 Found 76 hits with Last Name = 'reily' and Initial = 'md'
Found 76 hits with Last Name = 'reily' and Initial = 'md' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369268
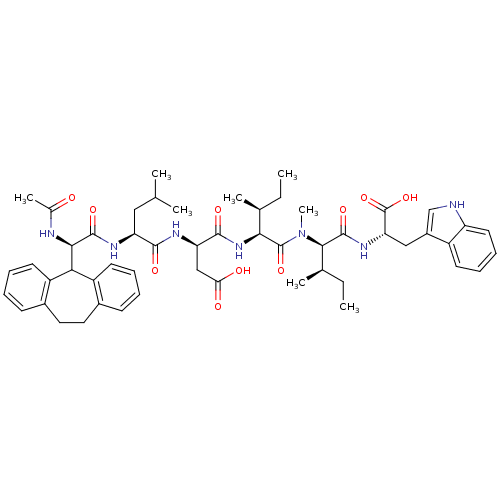
(CHEMBL1790519)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C1c2ccccc2CCc2ccccc12)C(=O)N(C)[C@H]([C@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C53H69N7O10/c1-9-30(5)45(52(68)60(8)47(31(6)10-2)51(67)58-42(53(69)70)26-35-28-54-39-22-16-15-19-36(35)39)59-49(65)41(27-43(62)63)56-48(64)40(25-29(3)4)57-50(66)46(55-32(7)61)44-37-20-13-11-17-33(37)23-24-34-18-12-14-21-38(34)44/h11-22,28-31,40-42,44-47,54H,9-10,23-27H2,1-8H3,(H,55,61)(H,56,64)(H,57,66)(H,58,67)(H,59,65)(H,62,63)(H,69,70)/t30-,31+,40-,41+,42-,45-,46+,47+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50032181
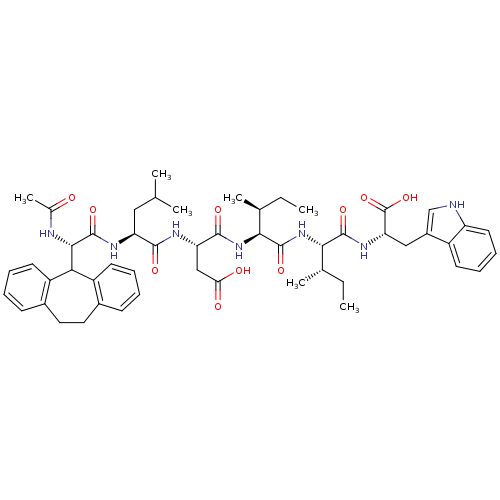
(2-{1-[1-(2-carboxy-1-{1-[10,11-dihydro-5H-dibenzo[...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C1c2ccccc2CCc2ccccc12)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C52H67N7O10/c1-8-29(5)44(49(65)57-41(52(68)69)25-34-27-53-38-21-15-14-18-35(34)38)59-50(66)45(30(6)9-2)58-48(64)40(26-42(61)62)55-47(63)39(24-28(3)4)56-51(67)46(54-31(7)60)43-36-19-12-10-16-32(36)22-23-33-17-11-13-20-37(33)43/h10-21,27-30,39-41,43-46,53H,8-9,22-26H2,1-7H3,(H,54,60)(H,55,63)(H,56,67)(H,57,65)(H,58,64)(H,59,66)(H,61,62)(H,68,69)/t29-,30-,39-,40-,41-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50366421
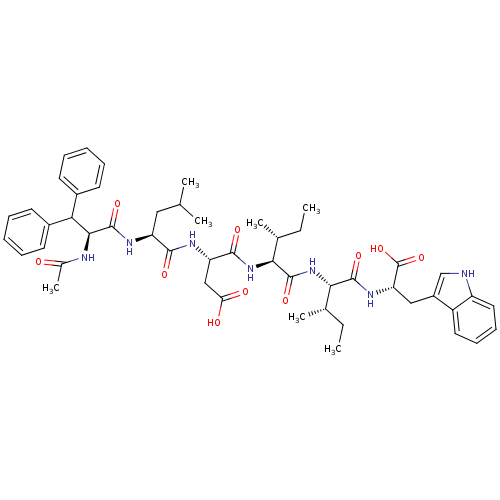
(CHEMBL1791271)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30+,37-,38-,39-,42-,43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]endothelin-1 [ET-1] binding to endothelin A receptor (ETA) of rabbit renal artery vascular smooth muscle cells |
Bioorg Med Chem Lett 5: 967-972 (1995)
Article DOI: 10.1016/0960-894X(95)00152-J
BindingDB Entry DOI: 10.7270/Q2MG7PZH |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50032181

