

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (Feline coronavirus (strain FIPV WSU-79/1146) (FCoV...) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (PEDV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-OC43) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-NL63) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (BtCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-HKU1) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510127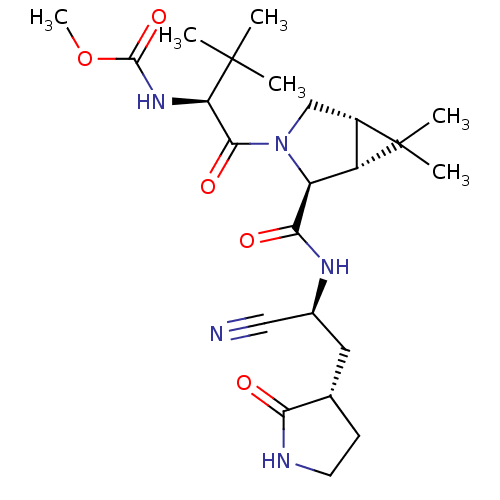 (Methyl {(2S)-1-[(1R,2S,5S)-2-({(1S)-1-cyano-2-[(3S...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (MHV-A59) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (HCoV-229E) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510063 (N-[(2S)-1-({(1S)-1-Cyano-2-[(3S)-2-oxopyrrolidin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510132 ((1R,2S,5S)-N-{(1 S)-1-Cyano-2-[(3S)-2- oxopyrrolid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM556419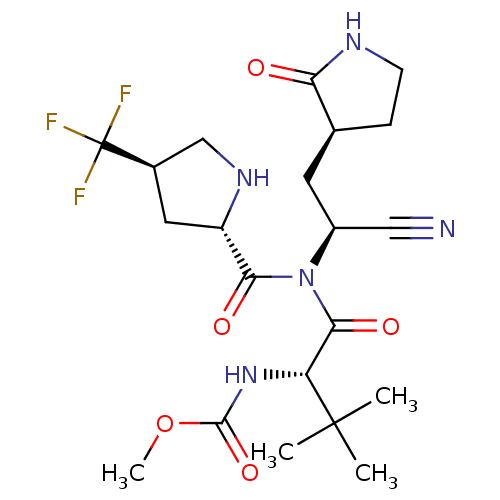 (US11351149, Example 95) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510129 ((1R,2S,5S)-N-{(1S)-1-Cyano-2-[(3R)-5-hydroxy-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | 3.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM556413 (US11351149, Example 92) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM556406 (US11351149, Example 85) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (IBV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PDB UniChem | WIPO WO2021205298 | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Proteolytic activity of SARS-CoV-2 Coronavirus 3CL protease is measured using a continuous fluorescence resonance energy transfer assay. The SARS-CoV... | Citation