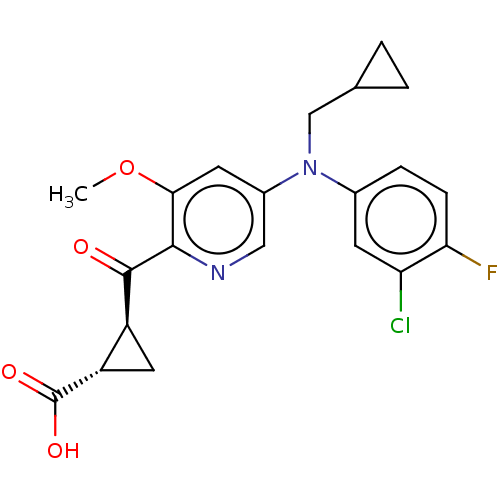 (CHEMBL4576647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519575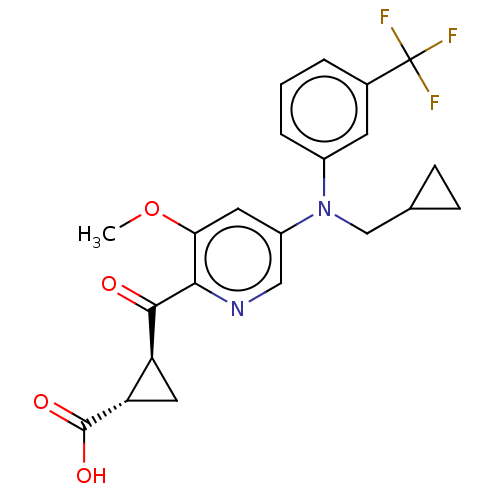 (CHEMBL4567339) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223290 ((1S,2S)-2-[(5-{[2-Fluoro-5-(trifluoromethyl)phenyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50262998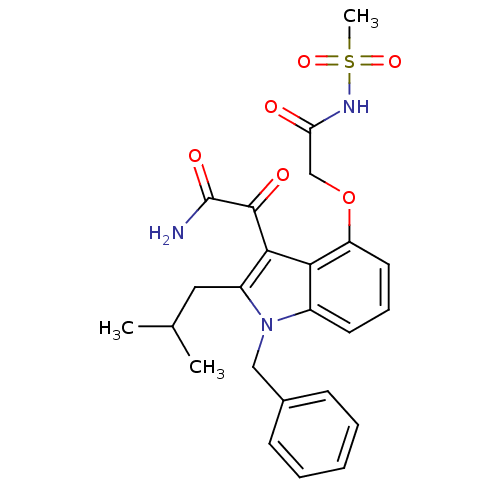 (CHEMBL477548 | mesyl-2-(3-(2-amino-2-oxoacetyl)-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Binding affinity to sPLA2X (unknown origin) | Bioorg Med Chem Lett 24: 5251-5 (2014) Article DOI: 10.1016/j.bmcl.2014.09.058 BindingDB Entry DOI: 10.7270/Q2668FS8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519574 (CHEMBL4563433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223288 ((1S,2S)-2-[(5-{(2-Fluoro-2-methylpropyl)[2-fluoro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519586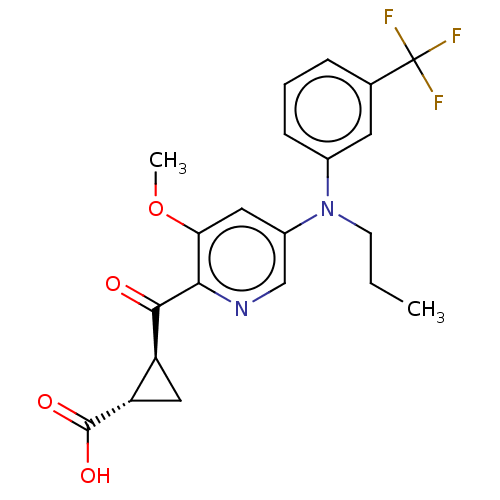 (CHEMBL4439499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519580 (CHEMBL4574439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519577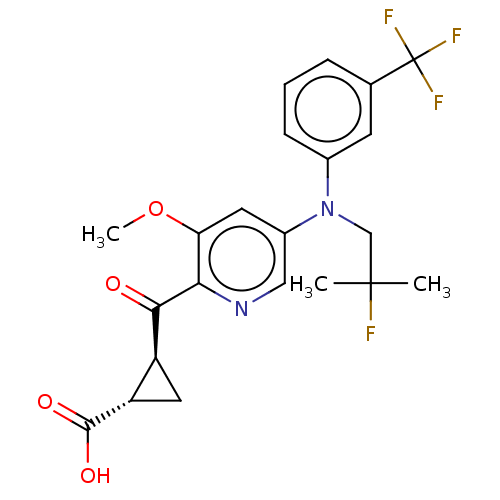 (CHEMBL4439148) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519571 (CHEMBL4454609) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM223287 ((1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-flu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 5-hydroxylase 2 (Homo sapiens (Human)) | BDBM445676 (Ex. 14/KM-06-64/(I-14) | US10683309, Example 14 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
MAX-DELBRÜCK-CENTRUM FÜR MOLEKULARE MEDIZIN; FORSCHUNGSVERBUND BERLIN E.V. US Patent | Assay Description The full-length coding sequences of human TPH1 and TPH2 were PCR amplified, ligated into a MBP fusion vector (pMalc2x, New England Biolabs, MA, USA) ... | US Patent US10683309 (2020) BindingDB Entry DOI: 10.7270/Q2NG4TQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 5-hydroxylase 2 (Homo sapiens (Human)) | BDBM445676 (Ex. 14/KM-06-64/(I-14) | US10683309, Example 14 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
