 Found 47 hits with Last Name = 'michener' and Initial = 'ml'
Found 47 hits with Last Name = 'michener' and Initial = 'ml' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50105264
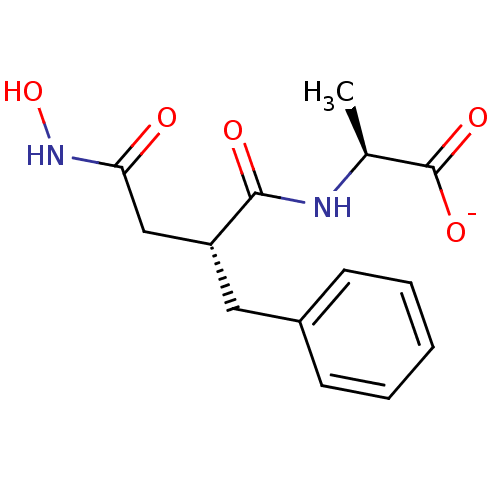
(2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...)Show SMILES C[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C([O-])=O Show InChI InChI=1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/p-1/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against LTA 4 hydrolase in aminopeptidase assay |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50105264

(2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...)Show SMILES C[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C([O-])=O Show InChI InChI=1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/p-1/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against LTA 4 hydrolase in epoxide hydrolase assay |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285632
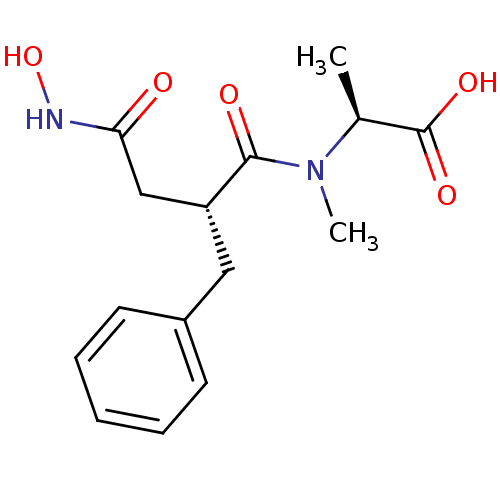
((S)-2-[((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)...)Show SMILES C[C@H](N(C)C(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C15H20N2O5/c1-10(15(20)21)17(2)14(19)12(9-13(18)16-22)8-11-6-4-3-5-7-11/h3-7,10,12,22H,8-9H2,1-2H3,(H,16,18)(H,20,21)/t10-,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285639
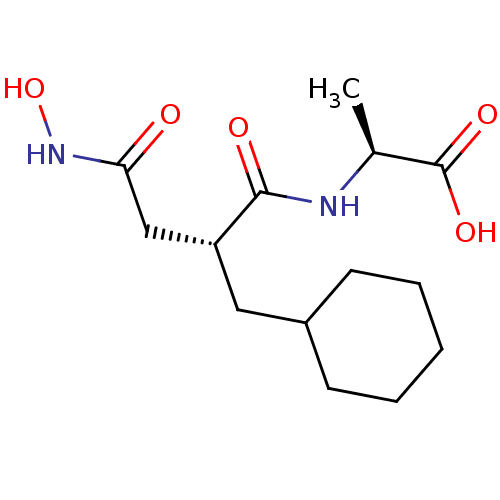
((S)-2-((R)-2-Cyclohexylmethyl-3-hydroxycarbamoyl-p...)Show SMILES C[C@H](NC(=O)[C@H](CC1CCCCC1)CC(=O)NO)C(O)=O Show InChI InChI=1S/C14H24N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h9-11,21H,2-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50105264