and Details BindingDB Entry DOI: 10.7270/Q232001P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50360584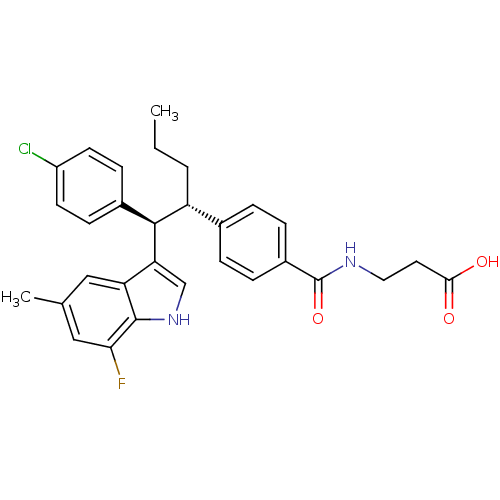 (CHEMBL1933350) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Glucagon-Cex from human GCGR | Bioorg Med Chem Lett 22: 415-20 (2011) Article DOI: 10.1016/j.bmcl.2011.10.113 BindingDB Entry DOI: 10.7270/Q2H70G82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM556398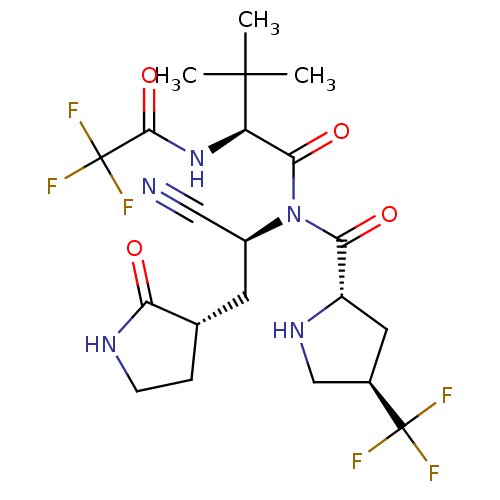 (US11351149, Example 77) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510126 ((1R,2S,5S)-N-{(1 S)-1-Cyano-2-[(3S)-2-oxopyrrolidi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510068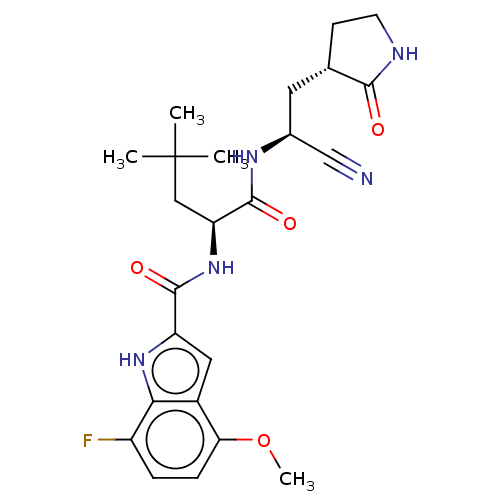 (N-[(2S)-1-({(1 S)-1-Cyano-2-[(3S)-2-oxopyrrolidin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM2437 (US8507533, 157) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon from human glucagon receptor expressed in Chem-1 cell membranes after 6 to 10 hrs by scintillation proximity assay | Bioorg Med Chem Lett 25: 4057-64 (2015) Article DOI: 10.1016/j.bmcl.2015.07.092 BindingDB Entry DOI: 10.7270/Q2HM5B7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510136 ((1R,2S,5S)-N-{(1S)-1-Cyano-2-[(3S)-2-oxopyrrolidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50398061 (CHEMBL2181928) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-aldosterone from human GST-tagged MR ligand binding domain after 4 hrs by liquid scintillation counting | J Med Chem 61: 1086-1097 (2018) Article DOI: 10.1021/acs.jmedchem.7b01515 BindingDB Entry DOI: 10.7270/Q2V1277H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510137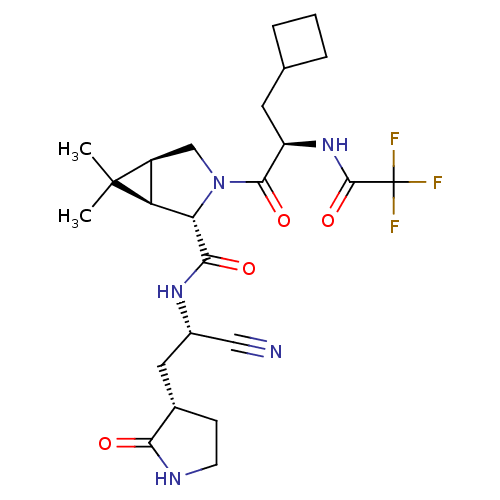 ((1R,2S,5S)-N-{(1S)-1-Cyano-2-[(3S)-2-oxopyrrolidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510139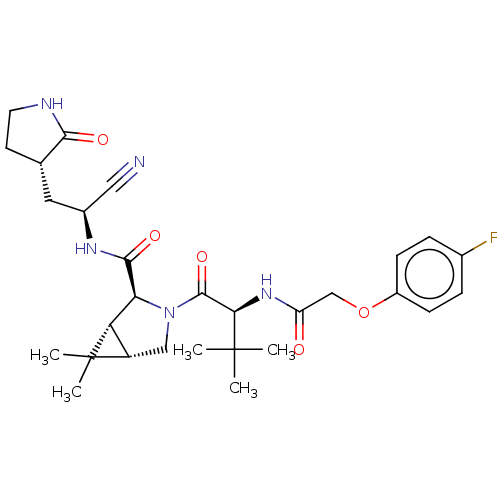 ((1R,2S,5S)-N-{(1S)-1-Cyano-2-[(3S)-2-oxopyrrolidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496900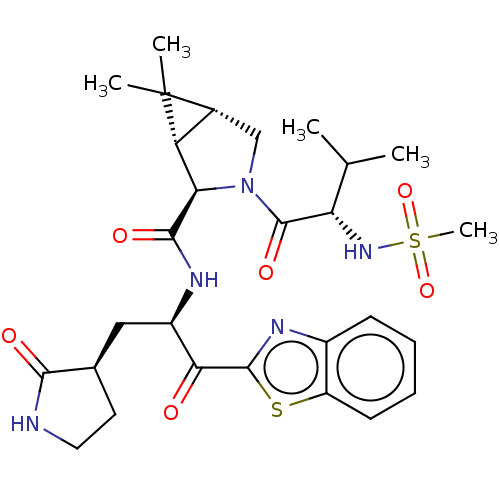 (science.abl4784, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 7.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50360599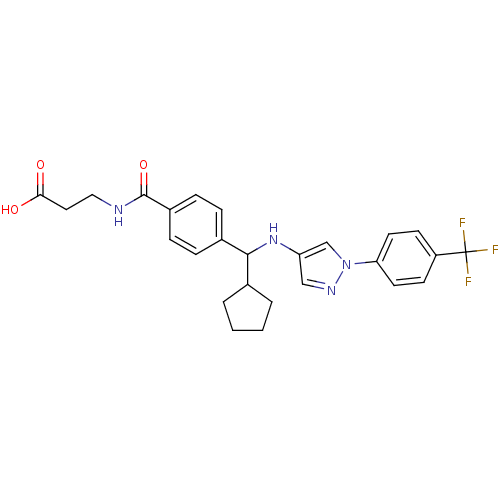 (CHEMBL1933365) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Glucagon-Cex from human GCGR | Bioorg Med Chem Lett 22: 415-20 (2011) Article DOI: 10.1016/j.bmcl.2011.10.113 BindingDB Entry DOI: 10.7270/Q2H70G82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50360599 (CHEMBL1933365) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Glucagon-Cex from human GCGR | Bioorg Med Chem Lett 22: 415-20 (2011) Article DOI: 10.1016/j.bmcl.2011.10.113 BindingDB Entry DOI: 10.7270/Q2H70G82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM556422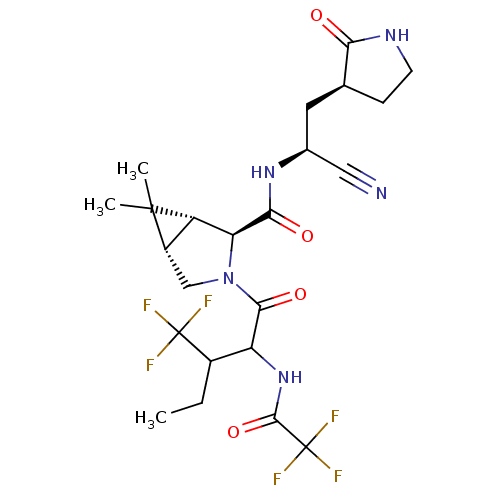 (US11351149, Example 97 | US11351149, Example 98) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510141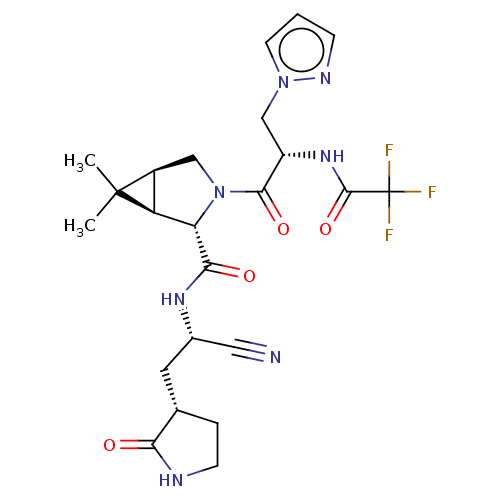 ((1R,2S,5S)-N-{(1S)-1-Cyano-2-[(3S)-2-oxopyrrolidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433595 (CHEMBL2381827) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50360598 (CHEMBL1933364) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Glucagon-Cex from human GCGR | Bioorg Med Chem Lett 22: 415-20 (2011) Article DOI: 10.1016/j.bmcl.2011.10.113 BindingDB Entry DOI: 10.7270/Q2H70G82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496901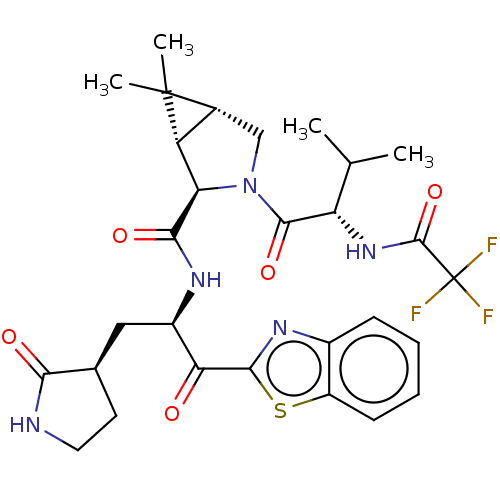 (science.abl4784, 5) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
