 Found 4535 hits with Last Name = 'xi' and Initial = 'm'
Found 4535 hits with Last Name = 'xi' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM14361
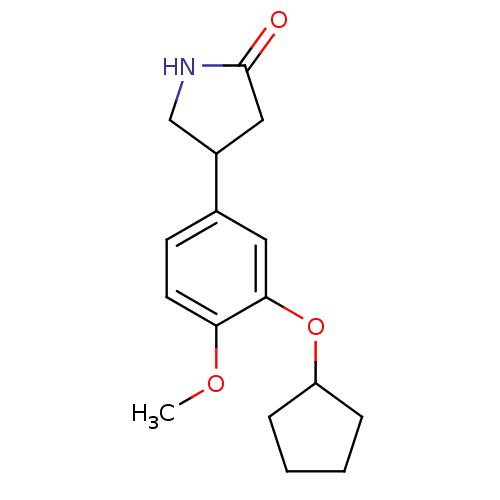
((R,S)-Rolipram | 4-(3-cyclopentyloxy-4-methoxy-phe...)Show InChI InChI=1S/C16H21NO3/c1-19-14-7-6-11(12-9-16(18)17-10-12)8-15(14)20-13-4-2-3-5-13/h6-8,12-13H,2-5,9-10H2,1H3,(H,17,18) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.00230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220995

(CHEMBL77788)Show InChI InChI=1S/C15H20N2O3/c1-11(18)17-16-10-12-7-8-14(19-2)15(9-12)20-13-5-3-4-6-13/h7-10,13H,3-6H2,1-2H3,(H,17,18)/b16-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50155307

(CHEMBL3781796)Show SMILES [H]C1(NC(=O)C(Cc2ccccc2)NC(=O)C([H])(NC(=O)C(CCCCN)NC(=O)C([H])(NC(=O)C(Cc2ccccc2)NC(=O)C(Cc2ccccc2)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(CSSCC(NC(=O)C(CO)NC1=O)C(O)=O)NC(=O)CNC(=O)C(C)NC(=O)C(CCCCN)NC(=O)C(CCCNC(N)=N)NC(=O)C(CCC(O)=O)NC(=O)C(CCCNC(N)=N)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(CCSC)NC(=O)C(C)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(C)NC(=O)C(N)CO)C(C)O)C(C)O)C(C)O Show InChI InChI=1S/C130H204N40O40S3/c1-64(146-106(187)75(34-18-21-44-131)151-108(189)78(37-24-47-142-129(138)139)152-111(192)80(41-42-98(181)182)154-109(190)79(38-25-48-143-130(140)141)155-122(203)93-40-26-49-169(93)126(207)67(4)148-107(188)81(43-51-211-8)150-103(184)66(3)147-121(202)92-39-27-50-170(92)127(208)87(57-96(137)179)162-118(199)88(60-172)163-115(196)85(55-94(135)177)157-104(185)65(2)145-105(186)74(134)59-171)102(183)144-58-97(180)149-90-62-212-213-63-91(128(209)210)165-119(200)89(61-173)164-125(206)101(70(7)176)168-117(198)84(54-73-32-16-11-17-33-73)161-124(205)100(69(6)175)166-112(193)77(36-20-23-46-133)156-123(204)99(68(5)174)167-116(197)83(53-72-30-14-10-15-31-72)159-113(194)82(52-71-28-12-9-13-29-71)158-114(195)86(56-95(136)178)160-110(191)76(153-120(90)201)35-19-22-45-132/h9-17,28-33,64-70,74-93,99-101,171-176H,18-27,34-63,131-134H2,1-8H3,(H2,135,177)(H2,136,178)(H2,137,179)(H,144,183)(H,145,186)(H,146,187)(H,147,202)(H,148,188)(H,149,180)(H,150,184)(H,151,189)(H,152,192)(H,153,201)(H,154,190)(H,155,203)(H,156,204)(H,157,185)(H,158,195)(H,159,194)(H,160,191)(H,161,205)(H,162,199)(H,163,196)(H,164,206)(H,165,200)(H,166,193)(H,167,197)(H,168,198)(H,181,182)(H,209,210)(H4,138,139,142)(H4,140,141,143)/t64-,65-,66-,67-,68+,69+,70+,74-,75-,76+,77-,78-,79-,80-,81-,82-,83-,84-,85?,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst2 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50173133
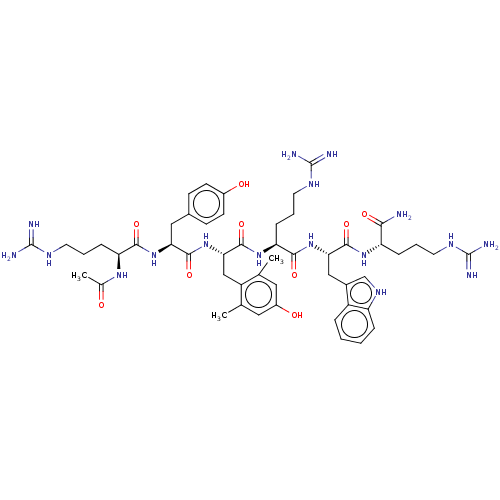
(CHEMBL3810319)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1c(C)cc(O)cc1C)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C51H73N17O9/c1-27-21-33(71)22-28(2)35(27)25-42(68-46(75)40(23-30-14-16-32(70)17-15-30)66-44(73)38(63-29(3)69)12-7-19-60-50(55)56)48(77)65-39(13-8-20-61-51(57)58)45(74)67-41(24-31-26-62-36-10-5-4-9-34(31)36)47(76)64-37(43(52)72)11-6-18-59-49(53)54/h4-5,9-10,14-17,21-22,26,37-42,62,70-71H,6-8,11-13,18-20,23-25H2,1-3H3,(H2,52,72)(H,63,69)(H,64,76)(H,65,77)(H,66,73)(H,67,74)(H,68,75)(H4,53,54,59)(H4,55,56,60)(H4,57,58,61)/t37-,38-,39-,40-,41-,42-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counti... |
J Med Chem 59: 3777-92 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01976
BindingDB Entry DOI: 10.7270/Q2T72KC6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221005
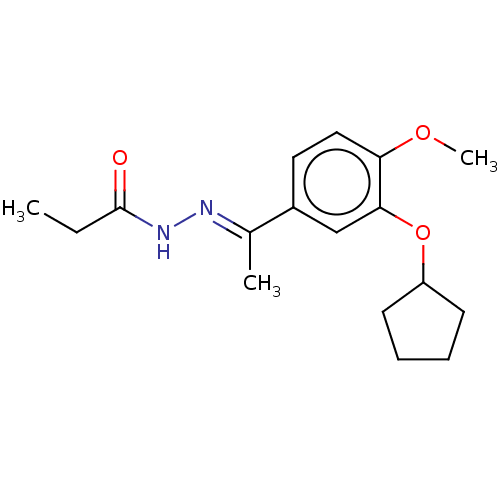
(CHEMBL75684)Show InChI InChI=1S/C17H24N2O3/c1-4-17(20)19-18-12(2)13-9-10-15(21-3)16(11-13)22-14-7-5-6-8-14/h9-11,14H,4-8H2,1-3H3,(H,19,20)/b18-12+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220998
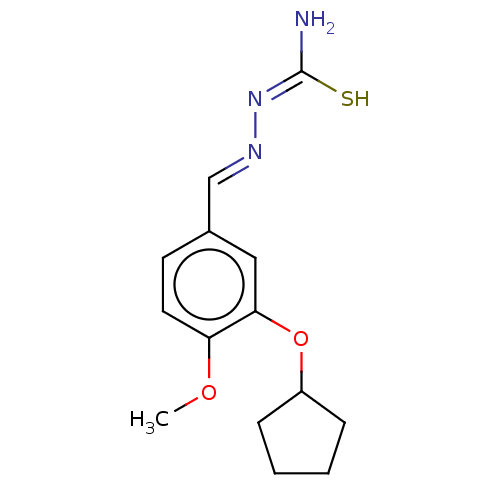
(CHEMBL76382)Show InChI InChI=1S/C14H19N3O2S/c1-18-12-7-6-10(9-16-17-14(15)20)8-13(12)19-11-4-2-3-5-11/h6-9,11H,2-5H2,1H3,(H3,15,17,20)/b16-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50173132