(2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...)Show SMILES C[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C([O-])=O Show InChI InChI=1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/p-1/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50024101
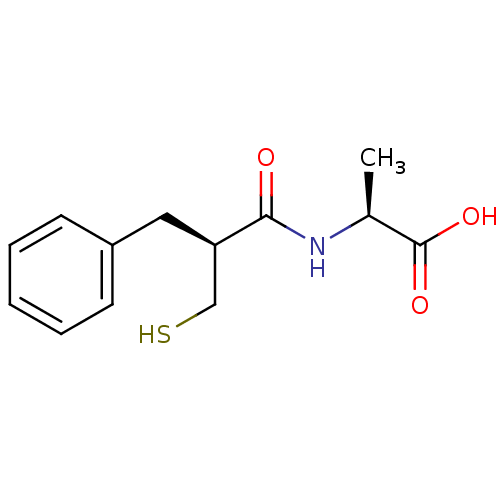
((S)-2-((S)-2-Mercaptomethyl-3-phenyl-propionylamin...)Show InChI InChI=1S/C13H17NO3S/c1-9(13(16)17)14-12(15)11(8-18)7-10-5-3-2-4-6-10/h2-6,9,11,18H,7-8H2,1H3,(H,14,15)(H,16,17)/t9-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit kidney neutral endopeptidase (NEP) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285636
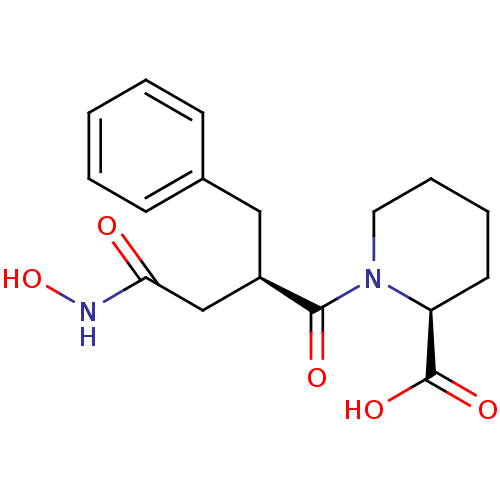
((S)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCCC[C@H]1C(O)=O Show InChI InChI=1S/C17H22N2O5/c20-15(18-24)11-13(10-12-6-2-1-3-7-12)16(21)19-9-5-4-8-14(19)17(22)23/h1-3,6-7,13-14,24H,4-5,8-11H2,(H,18,20)(H,22,23)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285630
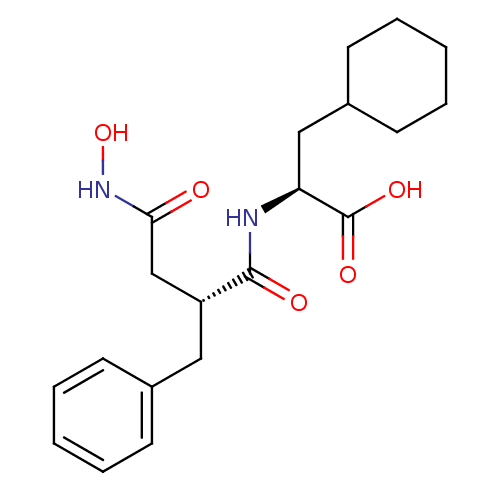
((S)-2-((R)-2-Benzyl-3-hydroxycarbamoyl-propionylam...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(O)=O Show InChI InChI=1S/C20H28N2O5/c23-18(22-27)13-16(11-14-7-3-1-4-8-14)19(24)21-17(20(25)26)12-15-9-5-2-6-10-15/h1,3-4,7-8,15-17,27H,2,5-6,9-13H2,(H,21,24)(H,22,23)(H,25,26)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285640
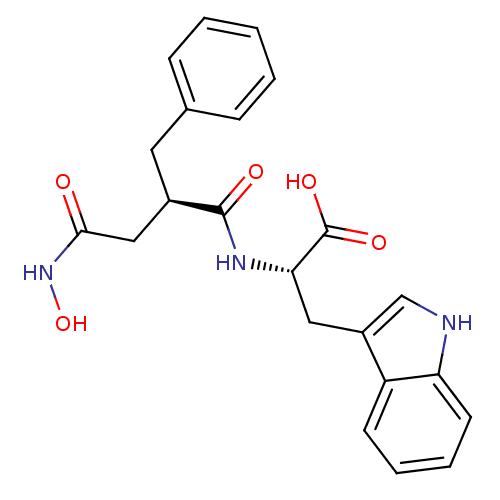
((S)-2-((R)-2-Benzyl-3-hydroxycarbamoyl-propionylam...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C22H23N3O5/c26-20(25-30)12-15(10-14-6-2-1-3-7-14)21(27)24-19(22(28)29)11-16-13-23-18-9-5-4-8-17(16)18/h1-9,13,15,19,23,30H,10-12H2,(H,24,27)(H,25,26)(H,28,29)/t15-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285637
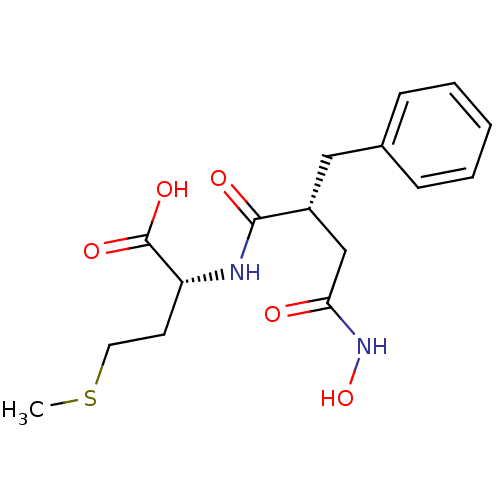
((R)-2-((R)-2-Benzyl-3-hydroxycarbamoyl-propionylam...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H22N2O5S/c1-24-8-7-13(16(21)22)17-15(20)12(10-14(19)18-23)9-11-5-3-2-4-6-11/h2-6,12-13,23H,7-10H2,1H3,(H,17,20)(H,18,19)(H,21,22)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50312743
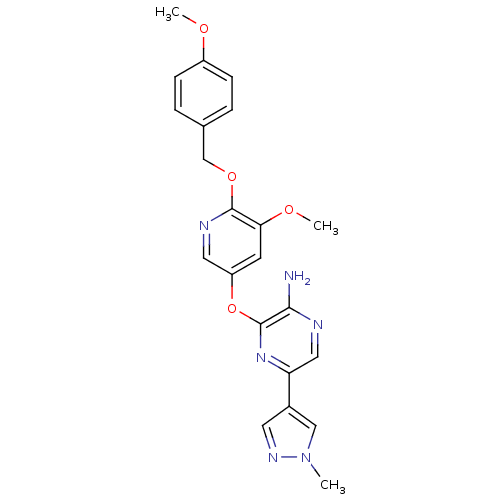
(3-(5-methoxy-6-(4-methoxybenzyloxy)pyridin-3-yloxy...)Show SMILES COc1ccc(COc2ncc(Oc3nc(cnc3N)-c3cnn(C)c3)cc2OC)cc1 Show InChI InChI=1S/C22H22N6O4/c1-28-12-15(9-26-28)18-11-24-20(23)22(27-18)32-17-8-19(30-3)21(25-10-17)31-13-14-4-6-16(29-2)7-5-14/h4-12H,13H2,1-3H3,(H2,23,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R autophosphorylation expressed in HEK293 cells after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1543-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.078
BindingDB Entry DOI: 10.7270/Q2513Z98 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50312742

