

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129580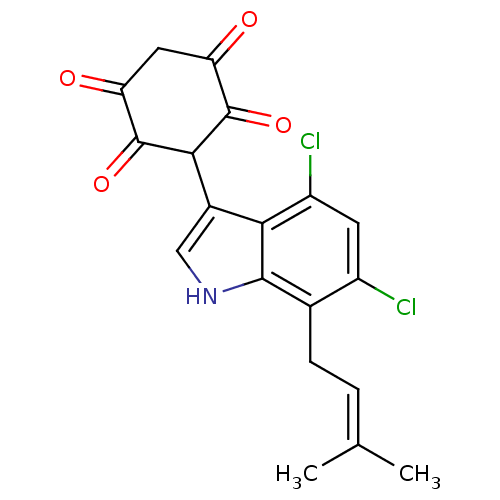 (3-[4,6-Dichloro-7-(3-methyl-but-2-enyl)-1H-indol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory constant of compound against Cell division cycle 25B was determined using mFP as a substrate | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129576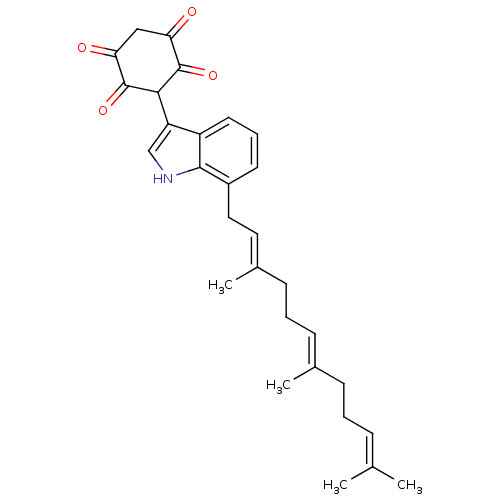 (2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory constant of compound against Cell division cycle 25 was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory constant of compound against Cell division cycle 25B was determined using mFP as a substrate | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-aminocyclopropane-1-carboxylate oxidase 1 (Malus domestica (Apple) (Pyrus malus)) | BDBM36630 (N-hydroxy aminoisobutryic acid) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University | Assay Description Enzyme inhibition assay using ethylene-forming enzyme (EFE). | Chem Biol 5: 49-57 (1998) Article DOI: 10.1016/s1074-5521(98)90086-2 BindingDB Entry DOI: 10.7270/Q24J0CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129575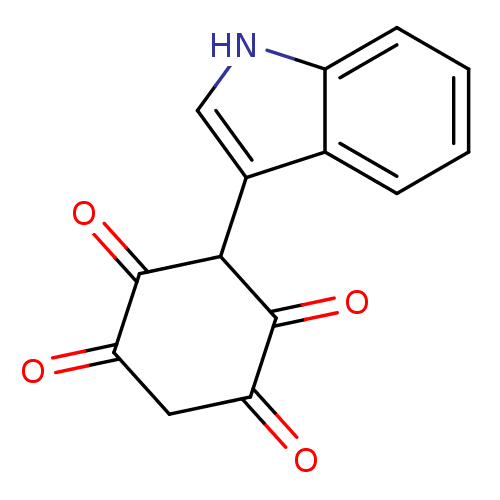 (2,5-Dihydroxy-3-(1H-indol-3-yl)-[1,4]benzoquinone ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory constant of compound against Cdc25B phosphatase was determined using mFP as a substrate | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-aminocyclopropane-1-carboxylate oxidase 1 (Malus domestica (Apple) (Pyrus malus)) | BDBM36632 (N-Hydroxyaminocyclobutanecarboxylic acid) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University | Assay Description Enzyme inhibition assay using ethylene-forming enzyme (EFE). | Chem Biol 