(CHEMBL3808650)Show SMILES CC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccc(F)cc1)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCNC(N)=N)C(N)=O |r| Show InChI InChI=1S/C49H68FN17O8/c1-27(68)62-36(10-5-21-59-48(54)55)42(71)65-39(24-29-14-18-32(69)19-15-29)45(74)66-38(23-28-12-16-31(50)17-13-28)44(73)64-37(11-6-22-60-49(56)57)43(72)67-40(25-30-26-61-34-8-3-2-7-33(30)34)46(75)63-35(41(51)70)9-4-20-58-47(52)53/h2-3,7-8,12-19,26,35-40,61,69H,4-6,9-11,20-25H2,1H3,(H2,51,70)(H,62,68)(H,63,75)(H,64,73)(H,65,71)(H,66,74)(H,67,72)(H4,52,53,58)(H4,54,55,59)(H4,56,57,60)/t35-,36-,37-,38-,39-,40-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in CHO cell membrane incubated for 60 mins by liquid scintillation counti... |
J Med Chem 59: 3777-92 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01976
BindingDB Entry DOI: 10.7270/Q2T72KC6 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221003
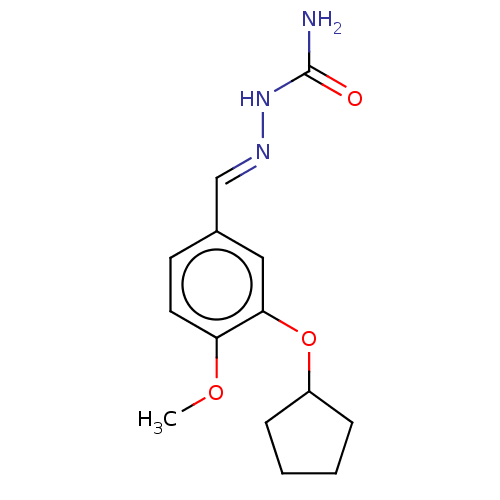
(CHEMBL432348)Show InChI InChI=1S/C14H19N3O3/c1-19-12-7-6-10(9-16-17-14(15)18)8-13(12)20-11-4-2-3-5-11/h6-9,11H,2-5H2,1H3,(H3,15,17,18)/b16-9+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50462974
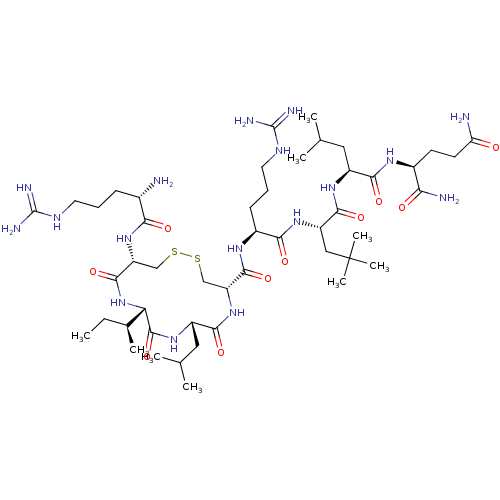
(CHEMBL4240100)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N |r| Show InChI InChI=1S/C48H89N17O10S2/c1-10-26(6)36-45(75)61-31(20-25(4)5)41(71)64-33(22-76-77-23-34(44(74)65-36)63-38(68)27(49)13-11-17-56-46(52)53)43(73)59-29(14-12-18-57-47(54)55)39(69)62-32(21-48(7,8)9)42(72)60-30(19-24(2)3)40(70)58-28(37(51)67)15-16-35(50)66/h24-34,36H,10-23,49H2,1-9H3,(H2,50,66)(H2,51,67)(H,58,70)(H,59,73)(H,60,72)(H,61,75)(H,62,69)(H,63,68)(H,64,71)(H,65,74)(H4,52,53,56)(H4,54,55,57)/t26-,27-,28-,29-,30-,31-,32-,33+,34+,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERalpha expressed in baculovirus expression system assessed as inhibition of estradiol-induced E... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220997