(3-((5-methoxy-6-(4-methoxybenzyloxy)pyridin-3-yl)m...)Show SMILES COc1ccc(COc2ncc(COc3nc(cnc3N)-c3cnn(C)c3)cc2OC)cc1 Show InChI InChI=1S/C23H24N6O4/c1-29-12-17(10-27-29)19-11-25-21(24)23(28-19)33-14-16-8-20(31-3)22(26-9-16)32-13-15-4-6-18(30-2)7-5-15/h4-12H,13-14H2,1-3H3,(H2,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R autophosphorylation expressed in HEK293 cells after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1543-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.078
BindingDB Entry DOI: 10.7270/Q2513Z98 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285635
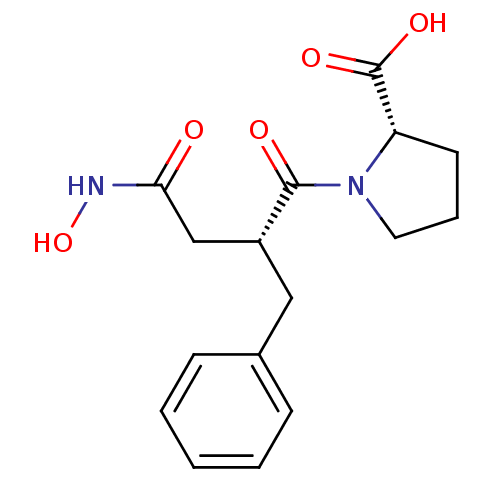
((S)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C16H20N2O5/c19-14(17-23)10-12(9-11-5-2-1-3-6-11)15(20)18-8-4-7-13(18)16(21)22/h1-3,5-6,12-13,23H,4,7-10H2,(H,17,19)(H,21,22)/t12-,13+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50312735
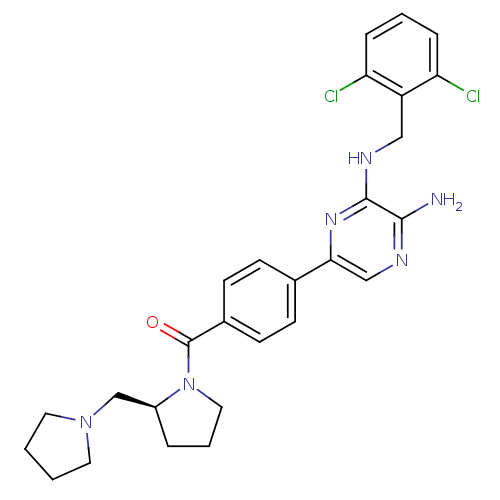
((S)-(4-(5-amino-6-(2,6-dichlorobenzylamino)pyrazin...)Show SMILES Nc1ncc(nc1NCc1c(Cl)cccc1Cl)-c1ccc(cc1)C(=O)N1CCC[C@H]1CN1CCCC1 |r| Show InChI InChI=1S/C27H30Cl2N6O/c28-22-6-3-7-23(29)21(22)15-32-26-25(30)31-16-24(33-26)18-8-10-19(11-9-18)27(36)35-14-4-5-20(35)17-34-12-1-2-13-34/h3,6-11,16,20H,1-2,4-5,12-15,17H2,(H2,30,31)(H,32,33)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R autophosphorylation expressed in HEK293 cells after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1543-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.078
BindingDB Entry DOI: 10.7270/Q2513Z98 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50105264

