 Found 193 hits with Last Name = 'mcphillie' and Initial = 'mj'
Found 193 hits with Last Name = 'mcphillie' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase ROS
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.254 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.259 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596396

(CHEMBL5201174)Show SMILES CCc1nc(C(N)=O)c(Cc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1CN1CCOCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM185674
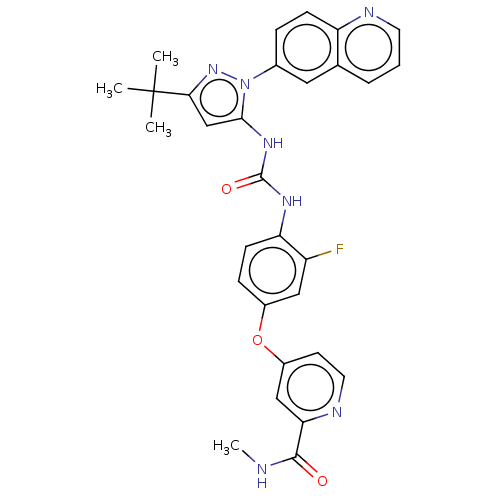
(4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1 Show InChI InChI=1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149
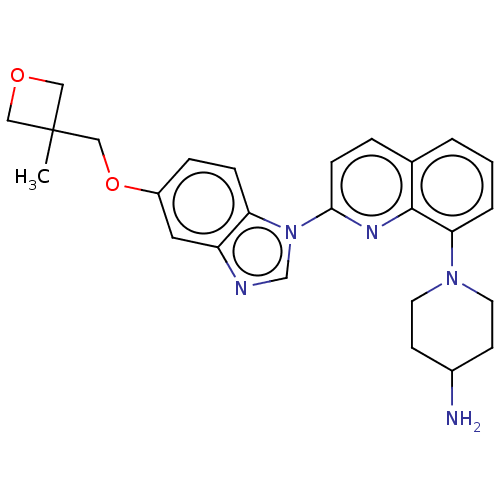
(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
ALK tyrosine kinase receptor
(Homo sapiens (Human)) | BDBM158154

(US10081622, Compound 11 | US10370379, Entrectinib ...)Show SMILES CN1CCN(CC1)c1ccc(C(=O)Nc2n[nH]c3ccc(Cc4cc(F)cc(F)c4)cc23)c(NC2CCOCC2)c1 Show InChI InChI=1S/C31H34F2N6O2/c1-38-8-10-39(11-9-38)25-3-4-26(29(19-25)34-24-6-12-41-13-7-24)31(40)35-30-27-17-20(2-5-28(27)36-37-30)14-21-15-22(32)18-23(33)16-21/h2-5,15-19,24,34H,6-14H2,1H3,(H2,35,36,37,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596396

(CHEMBL5201174)Show SMILES CCc1nc(C(N)=O)c(Cc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1CN1CCOCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596396

(CHEMBL5201174)Show SMILES CCc1nc(C(N)=O)c(Cc2ccc(N3CCC(CC3)N3CCN(C)CC3)c(OC)c2)nc1CN1CCOCC1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185674

(4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1 Show InChI InChI=1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149

(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50308060
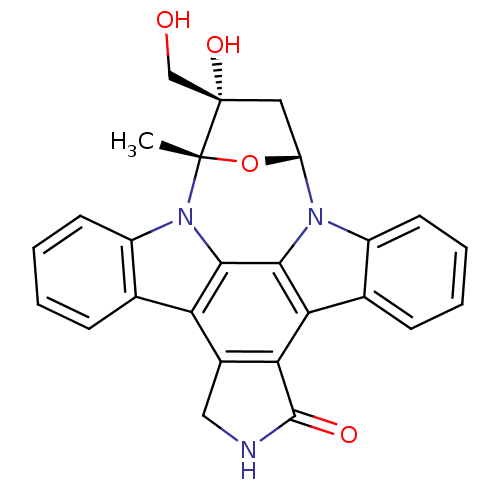
(16-hydroxy-16-(hydroxymethyl)-15-methyl-28-oxa-4,1...)Show SMILES C[C@]12O[C@H](C[C@]1(O)CO)n1c3ccccc3c3c4C(=O)NCc4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C26H21N3O4/c1-25-26(32,12-30)10-18(33-25)28-16-8-4-2-6-13(16)20-21-15(11-27-24(21)31)19-14-7-3-5-9-17(14)29(25)23(19)22(20)28/h2-9,18,30,32H,10-12H2,1H3,(H,27,31)/t18-,25+,26+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50300690