(CHEMBL78237)Show InChI InChI=1S/C16H22N2O3/c1-11(17-18-12(2)19)13-8-9-15(20-3)16(10-13)21-14-6-4-5-7-14/h8-10,14H,4-7H2,1-3H3,(H,18,19)/b17-11+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50070386
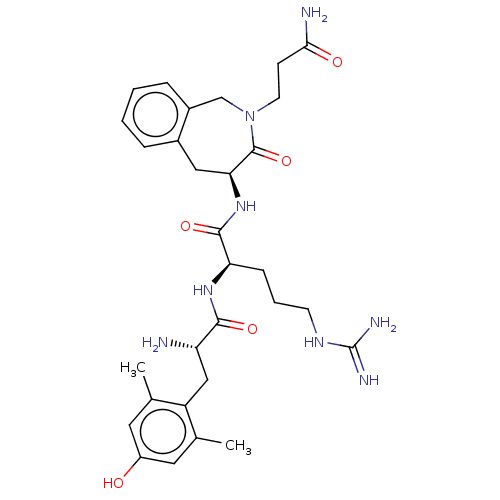
(CHEMBL3408737)Show SMILES [H][C@@]1(Cc2ccccc2CN(CCC(N)=O)C1=O)NC(=O)[C@@H](CCCNC(N)=N)NC(=O)[C@@H](N)Cc1c(C)cc(O)cc1C |r| Show InChI InChI=1S/C30H42N8O5/c1-17-12-21(39)13-18(2)22(17)15-23(31)27(41)36-24(8-5-10-35-30(33)34)28(42)37-25-14-19-6-3-4-7-20(19)16-38(29(25)43)11-9-26(32)40/h3-4,6-7,12-13,23-25,39H,5,8-11,14-16,31H2,1-2H3,(H2,32,40)(H,36,41)(H,37,42)(H4,33,34,35)/t23-,24+,25-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human mu opioid receptor expressed in HEK293 cell membrane incubated for 1 hr by TopCount scintillation counti... |
J Med Chem 59: 3777-92 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01976
BindingDB Entry DOI: 10.7270/Q2T72KC6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50428107
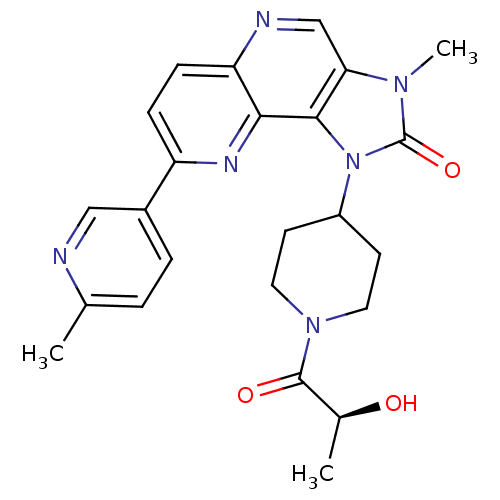
(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50221006
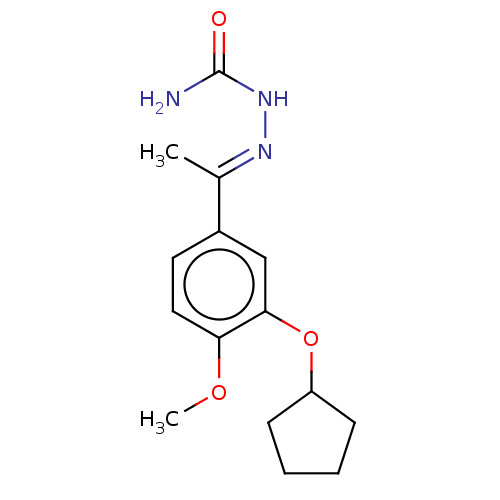
(CHEMBL77358)Show InChI InChI=1S/C15H21N3O3/c1-10(17-18-15(16)19)11-7-8-13(20-2)14(9-11)21-12-5-3-4-6-12/h7-9,12H,3-6H2,1-2H3,(H3,16,18,19)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428109
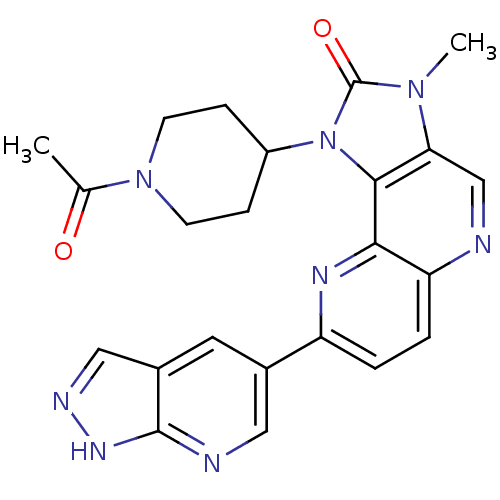
(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.191 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Apelin receptor
(Homo sapiens (Human)) | BDBM50556826

(CHEMBL4745863)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](CCC(=O)N[C@@H](CS)C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01913
BindingDB Entry DOI: 10.7270/Q2BG2SNB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428109

(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.243 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Apelin receptor
(Homo sapiens (Human)) | BDBM50556827

(CHEMBL4748838)Show SMILES CSCC[C@H](NC(=O)[C@H](CS)NC(=O)CC[C@H](NC(=O)CCOCCOCCOCCOCCOCCOCCN)C(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01913
BindingDB Entry DOI: 10.7270/Q2BG2SNB |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428107

(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.299 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220999