(2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...)Show SMILES C[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C([O-])=O Show InChI InChI=1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/p-1/t9-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit kidney neutral endopeptidase (NEP) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50312740

(3-(3-methoxy-4-(4-methoxybenzyloxy)benzyloxy)-5-(1...)Show SMILES COc1ccc(COc2ccc(COc3nc(cnc3N)-c3cnn(C)c3)cc2OC)cc1 Show InChI InChI=1S/C24H25N5O4/c1-29-13-18(11-27-29)20-12-26-23(25)24(28-20)33-15-17-6-9-21(22(10-17)31-3)32-14-16-4-7-19(30-2)8-5-16/h4-13H,14-15H2,1-3H3,(H2,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R autophosphorylation expressed in HEK293 cells after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1543-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.078
BindingDB Entry DOI: 10.7270/Q2513Z98 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50312736
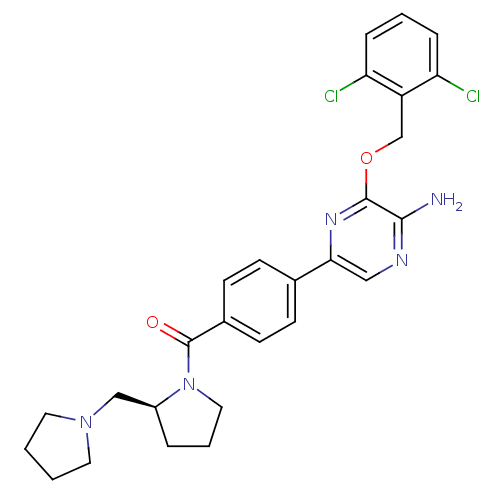
((S)-(4-(5-amino-6-(2,6-dichlorobenzyloxy)pyrazin-2...)Show SMILES Nc1ncc(nc1OCc1c(Cl)cccc1Cl)-c1ccc(cc1)C(=O)N1CCC[C@H]1CN1CCCC1 |r| Show InChI InChI=1S/C27H29Cl2N5O2/c28-22-6-3-7-23(29)21(22)17-36-26-25(30)31-15-24(32-26)18-8-10-19(11-9-18)27(35)34-14-4-5-20(34)16-33-12-1-2-13-33/h3,6-11,15,20H,1-2,4-5,12-14,16-17H2,(H2,30,31)/t20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R autophosphorylation expressed in HEK293 cells after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1543-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.078
BindingDB Entry DOI: 10.7270/Q2513Z98 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50312741