(2-{1-[1-(2-carboxy-1-{1-[10,11-dihydro-5H-dibenzo[...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C1c2ccccc2CCc2ccccc12)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C52H67N7O10/c1-8-29(5)44(49(65)57-41(52(68)69)25-34-27-53-38-21-15-14-18-35(34)38)59-50(66)45(30(6)9-2)58-48(64)40(26-42(61)62)55-47(63)39(24-28(3)4)56-51(67)46(54-31(7)60)43-36-19-12-10-16-32(36)22-23-33-17-11-13-20-37(33)43/h10-21,27-30,39-41,43-46,53H,8-9,22-26H2,1-7H3,(H,54,60)(H,55,63)(H,56,67)(H,57,65)(H,58,64)(H,59,66)(H,61,62)(H,68,69)/t29-,30-,39-,40-,41-,44-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration was evaluated by measuring the binding affinity at the Endothelin B receptor in rat cerebellar membranes |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369269

(CHEMBL1793932)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)CC)C(=O)NCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-50(67)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)49(66)54-28-41(61)39(26-42(62)63)56-48(65)40(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,66)(H,55,60)(H,56,65)(H,57,68)(H,58,64)(H,59,67)(H,62,63)/t30-,31-,37-,39-,40-,44-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369268

(CHEMBL1790519)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](NC(C)=O)C1c2ccccc2CCc2ccccc12)C(=O)N(C)[C@H]([C@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C53H69N7O10/c1-9-30(5)45(52(68)60(8)47(31(6)10-2)51(67)58-42(53(69)70)26-35-28-54-39-22-16-15-19-36(35)39)59-49(65)41(27-43(62)63)56-48(64)40(25-29(3)4)57-50(66)46(55-32(7)61)44-37-20-13-11-17-33(37)23-24-34-18-12-14-21-38(34)44/h11-22,28-31,40-42,44-47,54H,9-10,23-27H2,1-8H3,(H,55,61)(H,56,64)(H,57,66)(H,58,67)(H,59,65)(H,62,63)(H,69,70)/t30-,31+,40-,41+,42-,45-,46+,47+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50366424
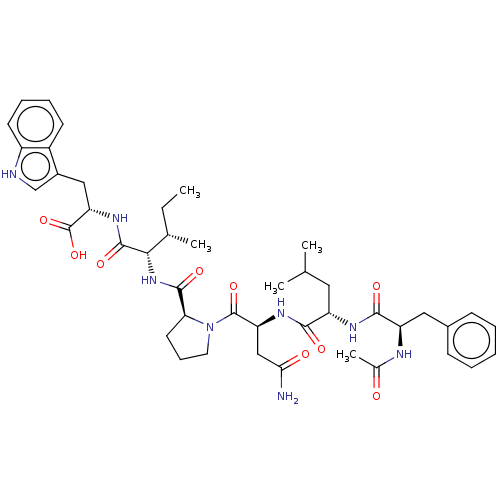
(CHEMBL2369709)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C43H58N8O9/c1-6-25(4)37(41(57)49-34(43(59)60)21-28-23-45-30-16-11-10-15-29(28)30)50-40(56)35-17-12-18-51(35)42(58)33(22-36(44)53)48-38(54)31(19-24(2)3)47-39(55)32(46-26(5)52)20-27-13-8-7-9-14-27/h7-11,13-16,23-25,31-35,37,45H,6,12,17-22H2,1-5H3,(H2,44,53)(H,46,52)(H,47,55)(H,48,54)(H,49,57)(H,50,56)(H,59,60)/t25-,31-,32+,33-,34-,35-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]endothelin-1 [ET-1] binding to endothelin A receptor (ETA) of rabbit renal artery vascular smooth muscle cells |
Bioorg Med Chem Lett 5: 967-972 (1995)
Article DOI: 10.1016/0960-894X(95)00152-J
BindingDB Entry DOI: 10.7270/Q2MG7PZH |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50366424