(CHEMBL77745)Show InChI InChI=1S/C15H21N3O2S/c1-10(17-18-15(16)21)11-7-8-13(19-2)14(9-11)20-12-5-3-4-6-12/h7-9,12H,3-6H2,1-2H3,(H3,16,18,21)/b17-10+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50594974
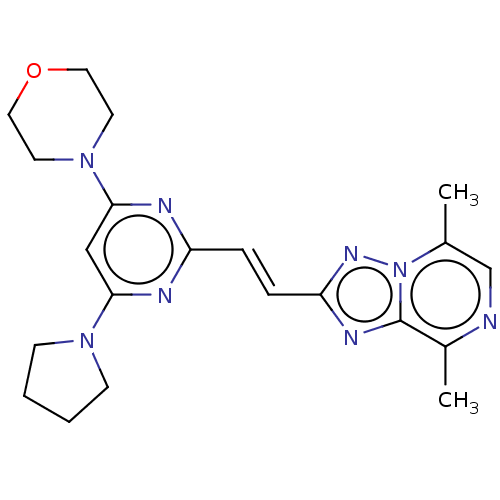
(CHEMBL5186554)Show SMILES Cc1cnc(C)c2nc(\C=C\c3nc(cc(n3)N3CCOCC3)N3CCCC3)nn12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114170
BindingDB Entry DOI: 10.7270/Q2RX9H3Q |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50594974

(CHEMBL5186554)Show SMILES Cc1cnc(C)c2nc(\C=C\c3nc(cc(n3)N3CCOCC3)N3CCCC3)nn12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2022.114170
BindingDB Entry DOI: 10.7270/Q2RX9H3Q |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428111
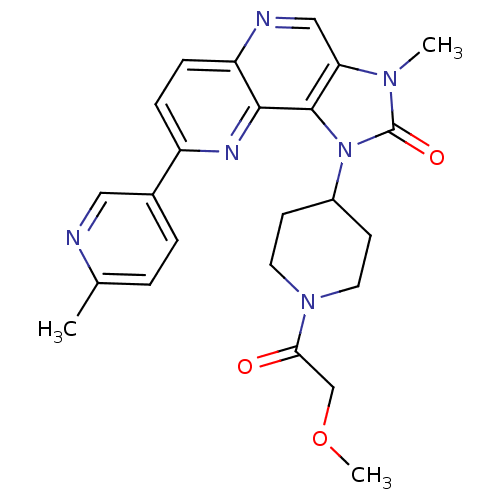
(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428108
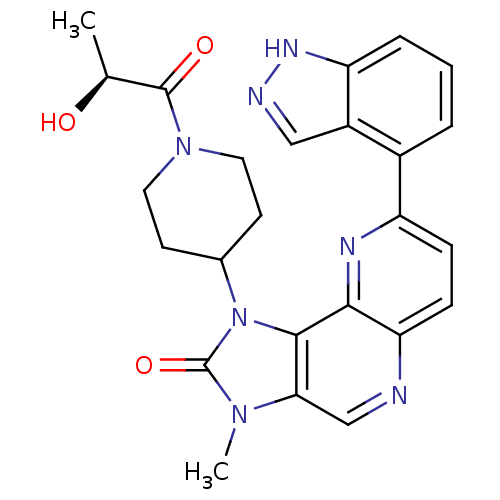
(CHEMBL2331669 | US8791131, 255)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O |r| Show InChI InChI=1S/C25H25N7O3/c1-14(33)24(34)31-10-8-15(9-11-31)32-23-21(30(2)25(32)35)13-26-20-7-6-18(28-22(20)23)16-4-3-5-19-17(16)12-27-29-19/h3-7,12-15,33H,8-11H2,1-2H3,(H,27,29)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.395 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50474799
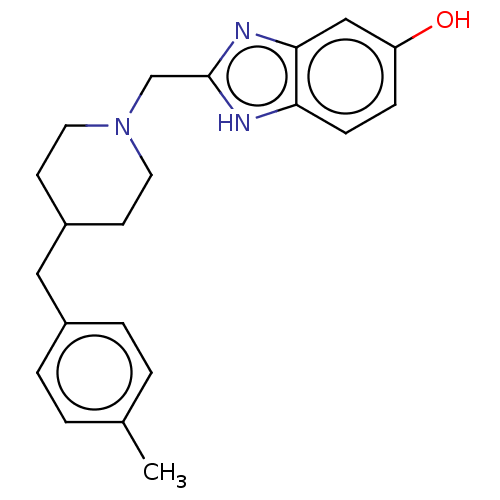
(CHEMBL416690)Show InChI InChI=1S/C21H25N3O/c1-15-2-4-16(5-3-15)12-17-8-10-24(11-9-17)14-21-22-19-7-6-18(25)13-20(19)23-21/h2-7,13,17,25H,8-12,14H2,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... |
J Med Chem 47: 2089-96 (2004)
Article DOI: 10.1021/jm030483s
BindingDB Entry DOI: 10.7270/Q2348P41 |
More data for this
Ligand-Target Pair | |
Apelin receptor
(Homo sapiens (Human)) | BDBM50556825

(CHEMBL4787784)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](CCC(=O)NCCCC[C@H](N)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01913
BindingDB Entry DOI: 10.7270/Q2BG2SNB |
More data for this
Ligand-Target Pair | |
Nociceptin receptor
(Homo sapiens (Human)) | BDBM50173126