(3-((4-methoxy-5-(4-methoxybenzyloxy)pyridin-2-yl)m...)Show SMILES COc1ccc(COc2cnc(COc3nc(cnc3N)-c3cnn(C)c3)cc2OC)cc1 Show InChI InChI=1S/C23H24N6O4/c1-29-12-16(9-27-29)19-10-26-22(24)23(28-19)33-14-17-8-20(31-3)21(11-25-17)32-13-15-4-6-18(30-2)7-5-15/h4-12H,13-14H2,1-3H3,(H2,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 143 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R autophosphorylation expressed in HEK293 cells after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1543-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.078
BindingDB Entry DOI: 10.7270/Q2513Z98 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50024101

((S)-2-((S)-2-Mercaptomethyl-3-phenyl-propionylamin...)Show InChI InChI=1S/C13H17NO3S/c1-9(13(16)17)14-12(15)11(8-18)7-10-5-3-2-4-6-10/h2-6,9,11,18H,7-8H2,1H3,(H,14,15)(H,16,17)/t9-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme(ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285633
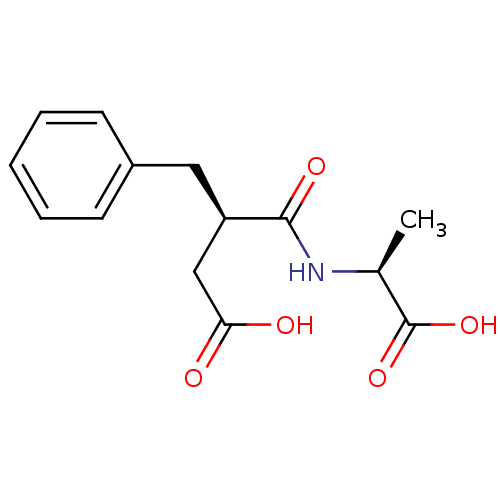
((R)-3-Benzyl-N-((S)-1-carboxy-ethyl)-succinamic ac...)Show SMILES C[C@H](NC(=O)[C@@H](CC(O)=O)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C14H17NO5/c1-9(14(19)20)15-13(18)11(8-12(16)17)7-10-5-3-2-4-6-10/h2-6,9,11H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50312737

(CHEMBL1088040 | N2-(2,6-dichlorobenzyl)-6-(1-methy...)Show InChI InChI=1S/C15H14Cl2N6/c1-23-8-9(5-21-23)13-7-19-14(18)15(22-13)20-6-10-11(16)3-2-4-12(10)17/h2-5,7-8H,6H2,1H3,(H2,18,19)(H,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 345 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R autophosphorylation expressed in HEK293 cells after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1543-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.078
BindingDB Entry DOI: 10.7270/Q2513Z98 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50312738
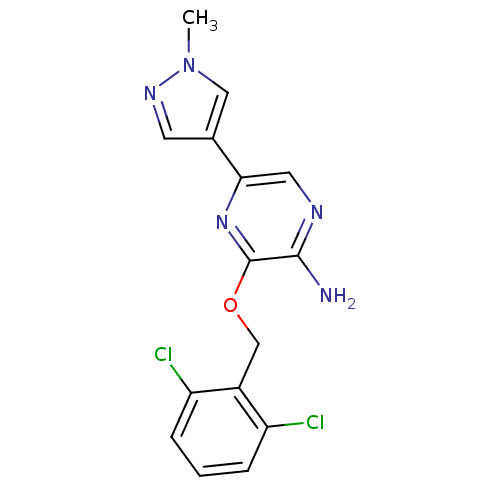
(3-(2,6-dichlorobenzyloxy)-5-(1-methyl-1H-pyrazol-4...)Show InChI InChI=1S/C15H13Cl2N5O/c1-22-7-9(5-20-22)13-6-19-14(18)15(21-13)23-8-10-11(16)3-2-4-12(10)17/h2-7H,8H2,1H3,(H2,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R autophosphorylation expressed in HEK293 cells after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1543-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.078
BindingDB Entry DOI: 10.7270/Q2513Z98 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285634