(CHEMBL2369709)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C43H58N8O9/c1-6-25(4)37(41(57)49-34(43(59)60)21-28-23-45-30-16-11-10-15-29(28)30)50-40(56)35-17-12-18-51(35)42(58)33(22-36(44)53)48-38(54)31(19-24(2)3)47-39(55)32(46-26(5)52)20-27-13-8-7-9-14-27/h7-11,13-16,23-25,31-35,37,45H,6,12,17-22H2,1-5H3,(H2,44,53)(H,46,52)(H,47,55)(H,48,54)(H,49,57)(H,50,56)(H,59,60)/t25-,31-,32+,33-,34-,35-,37-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-endothelin-3 [ET-3] binding to endothelin B receptor (ETB) of rat cerebellum |
Bioorg Med Chem Lett 5: 967-972 (1995)
Article DOI: 10.1016/0960-894X(95)00152-J
BindingDB Entry DOI: 10.7270/Q2MG7PZH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in rat red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50032172

((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50366421

(CHEMBL1791271)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30+,37-,38-,39-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-endothelin-3 [ET-3] binding to endothelin B receptor (ETB) of rat cerebellum |
Bioorg Med Chem Lett 5: 967-972 (1995)
Article DOI: 10.1016/0960-894X(95)00152-J
BindingDB Entry DOI: 10.7270/Q2MG7PZH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in monkey red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in human red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369267

(CHEMBL2369736)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N(C)[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(50(66)58(8)45(31(6)10-2)49(65)56-40(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-47(63)39(27-41(60)61)54-46(62)38(25-29(3)4)55-48(64)44(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,62)(H,55,64)(H,56,65)(H,57,63)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369267

(CHEMBL2369736)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N(C)[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(50(66)58(8)45(31(6)10-2)49(65)56-40(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-47(63)39(27-41(60)61)54-46(62)38(25-29(3)4)55-48(64)44(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,62)(H,55,64)(H,56,65)(H,57,63)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50032172

((S)-3-[(S)-2-((S)-2-Acetylamino-3,3-diphenyl-propi...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C50H65N7O10/c1-8-29(5)42(47(63)55-39(50(66)67)25-34-27-51-36-23-17-16-22-35(34)36)57-48(64)43(30(6)9-2)56-46(62)38(26-40(59)60)53-45(61)37(24-28(3)4)54-49(65)44(52-31(7)58)41(32-18-12-10-13-19-32)33-20-14-11-15-21-33/h10-23,27-30,37-39,41-44,51H,8-9,24-26H2,1-7H3,(H,52,58)(H,53,61)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,59,60)(H,66,67)/t29-,30-,37-,38-,39-,42-,43-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibitory concentration was evaluated by measuring the binding affinity at the Endothelin B receptor in rat cerebellar membranes |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in mouse red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369273

(CHEMBL1793926)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(48(64)55-39(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-49(65)44(31(6)10-2)56-46(62)38(27-41(60)61)54-47(63)40(25-29(3)4)58(8)50(66)45(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,63)(H,55,64)(H,56,62)(H,57,65)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369266

(CHEMBL1793925)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-50(67)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)49(66)57-40(26-42(62)63)48(65)54-28-41(61)39(24-29(3)4)56-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,65)(H,55,60)(H,56,68)(H,57,66)(H,58,64)(H,59,67)(H,62,63)/t30-,31-,37-,39-,40-,44-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >250 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369264
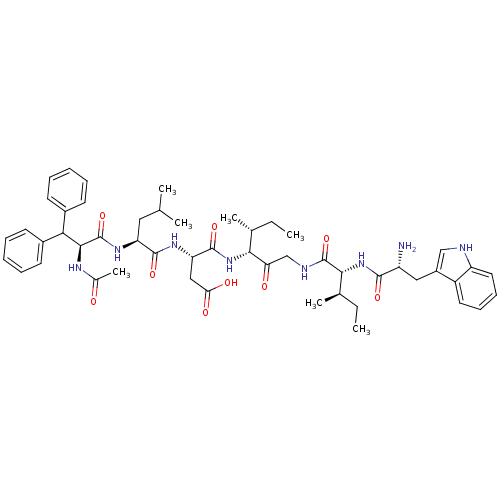
(CHEMBL1793929)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)CNC(=O)[C@H](NC(=O)[C@H](N)Cc1c[nH]c2ccccc12)[C@H](C)CC Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(41(61)28-54-50(67)45(31(6)9-2)59-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)58-49(66)40(26-42(62)63)56-48(65)39(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,67)(H,55,60)(H,56,65)(H,57,68)(H,58,66)(H,59,64)(H,62,63)/t30-,31-,37-,39+,40+,44-,45-,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >250 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369265