Pfizer | Assay Description The respective human coronavirus Mpro in assay buffer (20 mM Tris-HCl, pH 7.3, 100 mM NaCl, 1 mM EDTA, 5 mM TCEP) and 0.1% BSA was added to assay-rea... | Science 374: 1-13 (2021) BindingDB Entry DOI: 10.7270/Q23T9MCM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50360598 (CHEMBL1933364) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]Glucagon-Cex from human GCGR | Bioorg Med Chem Lett 22: 415-20 (2011) Article DOI: 10.1016/j.bmcl.2011.10.113 BindingDB Entry DOI: 10.7270/Q2H70G82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510048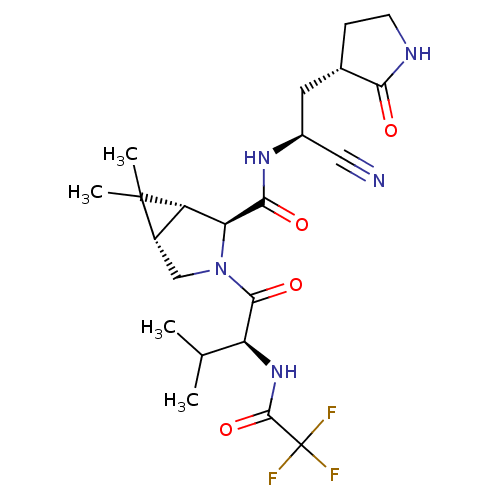 ((1R,2S,5S)-N-{(1S)-1-Cyano-2-[(3S)-2-oxopyrrolidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510141 ((1R,2S,5S)-N-{(1S)-1-Cyano-2-[(3S)-2-oxopyrrolidin...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50433577 (CHEMBL2381848) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon-cex from human glucagon receptor by cell based assay in presence of 0.2% bovine serum albumin | Bioorg Med Chem Lett 23: 3051-8 (2013) Article DOI: 10.1016/j.bmcl.2013.03.014 BindingDB Entry DOI: 10.7270/Q27M099R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM142039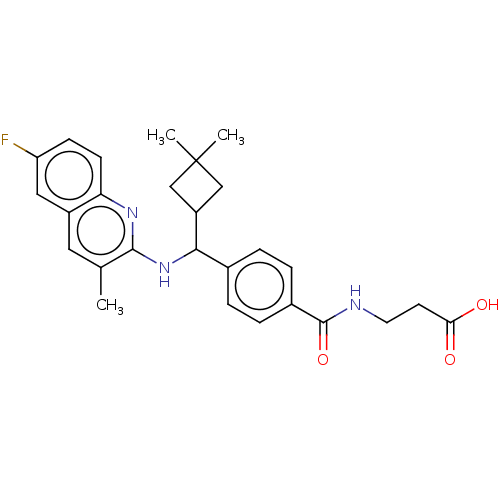 (US8927577, 118 | US8927577, 20 | US8927577, 35) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon from human glucagon receptor expressed in Chem-1 cell membranes after 6 to 10 hrs by scintillation proximity assay | Bioorg Med Chem Lett 25: 4057-64 (2015) Article DOI: 10.1016/j.bmcl.2015.07.092 BindingDB Entry DOI: 10.7270/Q2HM5B7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM50119555 (CHEMBL3617571) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon from human glucagon receptor expressed in Chem-1 cell membranes after 6 to 10 hrs by scintillation proximity assay | Bioorg Med Chem Lett 25: 4057-64 (2015) Article DOI: 10.1016/j.bmcl.2015.07.092 BindingDB Entry DOI: 10.7270/Q2HM5B7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM556411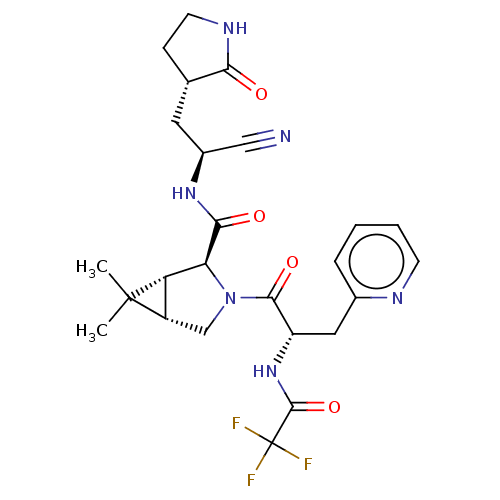 (US11351149, Example 90) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucagon receptor (Homo sapiens (Human)) | BDBM123557 (US8748624, 43) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [125I]-glucagon from full length human glucagon receptor expressed in HEK293 cell membranes after 2 hrs by scintillation counting ana... | Bioorg Med Chem Lett 25: 4057-64 (2015) Article DOI: 10.1016/j.bmcl.2015.07.092 BindingDB Entry DOI: 10.7270/Q2HM5B7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM556396 ((2S,4R)-4-tert-Butyl-N-{(1S)-1-cyano-2-[(3S)-2-oxo...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510064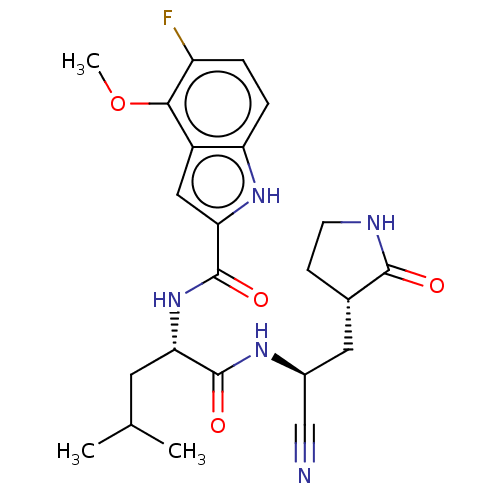 (N-[(2S)-1-({(1S)-1- Cyano-2-[(3S)-2-oxopyrrolidin-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM556407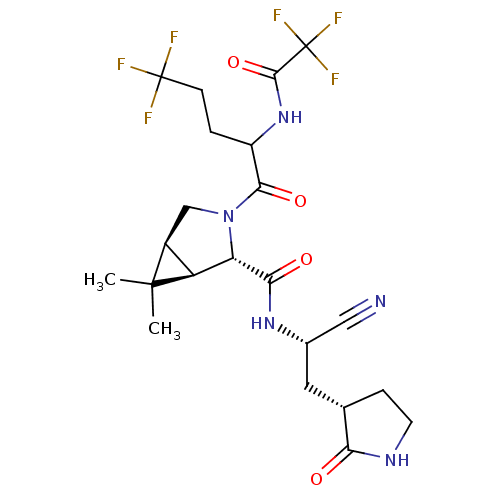 (US11351149, Example 86) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1a (2019-nCoV) | BDBM510066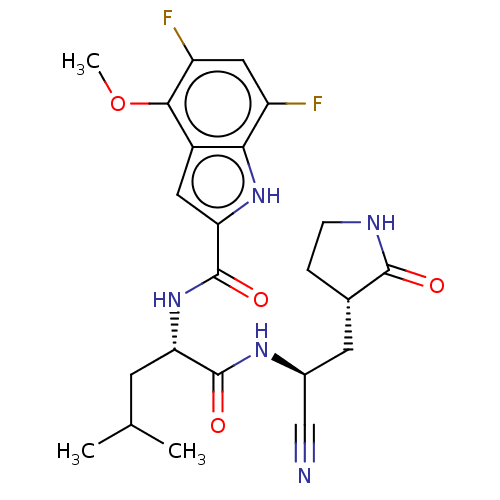 (N-[(2S)-1-({(1S)-1-Cyano-2-[(3S)-2-oxopyrrolidin-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The proteolytic activity of the main protease, 3CLpro, of SARS-CoV-2 was monitored using a continuous fluorescence resonance energy transfer (FRET) a... | Citation and Details BindingDB Entry DOI: 10.7270/Q2222Z08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 622 total ) | Next | Last >> |