((R)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCCC[C@@H]1C(O)=O Show InChI InChI=1S/C17H22N2O5/c20-15(18-24)11-13(10-12-6-2-1-3-7-12)16(21)19-9-5-4-8-14(19)17(22)23/h1-3,6-7,13-14,24H,4-5,8-11H2,(H,18,20)(H,22,23)/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50312739
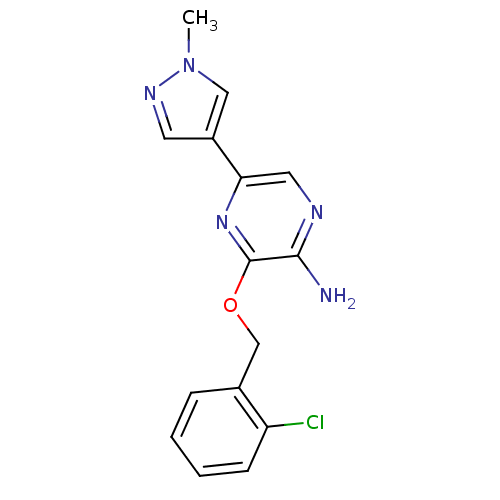
(3-(2-chlorobenzyloxy)-5-(1-methyl-1H-pyrazol-4-yl)...)Show InChI InChI=1S/C15H14ClN5O/c1-21-8-11(6-19-21)13-7-18-14(17)15(20-13)22-9-10-4-2-3-5-12(10)16/h2-8H,9H2,1H3,(H2,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R autophosphorylation expressed in HEK293 cells after 1 hr by ELISA |
Bioorg Med Chem Lett 20: 1543-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.078
BindingDB Entry DOI: 10.7270/Q2513Z98 |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50285639

((S)-2-((R)-2-Cyclohexylmethyl-3-hydroxycarbamoyl-p...)Show SMILES C[C@H](NC(=O)[C@H](CC1CCCCC1)CC(=O)NO)C(O)=O Show InChI InChI=1S/C14H24N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h9-11,21H,2-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/t9-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit kidney neutral endopeptidase (NEP) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285635

((S)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C16H20N2O5/c19-14(17-23)10-12(9-11-5-2-1-3-6-11)15(20)18-8-4-7-13(18)16(21)22/h1-3,5-6,12-13,23H,4,7-10H2,(H,17,19)(H,21,22)/t12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285641
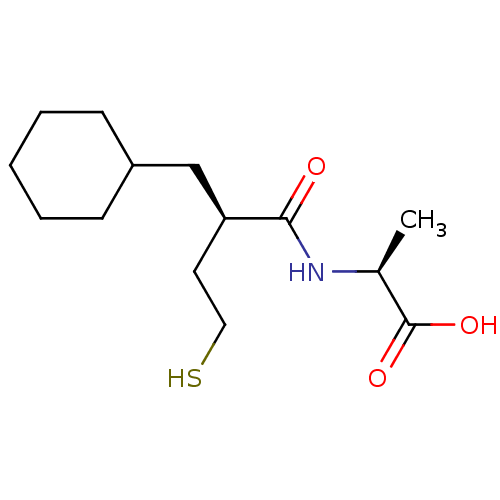
((S)-2-((R)-2-Cyclohexylmethyl-4-mercapto-butyrylam...)Show InChI InChI=1S/C14H25NO3S/c1-10(14(17)18)15-13(16)12(7-8-19)9-11-5-3-2-4-6-11/h10-12,19H,2-9H2,1H3,(H,15,16)(H,17,18)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50024101

((S)-2-((S)-2-Mercaptomethyl-3-phenyl-propionylamin...)Show InChI InChI=1S/C13H17NO3S/c1-9(13(16)17)14-12(15)11(8-18)7-10-5-3-2-4-6-10/h2-6,9,11,18H,7-8H2,1H3,(H,14,15)(H,16,17)/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50285632