5: 49-57 (1998) Article DOI: 10.1016/s1074-5521(98)90086-2 BindingDB Entry DOI: 10.7270/Q24J0CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-aminocyclopropane-1-carboxylate oxidase 1 (Malus domestica (Apple) (Pyrus malus)) | BDBM36629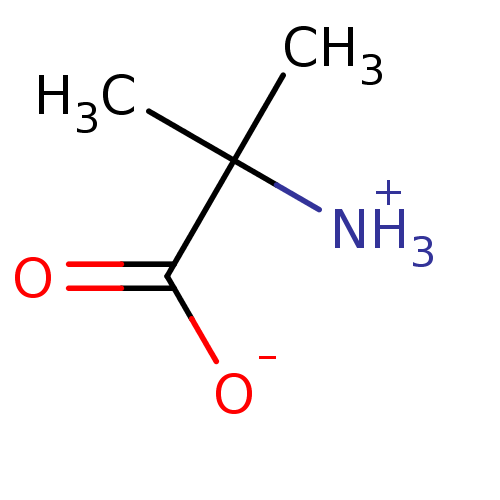 (Aminoisobutyric (AIB)) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University | Assay Description Enzyme inhibition assay using ethylene-forming enzyme (EFE). | Chem Biol 5: 49-57 (1998) Article DOI: 10.1016/s1074-5521(98)90086-2 BindingDB Entry DOI: 10.7270/Q24J0CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1-aminocyclopropane-1-carboxylate oxidase 1 (Malus domestica (Apple) (Pyrus malus)) | BDBM36631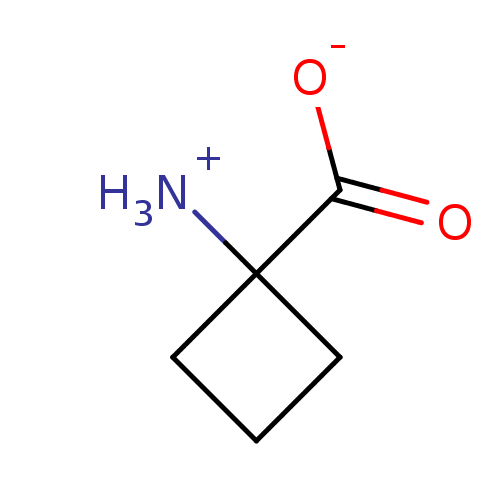 (Aminocyclobutanecarboxylic acid (ACBC)) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University | Assay Description Enzyme inhibition assay using ethylene-forming enzyme (EFE). | Chem Biol 5: 49-57 (1998) Article DOI: 10.1016/s1074-5521(98)90086-2 BindingDB Entry DOI: 10.7270/Q24J0CG6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129583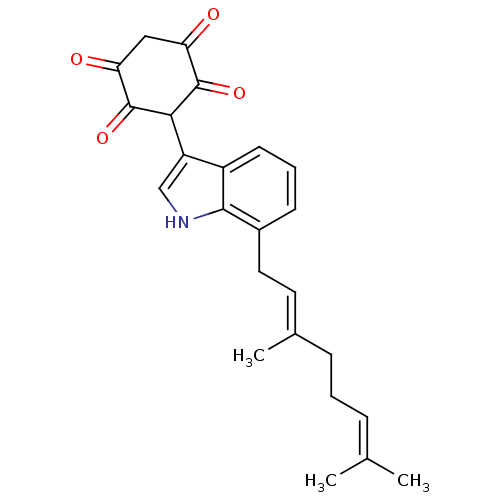 (3-[7-(3,7-Dimethyl-octa-2,6-dienyl)-1H-indol-3-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129579 (2,5-Dihydroxy-3-[7-(2-methyl-benzyl)-1H-indol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129579 (2,5-Dihydroxy-3-[7-(2-methyl-benzyl)-1H-indol-3-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129580 (3-[4,6-Dichloro-7-(3-methyl-but-2-enyl)-1H-indol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129585 (3-(7-Benzyl-1H-indol-3-yl)-2,5-dihydroxy-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129585 (3-(7-Benzyl-1H-indol-3-yl)-2,5-dihydroxy-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129576 (2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129576 (2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129572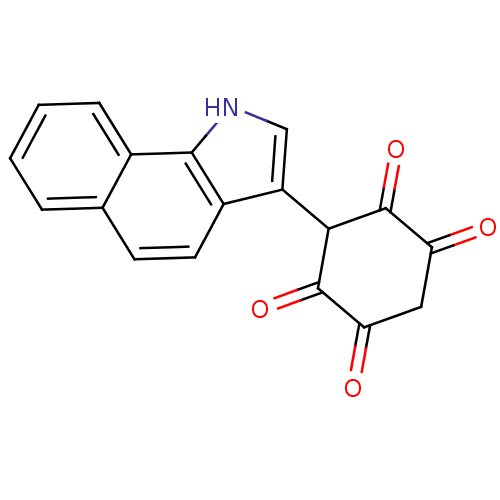 (3-(1H-Benzo[g]indol-3-yl)-2,5-dihydroxy-[1,4]benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129577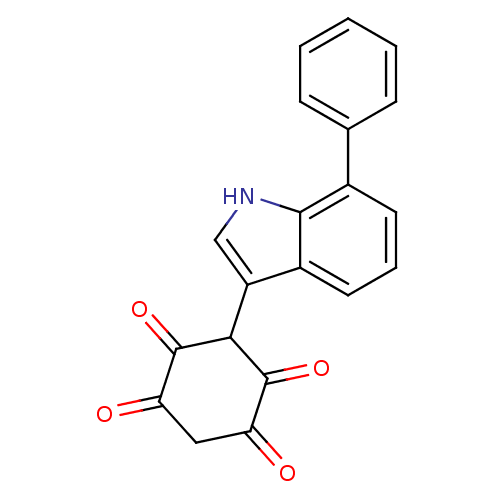 (2,5-Dihydroxy-3-(7-phenyl-1H-indol-3-yl)-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129577 (2,5-Dihydroxy-3-(7-phenyl-1H-indol-3-yl)-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50074938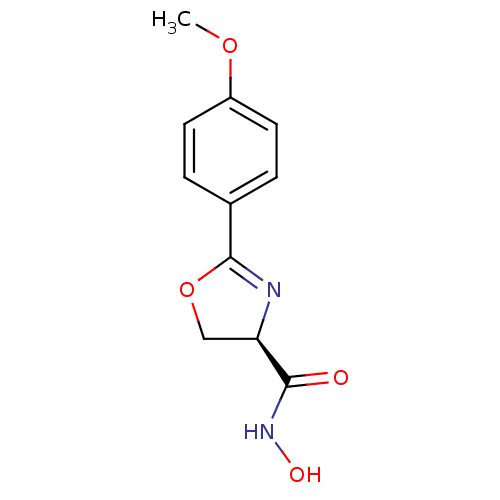 ((R)-2-(4-Methoxy-phenyl)-4,5-dihydro-oxazole-4-car...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129579 (2,5-Dihydroxy-3-[7-(2-methyl-benzyl)-1H-indol-3-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25 degree C was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129576 (2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25 degree C was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory concentration of compound against mutant M532A of Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129581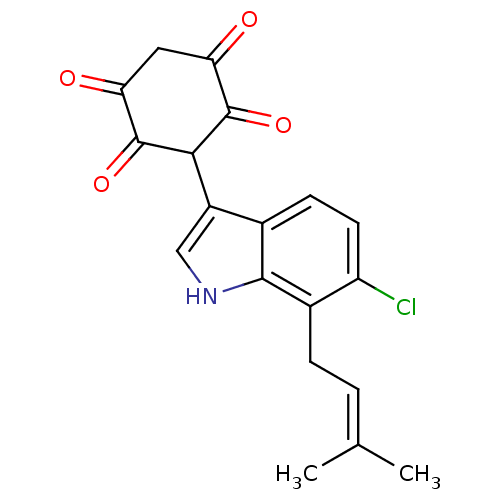 (3-[6-Chloro-7-(3-methyl-but-2-enyl)-1H-indol-3-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118596 ((R/S)-[3-(4-Methoxy-phenyl)-4,5-dihydro-isoxazol-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129584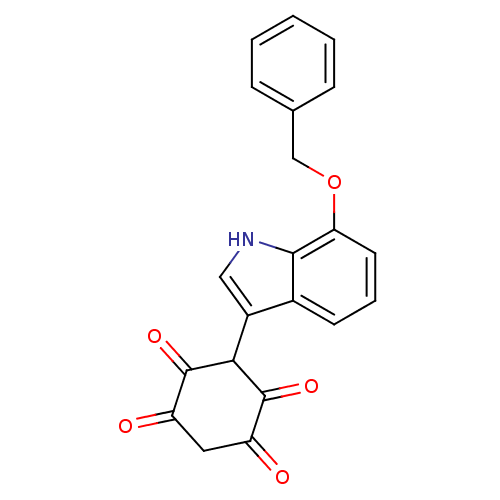 (3-(7-Benzyloxy-1H-indol-3-yl)-2,5-dihydroxy-[1,4]b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129584 (3-(7-Benzyloxy-1H-indol-3-yl)-2,5-dihydroxy-[1,4]b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129582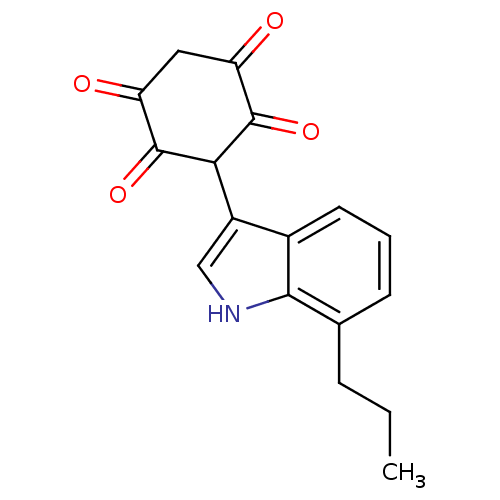 (2,5-Dihydroxy-3-(7-propyl-1H-indol-3-yl)-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129586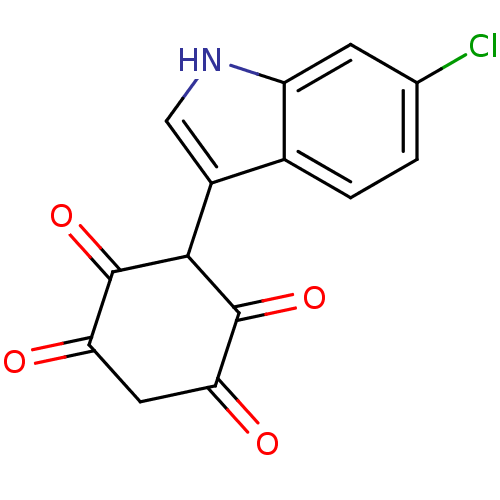 (3-(6-Chloro-1H-indol-3-yl)-2,5-dihydroxy-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50409600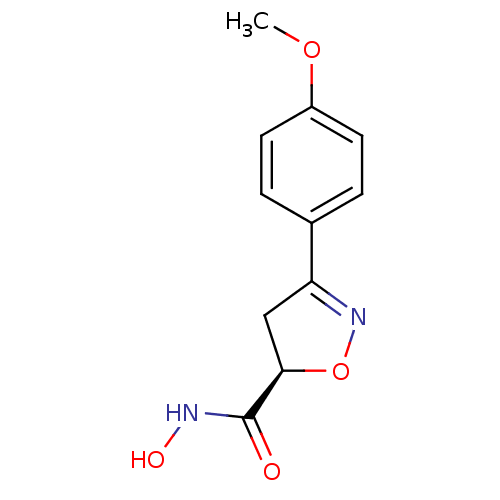 (CHEMBL2111931) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129580 (3-[4,6-Dichloro-7-(3-methyl-but-2-enyl)-1H-indol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129571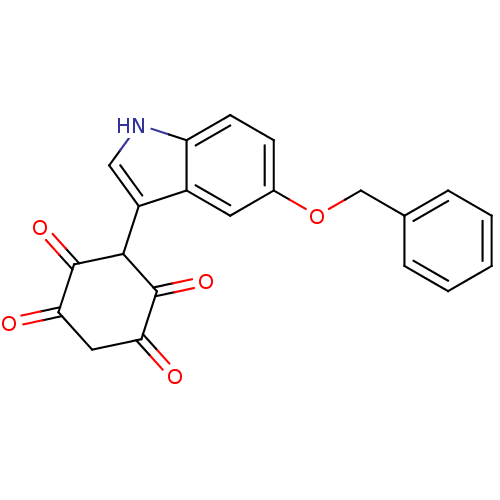 (3-(5-Benzyloxy-1H-indol-3-yl)-2,5-dihydroxy-[1,4]b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129569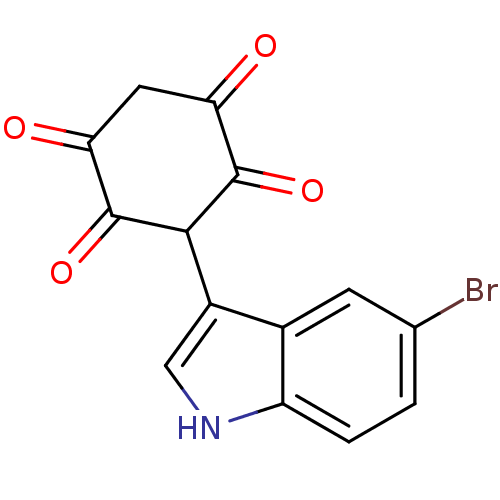 (3-(5-Bromo-1H-indol-3-yl)-2,5-dihydroxy-[1,4]benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory concentration of compound against mutant WT of Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory concentration of compound against mutant M531A of Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory concentration of compound against mutant E478Q of Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129584 (3-(7-Benzyloxy-1H-indol-3-yl)-2,5-dihydroxy-[1,4]b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25 degree C was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129581 (3-[6-Chloro-7-(3-methyl-but-2-enyl)-1H-indol-3-yl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129570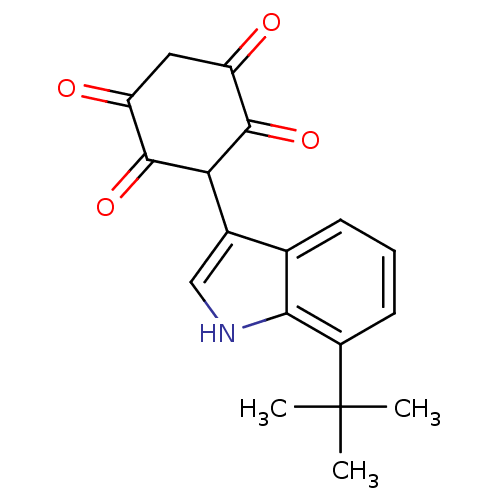 (3-(7-tert-Butyl-1H-indol-3-yl)-2,5-dihydroxy-[1,4]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129570 (3-(7-tert-Butyl-1H-indol-3-yl)-2,5-dihydroxy-[1,4]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 1 (Homo sapiens (Human)) | BDBM50129588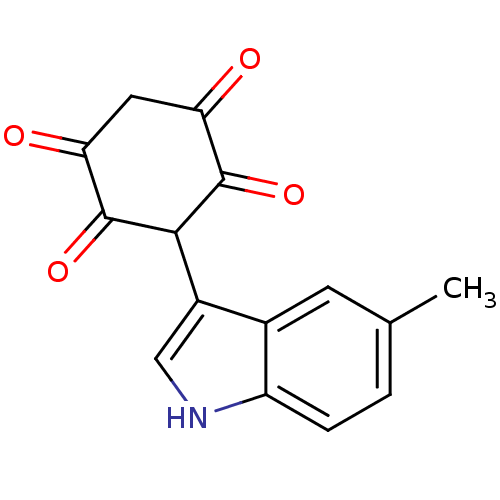 (2,5-Dihydroxy-3-(5-methyl-1H-indol-3-yl)-[1,4]benz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25A