MAX-DELBRÜCK-CENTRUM FÜR MOLEKULARE MEDIZIN; FORSCHUNGSVERBUND BERLIN E.V. US Patent | Assay Description The full-length coding sequences of human TPH1 and TPH2 were PCR amplified, ligated into a MBP fusion vector (pMalc2x, New England Biolabs, MA, USA) ... | US Patent US11028104 (2021) BindingDB Entry DOI: 10.7270/Q2JM2DRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519578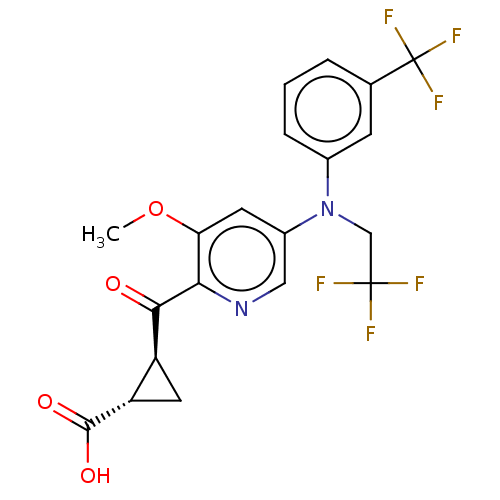 (CHEMBL4445815) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519589 (CHEMBL4531985) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 5-hydroxylase 2 (Homo sapiens (Human)) | BDBM357737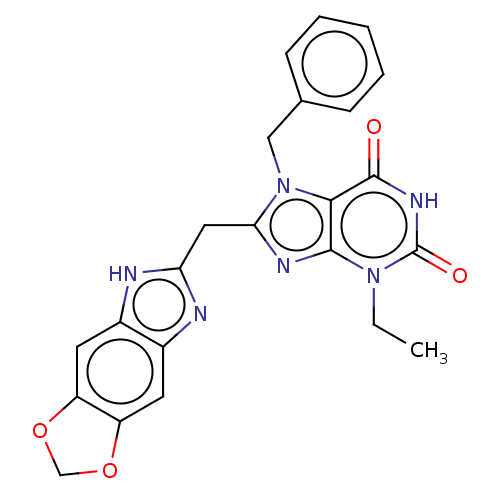 (KM-05-166/(I-206) | US10214530, Example 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00598 BindingDB Entry DOI: 10.7270/Q2G164VF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 5-hydroxylase 2 (Homo sapiens (Human)) | BDBM357735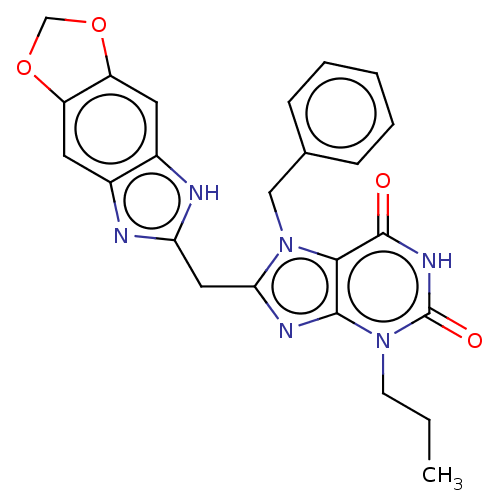 (KM-05-193/(I-209) | US10214530, Example 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00598 BindingDB Entry DOI: 10.7270/Q2G164VF | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 5-hydroxylase 2 (Homo sapiens (Human)) | BDBM445677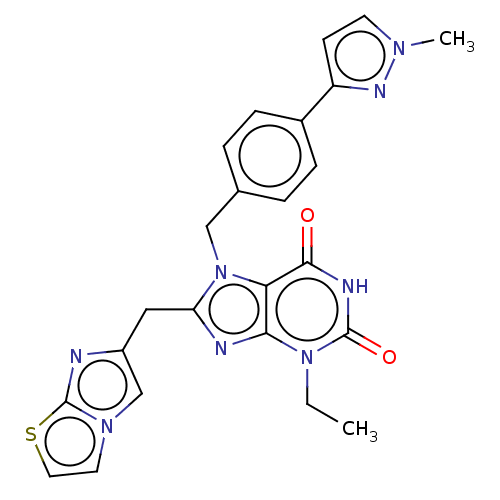 (Ex. 15/KM-06-96/(I-15) | US10683309, Example 15 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
MAX-DELBRÜCK-CENTRUM FÜR MOLEKULARE MEDIZIN; FORSCHUNGSVERBUND BERLIN E.V. US Patent | Assay Description The full-length coding sequences of human TPH1 and TPH2 were PCR amplified, ligated into a MBP fusion vector (pMalc2x, New England Biolabs, MA, USA) ... | US Patent US11028104 (2021) BindingDB Entry DOI: 10.7270/Q2JM2DRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 5-hydroxylase 2 (Homo sapiens (Human)) | BDBM445677 (Ex. 15/KM-06-96/(I-15) | US10683309, Example 15 | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
MAX-DELBRÜCK-CENTRUM FÜR MOLEKULARE MEDIZIN; FORSCHUNGSVERBUND BERLIN E.V. US Patent | Assay Description The full-length coding sequences of human TPH1 and TPH2 were PCR amplified, ligated into a MBP fusion vector (pMalc2x, New England Biolabs, MA, USA) ... | US Patent US10683309 (2020) BindingDB Entry DOI: 10.7270/Q2NG4TQ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458617 (CHEMBL4215835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519569 (CHEMBL4545950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of LTC4S in zymosan-stimulated human PBMC assessed as inhibition of LTC4 production preincubated for 45 mins followed by zymosan stimulati... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 5-hydroxylase 2 (Homo sapiens (Human)) | BDBM357735 (KM-05-193/(I-209) | US10214530, Example 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