(1-(5-Tert-Butyl-1,2-Oxazol-3-Yl)-3-(4-{7-[2-(Morph...)Show SMILES CC(C)(C)c1cc(NC(=O)Nc2ccc(cc2)-c2cn3c(n2)sc2cc(OCCN4CCOCC4)ccc32)no1 Show InChI InChI=1S/C29H32N6O4S/c1-29(2,3)25-17-26(33-39-25)32-27(36)30-20-6-4-19(5-7-20)22-18-35-23-9-8-21(16-24(23)40-28(35)31-22)38-15-12-34-10-13-37-14-11-34/h4-9,16-18H,10-15H2,1-3H3,(H2,30,32,33,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
BDNF/NT-3 growth factors receptor
(Homo sapiens (Human)) | BDBM136597
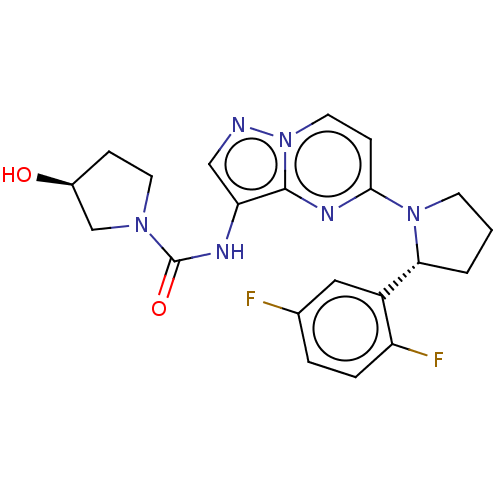
(US10005783, 14 | US10047097, 14 | US10774085, Exam...)Show SMILES O[C@H]1CCN(C1)C(=O)Nc1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N6O2/c22-13-3-4-16(23)15(10-13)18-2-1-7-28(18)19-6-9-29-20(26-19)17(11-24-29)25-21(31)27-8-5-14(30)12-27/h3-4,6,9-11,14,18,30H,1-2,5,7-8,12H2,(H,25,31)/t14-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM136597

(US10005783, 14 | US10047097, 14 | US10774085, Exam...)Show SMILES O[C@H]1CCN(C1)C(=O)Nc1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N6O2/c22-13-3-4-16(23)15(10-13)18-2-1-7-28(18)19-6-9-29-20(26-19)17(11-24-29)25-21(31)27-8-5-14(30)12-27/h3-4,6,9-11,14,18,30H,1-2,5,7-8,12H2,(H,25,31)/t14-,18+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50515042
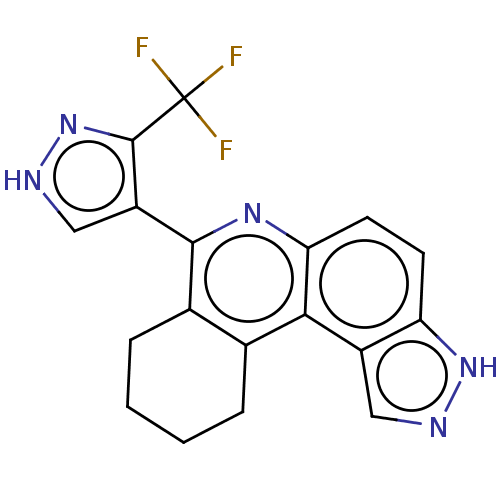
(CHEMBL4593398)Show SMILES FC(F)(F)c1n[nH]cc1-c1nc2ccc3[nH]ncc3c2c2CCCCc12 Show InChI InChI=1S/C18H14F3N5/c19-18(20,21)17-12(8-23-26-17)16-10-4-2-1-3-9(10)15-11-7-22-25-13(11)5-6-14(15)24-16/h5-8H,1-4H2,(H,22,25)(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
NT-3 growth factor receptor
(Homo sapiens (Human)) | BDBM136597