(CHEMBL3809510)Show SMILES CC[C@H](C)[C@@H](NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CCCNC(N)=N)NC(C)=O)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C44H70N14O9/c1-4-25(2)36(42(67)54-31(37(46)62)9-5-6-20-45)58-39(64)33(11-8-22-52-44(49)50)55-40(65)34(23-27-12-16-29(60)17-13-27)57-41(66)35(24-28-14-18-30(61)19-15-28)56-38(63)32(53-26(3)59)10-7-21-51-43(47)48/h12-19,25,31-36,60-61H,4-11,20-24,45H2,1-3H3,(H2,46,62)(H,53,59)(H,54,67)(H,55,65)(H,56,63)(H,57,66)(H,58,64)(H4,47,48,51)(H4,49,50,52)/t25-,31-,32-,33-,34-,35-,36+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vrije Universiteit Brussel
Curated by ChEMBL
| Assay Description
Displacement of [3H]OFQ/nociceptin from human nociceptin receptor expressed in HEK293 cell membrane incubated for 1 hr by TopCount scintillation coun... |
J Med Chem 59: 3777-92 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01976
BindingDB Entry DOI: 10.7270/Q2T72KC6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428113
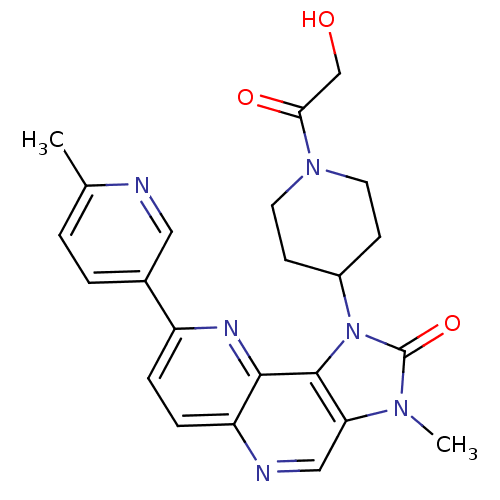
(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.532 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428115
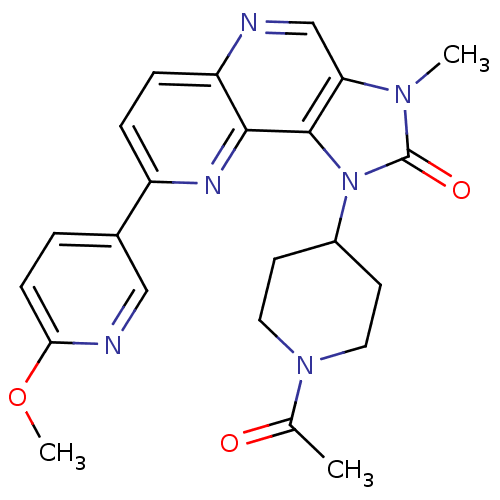
(CHEMBL2331661 | US8791131, 136)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(C)=O)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14(30)28-10-8-16(9-11-28)29-22-19(27(2)23(29)31)13-24-18-6-5-17(26-21(18)22)15-4-7-20(32-3)25-12-15/h4-7,12-13,16H,8-11H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.542 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428110
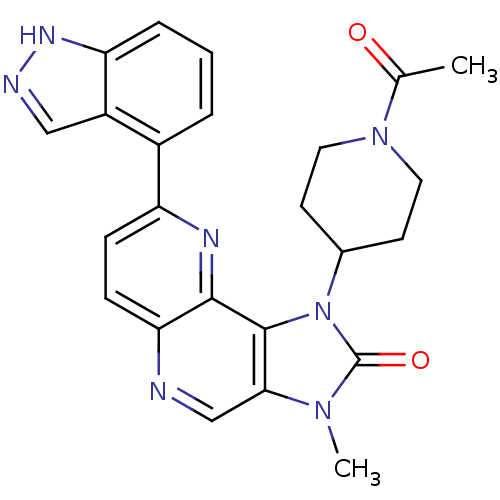
(CHEMBL2331667 | US8791131, 254)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O Show InChI InChI=1S/C24H23N7O2/c1-14(32)30-10-8-15(9-11-30)31-23-21(29(2)24(31)33)13-25-20-7-6-18(27-22(20)23)16-4-3-5-19-17(16)12-26-28-19/h3-7,12-13,15H,8-11H2,1-2H3,(H,26,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.584 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361649
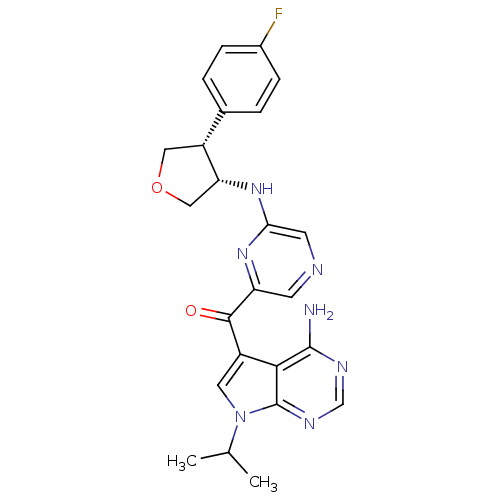
(CHEMBL1938415)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3COC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-9-16(21-23(26)28-12-29-24(21)32)22(33)18-7-27-8-20(30-18)31-19-11-34-10-17(19)14-3-5-15(25)6-4-14/h3-9,12-13,17,19H,10-11H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Apelin receptor
(Homo sapiens (Human)) | BDBM50556828
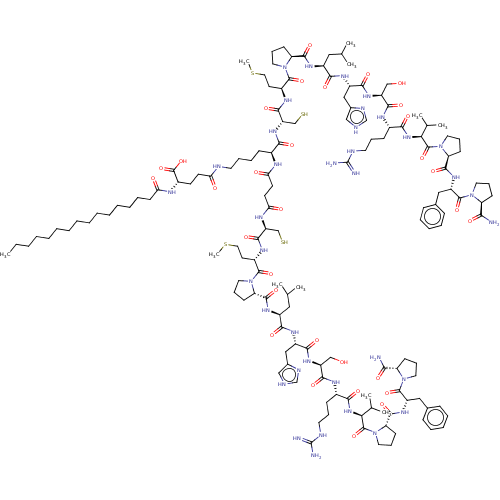
(CHEMBL4798771)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](CCC(=O)NCCCC[C@H](NC(=O)CCC(=O)N[C@@H](CS)C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](CCSC)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(N)=O)C(O)=O |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [Glp65, Nle75, Tyr77][125I]-apelin13 from human APJ receptor expressed in HEK293 cells incubated for 1 hr by gamma counting based rad... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01913
BindingDB Entry DOI: 10.7270/Q2BG2SNB |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361648
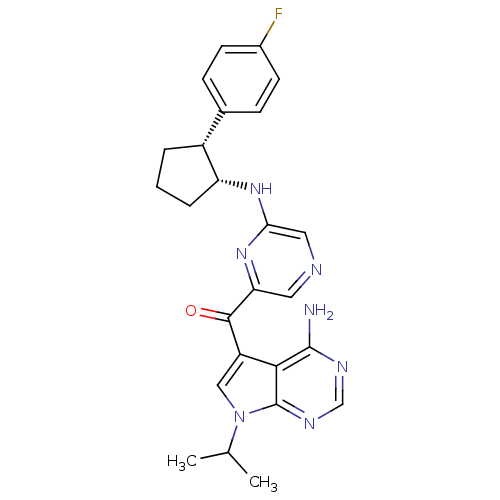
(CHEMBL1940246)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H26FN7O/c1-14(2)33-12-18(22-24(27)29-13-30-25(22)33)23(34)20-10-28-11-21(32-20)31-19-5-3-4-17(19)15-6-8-16(26)9-7-15/h6-14,17,19H,3-5H2,1-2H3,(H,31,32)(H2,27,29,30)/t17-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50474791