(CHEMBL1793928)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@H](C)CC)C(=O)CNC(=O)[C@H](N)Cc1c[nH]c2ccccc12 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(41(61)28-54-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)58-50(67)45(31(6)9-2)59-49(66)40(26-42(62)63)56-48(65)39(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,64)(H,55,60)(H,56,65)(H,57,68)(H,58,67)(H,59,66)(H,62,63)/t30-,31+,37+,39-,40-,44-,45+,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >250 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369269

(CHEMBL1793932)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)[C@@H](C)CC)C(=O)NCC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-50(67)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)49(66)54-28-41(61)39(26-42(62)63)56-48(65)40(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,66)(H,55,60)(H,56,65)(H,57,68)(H,58,64)(H,59,67)(H,62,63)/t30-,31-,37-,39-,40-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369264

(CHEMBL1793929)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)CNC(=O)[C@H](NC(=O)[C@H](N)Cc1c[nH]c2ccccc12)[C@H](C)CC Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(41(61)28-54-50(67)45(31(6)9-2)59-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)58-49(66)40(26-42(62)63)56-48(65)39(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,67)(H,55,60)(H,56,65)(H,57,68)(H,58,66)(H,59,64)(H,62,63)/t30-,31-,37-,39+,40+,44-,45-,46+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369266

(CHEMBL1793925)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CC(O)=O)C(=O)NCC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-50(67)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)49(66)57-40(26-42(62)63)48(65)54-28-41(61)39(24-29(3)4)56-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,65)(H,55,60)(H,56,68)(H,57,66)(H,58,64)(H,59,67)(H,62,63)/t30-,31-,37-,39-,40-,44-,45-,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM9347

((2Z)-but-2-enedioic acid; 9-amino-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C13H14N2O/c14-13-8-4-1-2-5-9(8)15-10-6-3-7-11(16)12(10)13/h1-2,4-5,11,16H,3,6-7H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 361 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in rat red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50052410

(9-Amino-1,2,3,4-tetrahydro-acridin-4-ol | CHEMBL31...)Show InChI InChI=1S/C13H14N2O/c14-12-8-4-1-2-6-10(8)15-13-9(12)5-3-7-11(13)16/h1-2,4,6,11,16H,3,5,7H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 411 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in rat red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50052410

(9-Amino-1,2,3,4-tetrahydro-acridin-4-ol | CHEMBL31...)Show InChI InChI=1S/C13H14N2O/c14-12-8-4-1-2-6-10(8)15-13-9(12)5-3-7-11(13)16/h1-2,4,6,11,16H,3,5,7H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 476 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in monkey red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50052409

(7-Hydroxytacrine | 9-Amino-1,2,3,4-tetrahydro-acri...)Show InChI InChI=1S/C13H14N2O/c14-13-9-3-1-2-4-11(9)15-12-7-8(16)5-6-10(12)13/h1-4,8,16H,5-7H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 483 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in rat red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50052410

(9-Amino-1,2,3,4-tetrahydro-acridin-4-ol | CHEMBL31...)Show InChI InChI=1S/C13H14N2O/c14-12-8-4-1-2-6-10(8)15-13-9(12)5-3-7-11(13)16/h1-2,4,6,11,16H,3,5,7H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 521 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in human red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369273

(CHEMBL1793926)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)N(C)C(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(48(64)55-39(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-49(65)44(31(6)10-2)56-46(62)38(27-41(60)61)54-47(63)40(25-29(3)4)58(8)50(66)45(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,63)(H,55,64)(H,56,62)(H,57,65)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50052409