(US10005783, 14 | US10047097, 14 | US10774085, Exam...)Show SMILES O[C@H]1CCN(C1)C(=O)Nc1cnn2ccc(nc12)N1CCC[C@@H]1c1cc(F)ccc1F |r| Show InChI InChI=1S/C21H22F2N6O2/c22-13-3-4-16(23)15(10-13)18-2-1-7-28(18)19-6-9-29-20(26-19)17(11-24-29)25-21(31)27-8-5-14(30)12-27/h3-4,6,9-11,14,18,30H,1-2,5,7-8,12H2,(H,25,31)/t14-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM185674

(4-[4-[(5-tert-butyl-2-quinolin-6-ylpyrazol-3-yl)ca...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3cc(nn3-c3ccc4ncccc4c3)C(C)(C)C)c(F)c2)ccn1 Show InChI InChI=1S/C30H28FN7O3/c1-30(2,3)26-17-27(38(37-26)19-7-9-23-18(14-19)6-5-12-33-23)36-29(40)35-24-10-8-20(15-22(24)31)41-21-11-13-34-25(16-21)28(39)32-4/h5-17H,1-4H3,(H,32,39)(H2,35,36,40) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM185149

(1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol...)Show SMILES CC1(COc2ccc3n(cnc3c2)-c2ccc3cccc(N4CCC(N)CC4)c3n2)COC1 Show InChI InChI=1S/C26H29N5O2/c1-26(14-32-15-26)16-33-20-6-7-22-21(13-20)28-17-31(22)24-8-5-18-3-2-4-23(25(18)29-24)30-11-9-19(27)10-12-30/h2-8,13,17,19H,9-12,14-16,27H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50322535

(3-(imidazo[1,2-b]pyridazin-3-ylethynyl)-4-methyl-N...)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(c3)C#Cc3cnc4cccnn34)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C29H27F3N6O/c1-20-5-6-22(16-21(20)8-10-25-18-33-27-4-3-11-34-38(25)27)28(39)35-24-9-7-23(26(17-24)29(30,31)32)19-37-14-12-36(2)13-15-37/h3-7,9,11,16-18H,12-15,19H2,1-2H3,(H,35,39) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM8726

(5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...)Show InChI InChI=1S/C12H7Cl3O2/c13-7-1-3-11(9(15)5-7)17-12-4-2-8(14)6-10(12)16/h1-6,16H | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductase |
Bioorg Med Chem Lett 23: 3551-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.035
BindingDB Entry DOI: 10.7270/Q2XK8GZ7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50435717
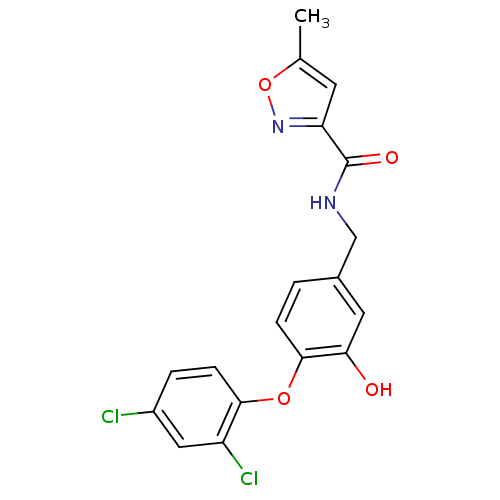
(CHEMBL2392450 | N‐{[4‐(2,4‐dichl...)Show SMILES Cc1cc(no1)C(=O)NCc1ccc(Oc2ccc(Cl)cc2Cl)c(O)c1 Show InChI InChI=1S/C18H14Cl2N2O4/c1-10-6-14(22-26-10)18(24)21-9-11-2-4-17(15(23)7-11)25-16-5-3-12(19)8-13(16)20/h2-8,23H,9H2,1H3,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductase |
Bioorg Med Chem Lett 23: 3551-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.035
BindingDB Entry DOI: 10.7270/Q2XK8GZ7 |
More data for this
Ligand-Target Pair | |
Lymphokine-activated killer T-cell-originated protein kinase
(Homo sapiens (Human)) | BDBM50515042