(CHEMBL65693)Show SMILES CS(=O)(=O)Nc1ccc2[nH]c(Cc3ccc(Oc4ccccc4)cc3)nc2c1 Show InChI InChI=1S/C21H19N3O3S/c1-28(25,26)24-16-9-12-19-20(14-16)23-21(22-19)13-15-7-10-18(11-8-15)27-17-5-3-2-4-6-17/h2-12,14,24H,13H2,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... |
J Med Chem 47: 2089-96 (2004)
Article DOI: 10.1021/jm030483s
BindingDB Entry DOI: 10.7270/Q2348P41 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361642
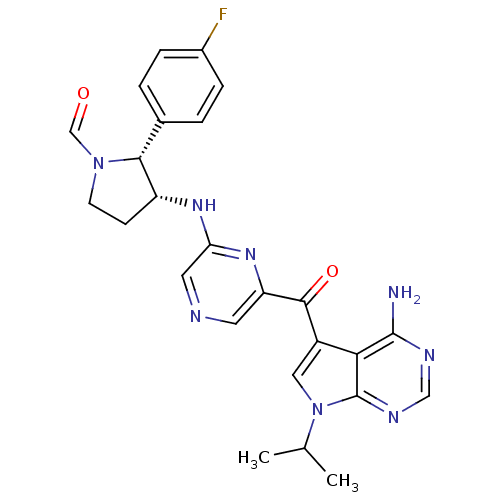
(CHEMBL1940251)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN(C=O)[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C25H25FN8O2/c1-14(2)34-11-17(21-24(27)29-12-30-25(21)34)23(36)19-9-28-10-20(32-19)31-18-7-8-33(13-35)22(18)15-3-5-16(26)6-4-15/h3-6,9-14,18,22H,7-8H2,1-2H3,(H,31,32)(H2,27,29,30)/t18-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50474809

(CHEMBL65454)Show SMILES Oc1ccc2[nH]c(CN3CCC(Cc4c(F)cccc4F)CC3)nc2c1 Show InChI InChI=1S/C20H21F2N3O/c21-16-2-1-3-17(22)15(16)10-13-6-8-25(9-7-13)12-20-23-18-5-4-14(26)11-19(18)24-20/h1-5,11,13,26H,6-10,12H2,(H,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... |
J Med Chem 47: 2089-96 (2004)
Article DOI: 10.1021/jm030483s
BindingDB Entry DOI: 10.7270/Q2348P41 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50428113

(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.842 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50474789

(CHEMBL68134)Show InChI InChI=1S/C20H22FN3O/c21-17-4-2-1-3-15(17)11-14-7-9-24(10-8-14)13-20-22-18-6-5-16(25)12-19(18)23-20/h1-6,12,14,25H,7-11,13H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... |
J Med Chem 47: 2089-96 (2004)
Article DOI: 10.1021/jm030483s
BindingDB Entry DOI: 10.7270/Q2348P41 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361641
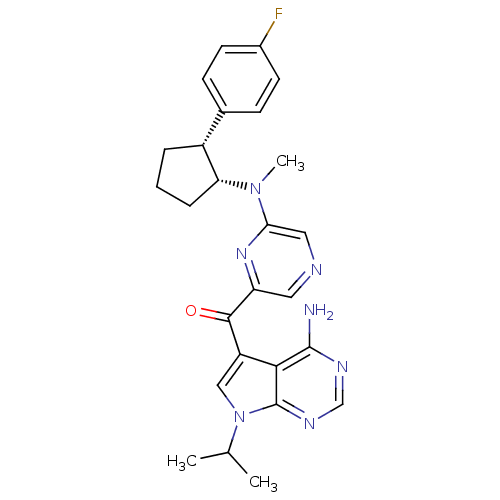
(CHEMBL1940247)Show SMILES CC(C)n1cc(C(=O)c2cncc(n2)N(C)[C@@H]2CCC[C@@H]2c2ccc(F)cc2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28FN7O/c1-15(2)34-13-19(23-25(28)30-14-31-26(23)34)24(35)20-11-29-12-22(32-20)33(3)21-6-4-5-18(21)16-7-9-17(27)10-8-16/h7-15,18,21H,4-6H2,1-3H3,(H2,28,30,31)/t18-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Mus musculus (Mouse)) | BDBM50428111

(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.922 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse PI3Kalpha |
ACS Med Chem Lett 4: 91-7 (2013)
Article DOI: 10.1021/ml300309h
BindingDB Entry DOI: 10.7270/Q2HX1F0B |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50204862