(7-Hydroxytacrine | 9-Amino-1,2,3,4-tetrahydro-acri...)Show InChI InChI=1S/C13H14N2O/c14-13-9-3-1-2-4-11(9)15-12-7-8(16)5-6-10(12)13/h1-4,8,16H,5-7H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 623 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in human red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369270
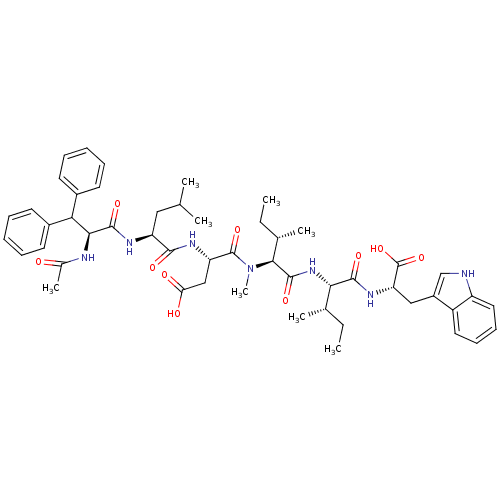
(CHEMBL1793933)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H]([C@@H](C)CC)N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(47(63)56-40(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-49(65)45(31(6)10-2)58(8)50(66)39(27-41(60)61)55-46(62)38(25-29(3)4)54-48(64)44(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,64)(H,55,62)(H,56,63)(H,57,65)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9347

((2Z)-but-2-enedioic acid; 9-amino-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C13H14N2O/c14-13-8-4-1-2-5-9(8)15-10-6-3-7-11(16)12(10)13/h1-2,4-5,11,16H,3,6-7H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 702 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in human red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50052409

(7-Hydroxytacrine | 9-Amino-1,2,3,4-tetrahydro-acri...)Show InChI InChI=1S/C13H14N2O/c14-13-9-3-1-2-4-11(9)15-12-7-8(16)5-6-10(12)13/h1-4,8,16H,5-7H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 759 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in monkey red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369265

(CHEMBL1793928)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@H](C)CC)C(=O)CNC(=O)[C@H](N)Cc1c[nH]c2ccccc12 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(41(61)28-54-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)58-50(67)45(31(6)9-2)59-49(66)40(26-42(62)63)56-48(65)39(24-29(3)4)57-51(68)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,64)(H,55,60)(H,56,65)(H,57,68)(H,58,67)(H,59,66)(H,62,63)/t30-,31+,37+,39-,40-,44-,45+,46-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM9347

((2Z)-but-2-enedioic acid; 9-amino-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C13H14N2O/c14-13-8-4-1-2-5-9(8)15-10-6-3-7-11(16)12(10)13/h1-2,4-5,11,16H,3,6-7H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 954 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in monkey red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369270

(CHEMBL1793933)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H]([C@@H](C)CC)N(C)C(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(47(63)56-40(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-49(65)45(31(6)10-2)58(8)50(66)39(27-41(60)61)55-46(62)38(25-29(3)4)54-48(64)44(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,64)(H,55,62)(H,56,63)(H,57,65)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM9347

((2Z)-but-2-enedioic acid; 9-amino-1,2,3,4-tetrahyd...)Show InChI InChI=1S/C13H14N2O/c14-13-8-4-1-2-5-9(8)15-10-6-3-7-11(16)12(10)13/h1-2,4-5,11,16H,3,6-7H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in mouse red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50052409