(CHEMBL4593398)Show SMILES FC(F)(F)c1n[nH]cc1-c1nc2ccc3[nH]ncc3c2c2CCCCc12 Show InChI InChI=1S/C18H14F3N5/c19-18(20,21)17-12(8-23-26-17)16-10-4-2-1-3-9(10)15-11-7-22-25-13(11)5-6-14(15)24-16/h5-8H,1-4H2,(H,22,25)(H,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
High affinity nerve growth factor receptor
(Homo sapiens (Human)) | BDBM50596395
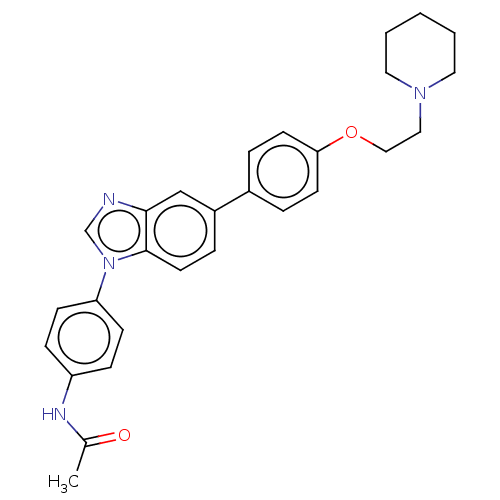
(CHEMBL5196221)Show SMILES CC(=O)Nc1ccc(cc1)-n1cnc2cc(ccc12)-c1ccc(OCCN2CCCCC2)cc1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50435720
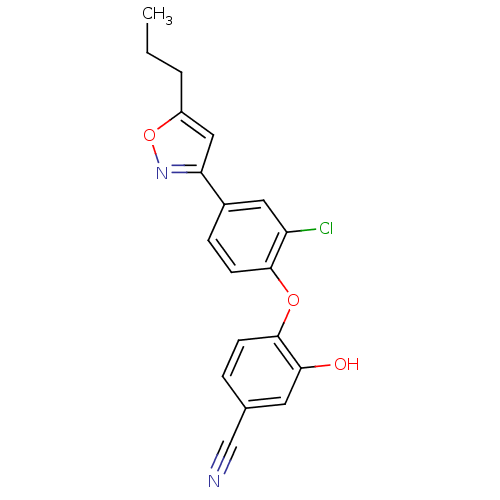
(CHEMBL2392447)Show InChI InChI=1S/C19H15ClN2O3/c1-2-3-14-10-16(22-25-14)13-5-7-18(15(20)9-13)24-19-6-4-12(11-21)8-17(19)23/h4-10,23H,2-3H2,1H3 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductase |
Bioorg Med Chem Lett 23: 3551-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.035
BindingDB Entry DOI: 10.7270/Q2XK8GZ7 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50435719
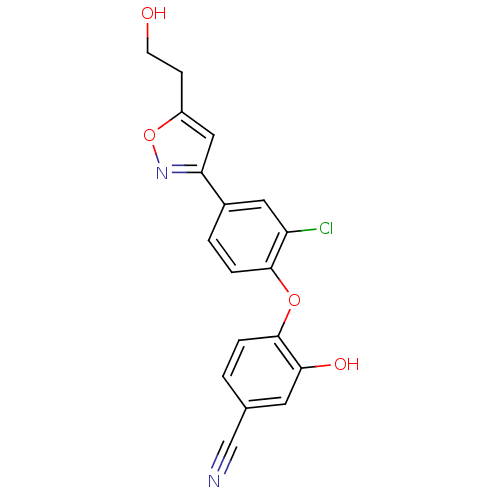
(CHEMBL2392448)Show InChI InChI=1S/C18H13ClN2O4/c19-14-8-12(15-9-13(5-6-22)25-21-15)2-4-17(14)24-18-3-1-11(10-20)7-16(18)23/h1-4,7-9,22-23H,5-6H2 | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductase |
Bioorg Med Chem Lett 23: 3551-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.035
BindingDB Entry DOI: 10.7270/Q2XK8GZ7 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887
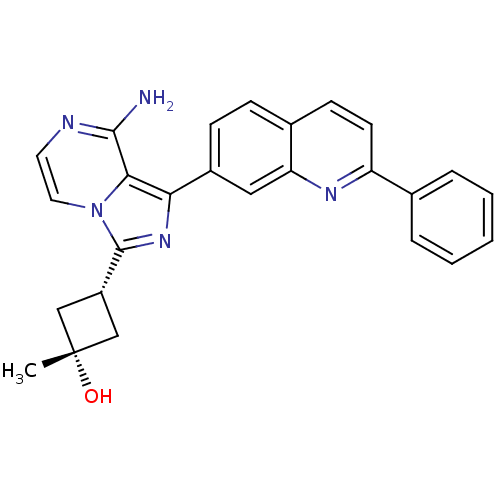
((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for the biological activity at the Beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596395