(CHEMBL436521 | N-(2-((4-benzylpiperidin-1-yl)methy...)Show SMILES CS(=O)(=O)Nc1ccc2nc(CN3CCC(Cc4ccccc4)CC3)[nH]c2c1 Show InChI InChI=1S/C21H26N4O2S/c1-28(26,27)24-18-7-8-19-20(14-18)23-21(22-19)15-25-11-9-17(10-12-25)13-16-5-3-2-4-6-16/h2-8,14,17,24H,9-13,15H2,1H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... |
J Med Chem 47: 2089-96 (2004)
Article DOI: 10.1021/jm030483s
BindingDB Entry DOI: 10.7270/Q2348P41 |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361652
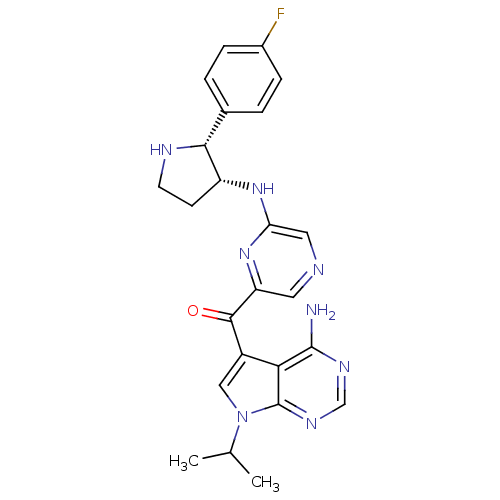
(CHEMBL1940250)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-16(20-23(26)29-12-30-24(20)33)22(34)18-9-27-10-19(32-18)31-17-7-8-28-21(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,21,28H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t17-,21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, NMDA 1/2B
(Homo sapiens (Human)) | BDBM50474796

(CHEMBL64941)Show InChI InChI=1S/C20H22FN3O/c21-16-3-1-14(2-4-16)11-15-7-9-24(10-8-15)13-20-22-18-6-5-17(25)12-19(18)23-20/h1-6,12,15,25H,7-11,13H2,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was evaluated for in vitro inhibition of [3H][(E)-N-(2-methoxybenzyl)cinnamamidine binding to human NR1a/NR2B receptors expressed in LtK-cel... |
J Med Chem 47: 2089-96 (2004)
Article DOI: 10.1021/jm030483s
BindingDB Entry DOI: 10.7270/Q2348P41 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4C
(Rattus norvegicus) | BDBM50220996

(CHEMBL76635)Show InChI InChI=1S/C20H23N3O3/c1-25-17-12-11-15(13-18(17)26-16-9-5-6-10-16)19(22-23-20(21)24)14-7-3-2-4-8-14/h2-4,7-8,11-13,16H,5-6,9-10H2,1H3,(H3,21,23,24)/b22-19+ | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
LEAD GENE CO., LTD
Curated by ChEMBL
| Assay Description
Binding affinity towards rolipram binding site on phosphodiesterase 4 using [3H]rolipram as radioligand in crude rat brain homogenate |
Bioorg Med Chem Lett 13: 2355-8 (2003)
BindingDB Entry DOI: 10.7270/Q2CZ39CG |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50462974

(CHEMBL4240100)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@@H](CSSC[C@@H](NC(=O)[C@H](CC(C)C)NC1=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](CC(C)(C)C)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCC(N)=O)C(N)=O)NC(=O)[C@@H](N)CCCNC(N)=N |r| Show InChI InChI=1S/C48H89N17O10S2/c1-10-26(6)36-45(75)61-31(20-25(4)5)41(71)64-33(22-76-77-23-34(44(74)65-36)63-38(68)27(49)13-11-17-56-46(52)53)43(73)59-29(14-12-18-57-47(54)55)39(69)62-32(21-48(7,8)9)42(72)60-30(19-24(2)3)40(70)58-28(37(51)67)15-16-35(50)66/h24-34,36H,10-23,49H2,1-9H3,(H2,50,66)(H2,51,67)(H,58,70)(H,59,73)(H,60,72)(H,61,75)(H,62,69)(H,63,68)(H,64,71)(H,65,74)(H4,52,53,56)(H4,54,55,57)/t26-,27-,28-,29-,30-,31-,32-,33+,34+,36-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Peking University Shenzhen Graduate School
Curated by ChEMBL
| Assay Description
Antagonist activity at full length recombinant human ERbeta expressed in baculovirus expression system assessed as inhibition of estradiol-induced Eu... |
Bioorg Med Chem Lett 28: 2827-2836 (2018)
Article DOI: 10.1016/j.bmcl.2018.05.062
BindingDB Entry DOI: 10.7270/Q2M90CBB |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361650
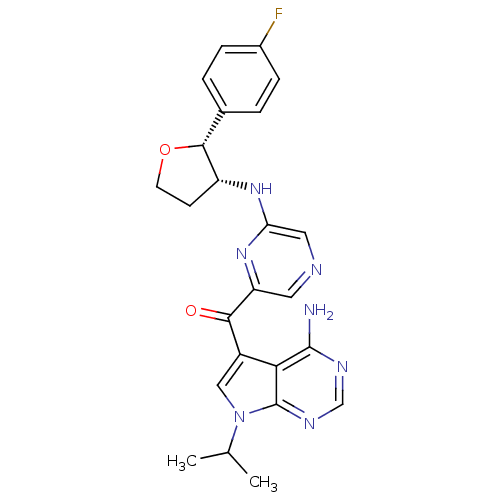
(CHEMBL1940248)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCO[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H24FN7O2/c1-13(2)32-11-16(20-23(26)28-12-29-24(20)32)21(33)18-9-27-10-19(31-18)30-17-7-8-34-22(17)14-3-5-15(25)6-4-14/h3-6,9-13,17,22H,7-8H2,1-2H3,(H,30,31)(H2,26,28,29)/t17-,22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PDK1-mediated AKT1 phosphorylation at T308 in human H460 cells after 2 hrs by ELISA |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM111939