was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129580 (3-[4,6-Dichloro-7-(3-methyl-but-2-enyl)-1H-indol-3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory concentration of compound against Cell division cycle 25B was determined using CDK2-p-TpY/Cyclin A as a substrate | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129580 (3-[4,6-Dichloro-7-(3-methyl-but-2-enyl)-1H-indol-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25 degree C was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129583 (3-[7-(3,7-Dimethyl-octa-2,6-dienyl)-1H-indol-3-yl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25 degree C was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory concentration of compound against mutant R492L of Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129581 (3-[6-Chloro-7-(3-methyl-but-2-enyl)-1H-indol-3-yl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Eight point inhibitory concentration against Cell division cycle 25 degree C was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| UDP-3-O-acyl-N-acetylglucosamine deacetylase (Escherichia coli) | BDBM50118597 ((R/S)-Thioacetic acid S-{2-[3-(4-methoxy-phenyl)-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibition of E. coli LpxC(UDP-3-O-[R-3-hydroxymyristoyl]-GlcNAc deacetylase. | J Med Chem 45: 4359-70 (2002) BindingDB Entry DOI: 10.7270/Q2W66K3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 2 (Homo sapiens (Human)) | BDBM50129574 (2,5-Dihydroxy-3-[7-(3-methyl-but-2-enyl)-1H-indol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory concentration of compound against mutant R544L of Cell division cycle 25B was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 101 total ) | Next | Last >> |