(CHEMBL5196221)Show SMILES CC(=O)Nc1ccc(cc1)-n1cnc2cc(ccc12)-c1ccc(OCCN2CCCCC2)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM4814

(CHEMBL535 | N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fl...)Show SMILES CCN(CC)CCNC(=O)c1c(C)[nH]c(\C=C2/C(=O)Nc3ccc(F)cc23)c1C Show InChI InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM161012
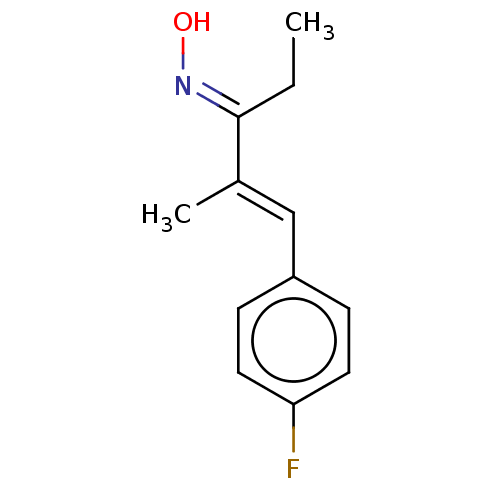
(US9108905, 1 | US9186360, 89)Show InChI InChI=1S/C12H14FNO/c1-3-12(14-15)9(2)8-10-4-6-11(13)7-5-10/h4-8,15H,3H2,1-2H3/b9-8+,14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hull
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPA1 expressed in HEK293F cells assessed as inhibition of AITC-induced increase in calcium influx by FLIPR analysis in ... |
Eur J Med Chem 170: 141-156 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.074
BindingDB Entry DOI: 10.7270/Q2F76GWS |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50315887

((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM161012

(US9108905, 1 | US9186360, 89)Show InChI InChI=1S/C12H14FNO/c1-3-12(14-15)9(2)8-10-4-6-11(13)7-5-10/h4-8,15H,3H2,1-2H3/b9-8+,14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hull
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPA1 expressed in HEK293 cells assessed as inhibition of CA-induced increase in calcium influx incubated for 10 mins pr... |
Eur J Med Chem 170: 141-156 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.074
BindingDB Entry DOI: 10.7270/Q2F76GWS |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily A member 1
(Homo sapiens (Human)) | BDBM161012