(US8618107, 105)Show SMILES Cn1cc(cc(Nc2ccncn2)c1=O)-c1cccc(N2CCc3c4CC(C)(C)Cc4sc3C2=O)c1CO Show InChI InChI=1S/C29H29N5O3S/c1-29(2)12-20-19-8-10-34(28(37)26(19)38-24(20)13-29)23-6-4-5-18(21(23)15-35)17-11-22(27(36)33(3)14-17)32-25-7-9-30-16-31-25/h4-7,9,11,14,16,35H,8,10,12-13,15H2,1-3H3,(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc., Research and Early Development, 1 DNA Way, South San Francisco, California 94080, United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human full length His-tagged BTK expressed in baculovirus expression system by Z-LYTE assay |
ACS Med Chem Lett 8: 608-613 (2017)
Article DOI: 10.1021/acsmedchemlett.7b00103
BindingDB Entry DOI: 10.7270/Q24M96ZH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50155307

(CHEMBL3781796)Show SMILES [H]C1(NC(=O)C(Cc2ccccc2)NC(=O)C([H])(NC(=O)C(CCCCN)NC(=O)C([H])(NC(=O)C(Cc2ccccc2)NC(=O)C(Cc2ccccc2)NC(=O)C(CC(N)=O)NC(=O)C(CCCCN)NC(=O)C(CSSCC(NC(=O)C(CO)NC1=O)C(O)=O)NC(=O)CNC(=O)C(C)NC(=O)C(CCCCN)NC(=O)C(CCCNC(N)=N)NC(=O)C(CCC(O)=O)NC(=O)C(CCCNC(N)=N)NC(=O)C1CCCN1C(=O)C(C)NC(=O)C(CCSC)NC(=O)C(C)NC(=O)C1CCCN1C(=O)C(CC(N)=O)NC(=O)C(CO)NC(=O)C(CC(N)=O)NC(=O)C(C)NC(=O)C(N)CO)C(C)O)C(C)O)C(C)O Show InChI InChI=1S/C130H204N40O40S3/c1-64(146-106(187)75(34-18-21-44-131)151-108(189)78(37-24-47-142-129(138)139)152-111(192)80(41-42-98(181)182)154-109(190)79(38-25-48-143-130(140)141)155-122(203)93-40-26-49-169(93)126(207)67(4)148-107(188)81(43-51-211-8)150-103(184)66(3)147-121(202)92-39-27-50-170(92)127(208)87(57-96(137)179)162-118(199)88(60-172)163-115(196)85(55-94(135)177)157-104(185)65(2)145-105(186)74(134)59-171)102(183)144-58-97(180)149-90-62-212-213-63-91(128(209)210)165-119(200)89(61-173)164-125(206)101(70(7)176)168-117(198)84(54-73-32-16-11-17-33-73)161-124(205)100(69(6)175)166-112(193)77(36-20-23-46-133)156-123(204)99(68(5)174)167-116(197)83(53-72-30-14-10-15-31-72)159-113(194)82(52-71-28-12-9-13-29-71)158-114(195)86(56-95(136)178)160-110(191)76(153-120(90)201)35-19-22-45-132/h9-17,28-33,64-70,74-93,99-101,171-176H,18-27,34-63,131-134H2,1-8H3,(H2,135,177)(H2,136,178)(H2,137,179)(H,144,183)(H,145,186)(H,146,187)(H,147,202)(H,148,188)(H,149,180)(H,150,184)(H,151,189)(H,152,192)(H,153,201)(H,154,190)(H,155,203)(H,156,204)(H,157,185)(H,158,195)(H,159,194)(H,160,191)(H,161,205)(H,162,199)(H,163,196)(H,164,206)(H,165,200)(H,166,193)(H,167,197)(H,168,198)(H,181,182)(H,209,210)(H4,138,139,142)(H4,140,141,143)/t64-,65-,66-,67-,68+,69+,70+,74-,75-,76+,77-,78-,79-,80-,81-,82-,83-,84-,85?,86-,87-,88-,89-,90-,91-,92-,93-,99-,100-,101-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Gothenburg
Curated by ChEMBL
| Assay Description
Displacement of [125I]tyr11-SRIF from human sst4 receptor after 60 mins by liquid scintillation counting method |
Eur J Med Chem 114: 59-64 (2016)
Article DOI: 10.1016/j.ejmech.2016.02.046
BindingDB Entry DOI: 10.7270/Q2WH2RWC |
More data for this
Ligand-Target Pair | |
3-phosphoinositide-dependent protein kinase 1
(Homo sapiens (Human)) | BDBM50361644
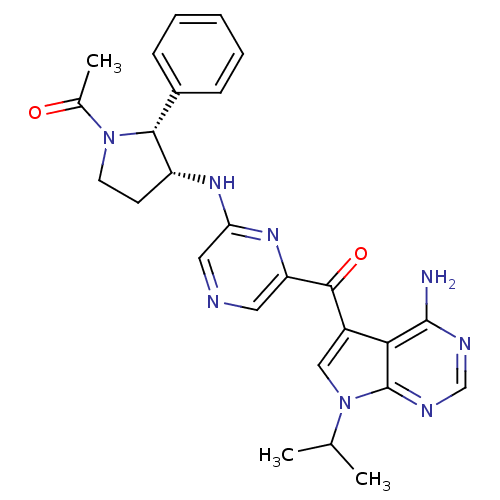
(CHEMBL1940253)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CCN([C@@H]3c3ccccc3)C(C)=O)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C26H28N8O2/c1-15(2)34-13-18(22-25(27)29-14-30-26(22)34)24(36)20-11-28-12-21(32-20)31-19-9-10-33(16(3)35)23(19)17-7-5-4-6-8-17/h4-8,11-15,19,23H,9-10H2,1-3H3,(H,31,32)(H2,27,29,30)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged PDK1 catalytic domain using Ac-Sox-PKTFCGTPEYLAPEVRREPRILSEEEQEMFRDFDYIAD-NH2 as substrate by fluorescence... |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data