MAX-DELBRÜCK-CENTRUM FÜR MOLEKULARE MEDIZIN US Patent | Assay Description These compounds according to the afore-mentioned examples were tested for tryptophan hydroxylase (TPH) inhibitory activity in a fluorescence-based in... | US Patent US10214530 (2019) BindingDB Entry DOI: 10.7270/Q25B04SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 5-hydroxylase 2 (Homo sapiens (Human)) | BDBM357737 (KM-05-166/(I-206) | US10214530, Example 24) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
MAX-DELBRÜCK-CENTRUM FÜR MOLEKULARE MEDIZIN US Patent | Assay Description These compounds according to the afore-mentioned examples were tested for tryptophan hydroxylase (TPH) inhibitory activity in a fluorescence-based in... | US Patent US10214530 (2019) BindingDB Entry DOI: 10.7270/Q25B04SK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458617 (CHEMBL4215835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene C4 synthase (Homo sapiens (Human)) | BDBM50519566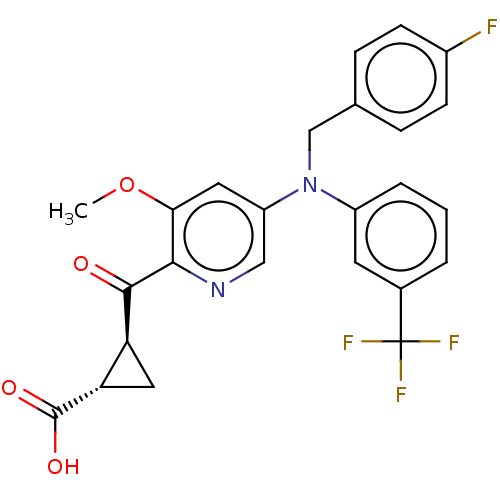 (CHEMBL4565208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Orexo AB Curated by ChEMBL | Assay Description Inhibition of human N-terminal His6-tagged LTC4S expressed in Pichia pastoris X33 using LTA4 methyl ester and glutathione as substrate preincubated f... | J Med Chem 62: 7769-7787 (2019) Article DOI: 10.1021/acs.jmedchem.9b00555 BindingDB Entry DOI: 10.7270/Q2WS8XMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptophan 5-hydroxylase 2 (Homo sapiens (Human)) | BDBM445685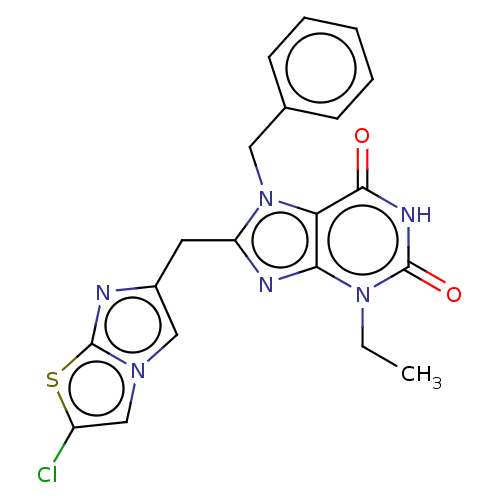 (Ex. 21/KM-06-108/(I-21) | US10683309, Example 21 |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
MAX-DELBRÜCK-CENTRUM FÜR MOLEKULARE MEDIZIN; FORSCHUNGSVERBUND BERLIN E.V. US Patent | Assay Description The full-length coding sequences of human TPH1 and TPH2 were PCR amplified, ligated into a MBP fusion vector (pMalc2x, New England Biolabs, MA, USA) ... | US Patent US11028104 (2021) BindingDB Entry DOI: 10.7270/Q2JM2DRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458614 (CHEMBL4210991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 671 total ) | Next | Last >> |