(US9108905, 1 | US9186360, 89)Show InChI InChI=1S/C12H14FNO/c1-3-12(14-15)9(2)8-10-4-6-11(13)7-5-10/h4-8,15H,3H2,1-2H3/b9-8+,14-12+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Hull
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPA1 expressed in HEK293 cells assessed as inhibition of CA-induced increase in calcium influx incubated for 10 mins pr... |
Eur J Med Chem 170: 141-156 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.074
BindingDB Entry DOI: 10.7270/Q2F76GWS |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50157879
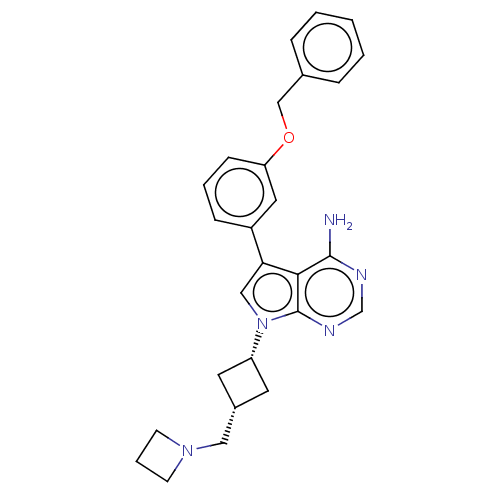
(CHEMBL1614712)Show SMILES [H][C@]1(CN2CCC2)C[C@]([H])(C1)n1cc(-c2cccc(OCc3ccccc3)c2)c2c(N)ncnc12 |wD:8.9,1.0,(6.47,-15.11,;5.71,-13.78,;4.93,-15.11,;5.69,-16.44,;5.68,-17.98,;7.22,-17.99,;7.23,-16.45,;5.31,-12.3,;6.81,-11.9,;6.02,-10.55,;7.2,-13.39,;7.59,-10.56,;6.61,-9.37,;7.45,-8.07,;7.04,-6.58,;5.56,-6.19,;5.15,-4.71,;6.24,-3.6,;7.73,-3.99,;8.81,-2.89,;10.15,-3.66,;11.48,-2.89,;12.81,-3.66,;14.12,-2.9,;14.13,-1.36,;12.8,-.59,;11.48,-1.36,;8.13,-5.47,;8.94,-8.47,;10.35,-7.7,;10.34,-6.15,;11.69,-8.46,;11.69,-10.01,;10.35,-10.79,;9.02,-10.01,)| Show InChI InChI=1S/C27H29N5O/c28-26-25-24(21-8-4-9-23(14-21)33-17-19-6-2-1-3-7-19)16-32(27(25)30-18-29-26)22-12-20(13-22)15-31-10-5-11-31/h1-4,6-9,14,16,18,20,22H,5,10-13,15,17H2,(H2,28,29,30)/t20-,22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Activity at beta-1 adrenergic receptor |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596395

(CHEMBL5196221)Show SMILES CC(=O)Nc1ccc(cc1)-n1cnc2cc(ccc12)-c1ccc(OCCN2CCCCC2)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596395

(CHEMBL5196221)Show SMILES CC(=O)Nc1ccc(cc1)-n1cnc2cc(ccc12)-c1ccc(OCCN2CCCCC2)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |
Enoyl-acyl carrier reductase ENR
(Toxoplasma gondii) | BDBM50435718
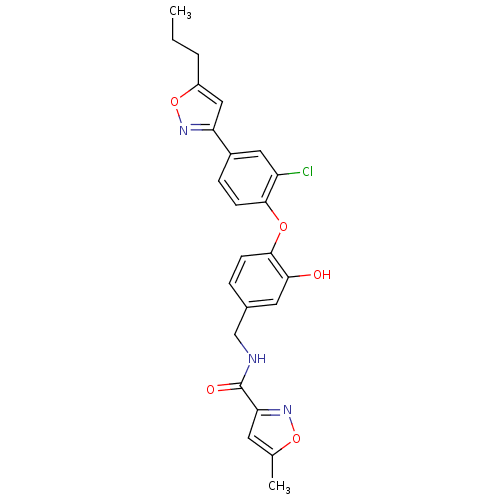
(CHEMBL2392449)Show SMILES CCCc1cc(no1)-c1ccc(Oc2ccc(CNC(=O)c3cc(C)on3)cc2O)c(Cl)c1 Show InChI InChI=1S/C24H22ClN3O5/c1-3-4-17-12-19(27-33-17)16-6-8-22(18(25)11-16)31-23-7-5-15(10-21(23)29)13-26-24(30)20-9-14(2)32-28-20/h5-12,29H,3-4,13H2,1-2H3,(H,26,30) | PDB
MMDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Leeds
Curated by ChEMBL
| Assay Description
Inhibition of Toxoplasma gondii enoyl acyl-carrier protein reductase |
Bioorg Med Chem Lett 23: 3551-5 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.035
BindingDB Entry DOI: 10.7270/Q2XK8GZ7 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50596394

(CHEMBL4532415)Show SMILES COc1cc2c(ccnc2cc1OCCCN1CCCCC1)N1CCN(CC1)C(=O)Nc1ccc(OC(C)C)cc1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116596
BindingDB Entry DOI: 10.7270/Q2MK6HXN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data