(7-Hydroxytacrine | 9-Amino-1,2,3,4-tetrahydro-acri...)Show InChI InChI=1S/C13H14N2O/c14-13-9-3-1-2-4-11(9)15-12-7-8(16)5-6-10(12)13/h1-4,8,16H,5-7H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in mouse red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Endothelin receptor type B
(RAT) | BDBM50369263
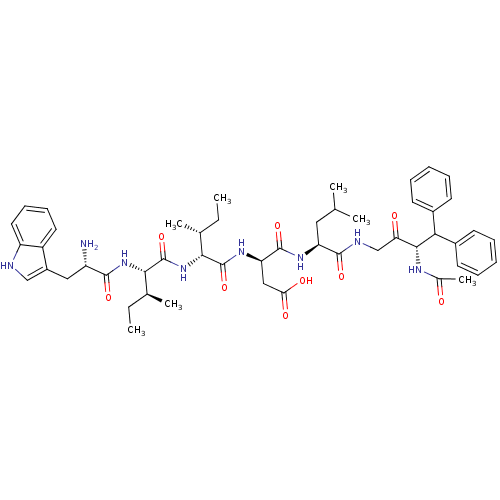
(CHEMBL1793924)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-51(68)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)50(67)57-40(26-42(62)63)49(66)56-39(24-29(3)4)48(65)54-28-41(61)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,65)(H,55,60)(H,56,66)(H,57,67)(H,58,64)(H,59,68)(H,62,63)/t30-,31+,37+,39+,40-,44-,45+,46-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Mus musculus (mouse)) | BDBM50052410

(9-Amino-1,2,3,4-tetrahydro-acridin-4-ol | CHEMBL31...)Show InChI InChI=1S/C13H14N2O/c14-12-8-4-1-2-6-10(8)15-13-9(12)5-3-7-11(13)16/h1-2,4,6,11,16H,3,5,7H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in mouse red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50369271

(CHEMBL1793934)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(47(63)55-39(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-48(64)44(31(6)10-2)56-46(62)40(27-41(60)61)58(8)50(66)38(25-29(3)4)54-49(65)45(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin B receptor of rat cerebellar membranes. |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50052411

(9-Amino-1,2,3,4-tetrahydro-acridin-2-ol | CHEMBL31...)Show InChI InChI=1S/C13H14N2O/c14-13-9-3-1-2-4-11(9)15-12-6-5-8(16)7-10(12)13/h1-4,8,16H,5-7H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in human red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Rattus norvegicus (rat)) | BDBM50052411

(9-Amino-1,2,3,4-tetrahydro-acridin-2-ol | CHEMBL31...)Show InChI InChI=1S/C13H14N2O/c14-13-9-3-1-2-4-11(9)15-12-6-5-8(16)7-10(12)13/h1-4,8,16H,5-7H2,(H2,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in rat red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50366423
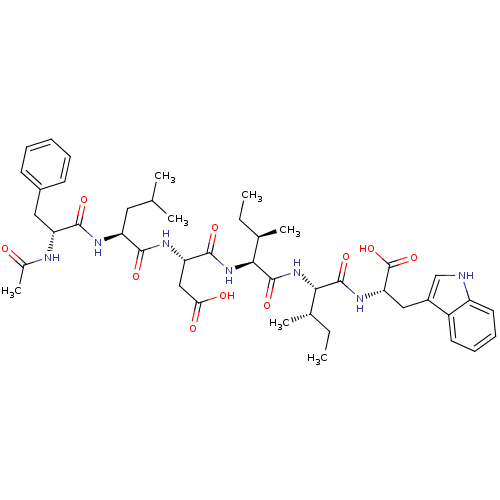
(CHEMBL1791279)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C44H61N7O10/c1-8-25(5)37(42(58)49-35(44(60)61)21-29-23-45-31-18-14-13-17-30(29)31)51-43(59)38(26(6)9-2)50-41(57)34(22-36(53)54)48-39(55)32(19-24(3)4)47-40(56)33(46-27(7)52)20-28-15-11-10-12-16-28/h10-18,23-26,32-35,37-38,45H,8-9,19-22H2,1-7H3,(H,46,52)(H,47,56)(H,48,55)(H,49,58)(H,50,57)(H,51,59)(H,53,54)(H,60,61)/t25-,26+,32-,33+,34-,35-,37-,38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]endothelin-1 [ET-1] binding to endothelin A receptor (ETA) of rabbit renal artery vascular smooth muscle cells |
Bioorg Med Chem Lett 5: 967-972 (1995)
Article DOI: 10.1016/0960-894X(95)00152-J
BindingDB Entry DOI: 10.7270/Q2MG7PZH |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50366423

(CHEMBL1791279)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](Cc1ccccc1)NC(C)=O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C44H61N7O10/c1-8-25(5)37(42(58)49-35(44(60)61)21-29-23-45-31-18-14-13-17-30(29)31)51-43(59)38(26(6)9-2)50-41(57)34(22-36(53)54)48-39(55)32(19-24(3)4)47-40(56)33(46-27(7)52)20-28-15-11-10-12-16-28/h10-18,23-26,32-35,37-38,45H,8-9,19-22H2,1-7H3,(H,46,52)(H,47,56)(H,48,55)(H,49,58)(H,50,57)(H,51,59)(H,53,54)(H,60,61)/t25-,26+,32-,33+,34-,35-,37-,38-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-endothelin-3 [ET-3] binding to endothelin B receptor (ETB) of rat cerebellum |
Bioorg Med Chem Lett 5: 967-972 (1995)
Article DOI: 10.1016/0960-894X(95)00152-J
BindingDB Entry DOI: 10.7270/Q2MG7PZH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50052411

(9-Amino-1,2,3,4-tetrahydro-acridin-2-ol | CHEMBL31...)Show InChI InChI=1S/C13H14N2O/c14-13-9-3-1-2-4-11(9)15-12-6-5-8(16)7-10(12)13/h1-4,8,16H,5-7H2,(H2,14,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of Acetylcholinesterase in monkey red blood cell |
J Med Chem 39: 3014-8 (1996)
Article DOI: 10.1021/jm9602266
BindingDB Entry DOI: 10.7270/Q20V8BVM |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369263

(CHEMBL1793924)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](N)Cc1c[nH]c2ccccc12)C(=O)N[C@H]([C@H](C)CC)C(=O)N[C@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(=O)NCC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C51H68N8O9/c1-8-30(5)44(59-51(68)45(31(6)9-2)58-47(64)37(52)25-35-27-53-38-23-17-16-22-36(35)38)50(67)57-40(26-42(62)63)49(66)56-39(24-29(3)4)48(65)54-28-41(61)46(55-32(7)60)43(33-18-12-10-13-19-33)34-20-14-11-15-21-34/h10-23,27,29-31,37,39-40,43-46,53H,8-9,24-26,28,52H2,1-7H3,(H,54,65)(H,55,60)(H,56,66)(H,57,67)(H,58,64)(H,59,68)(H,62,63)/t30-,31+,37+,39+,40-,44-,45+,46-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Homo sapiens (Human)) | BDBM50369271

(CHEMBL1793934)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)N(C)C(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C51H67N7O10/c1-9-30(5)43(47(63)55-39(51(67)68)26-35-28-52-37-24-18-17-23-36(35)37)57-48(64)44(31(6)10-2)56-46(62)40(27-41(60)61)58(8)50(66)38(25-29(3)4)54-49(65)45(53-32(7)59)42(33-19-13-11-14-20-33)34-21-15-12-16-22-34/h11-24,28-31,38-40,42-45,52H,9-10,25-27H2,1-8H3,(H,53,59)(H,54,65)(H,55,63)(H,56,62)(H,57,64)(H,60,61)(H,67,68)/t30-,31-,38-,39-,40-,43-,44-,45-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Inhibition of binding to Endothelin A receptor of rabbit renal vascular smooth muscle cells |
J Med Chem 40: 2228-40 (1997)
Article DOI: 10.1021/jm970161m
BindingDB Entry DOI: 10.7270/Q24T6K1W |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50366416
(CHEMBL2369713)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(N)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(C)=O)C(c1ccccc1)c1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C49H62N8O9/c1-6-29(4)42(46(62)55-38(49(65)66)25-33-27-51-35-21-14-13-20-34(33)35)56-45(61)39-22-15-23-57(39)48(64)37(26-40(50)59)54-44(60)36(24-28(2)3)53-47(63)43(52-30(5)58)41(31-16-9-7-10-17-31)32-18-11-8-12-19-32/h7-14,16-21,27-29,36-39,41-43,51H,6,15,22-26H2,1-5H3,(H2,50,59)(H,52,58)(H,53,63)(H,54,60)(H,55,62)(H,56,61)(H,65,66)/t29-,36-,37-,38-,39-,42-,43-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of [125I]-endothelin-3 [ET-3] binding to endothelin B receptor (ETB) of rat cerebellum |
Bioorg Med Chem Lett 5: 967-972 (1995)
Article DOI: 10.1016/0960-894X(95)00152-J
BindingDB Entry DOI: 10.7270/Q2MG7PZH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data