((S)-2-[((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)...)Show SMILES C[C@H](N(C)C(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C15H20N2O5/c1-10(15(20)21)17(2)14(19)12(9-13(18)16-22)8-11-6-4-3-5-7-11/h3-7,10,12,22H,8-9H2,1-2H3,(H,16,18)(H,20,21)/t10-,12+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit kidney neutral endopeptidase (NEP) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM21642

((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...)Show InChI InChI=1S/C9H15NO3S/c1-6(5-14)8(11)10-4-2-3-7(10)9(12)13/h6-7,14H,2-5H2,1H3,(H,12,13)/t6-,7+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50105264

(2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...)Show SMILES C[C@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C([O-])=O Show InChI InChI=1S/C14H18N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h2-6,9,11,21H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/p-1/t9-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285631
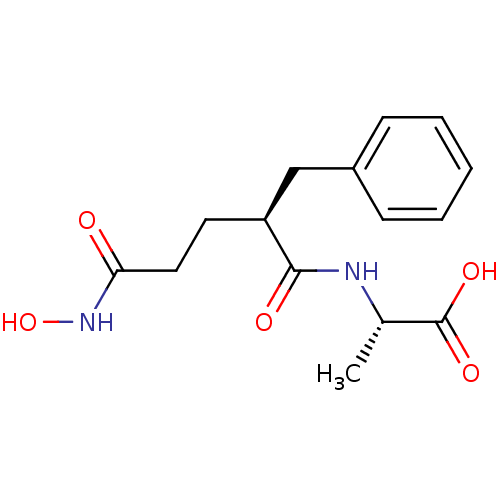
((S)-2-((S)-2-Benzyl-4-hydroxycarbamoyl-butyrylamin...)Show SMILES C[C@H](NC(=O)[C@@H](CCC(=O)NO)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C15H20N2O5/c1-10(15(20)21)16-14(19)12(7-8-13(18)17-22)9-11-5-3-2-4-6-11/h2-6,10,12,22H,7-9H2,1H3,(H,16,19)(H,17,18)(H,20,21)/t10-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50285636

((S)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCCC[C@H]1C(O)=O Show InChI InChI=1S/C17H22N2O5/c20-15(18-24)11-13(10-12-6-2-1-3-7-12)16(21)19-9-5-4-8-14(19)17(22)23/h1-3,6-7,13-14,24H,4-5,8-11H2,(H,18,20)(H,22,23)/t13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit kidney neutral endopeptidase (NEP) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285637

((R)-2-((R)-2-Benzyl-3-hydroxycarbamoyl-propionylam...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H22N2O5S/c1-24-8-7-13(16(21)22)17-15(20)12(10-14(19)18-23)9-11-5-3-2-4-6-11/h2-6,12-13,23H,7-10H2,1H3,(H,17,20)(H,18,19)(H,21,22)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme(ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285631

((S)-2-((S)-2-Benzyl-4-hydroxycarbamoyl-butyrylamin...)Show SMILES C[C@H](NC(=O)[C@@H](CCC(=O)NO)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C15H20N2O5/c1-10(15(20)21)16-14(19)12(7-8-13(18)17-22)9-11-5-3-2-4-6-11/h2-6,10,12,22H,7-9H2,1H3,(H,16,19)(H,17,18)(H,20,21)/t10-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme(ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285638
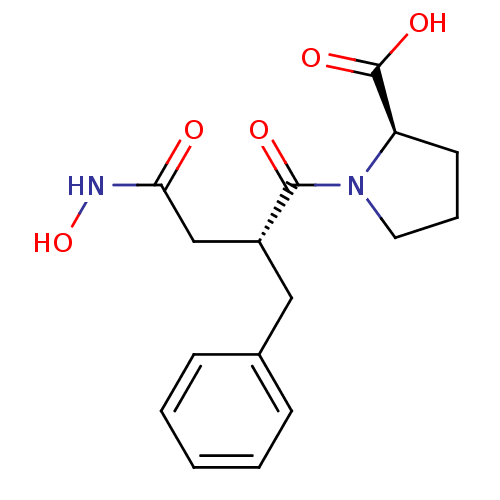
((R)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCC[C@@H]1C(O)=O Show InChI InChI=1S/C16H20N2O5/c19-14(17-23)10-12(9-11-5-2-1-3-6-11)15(20)18-8-4-7-13(18)16(21)22/h1-3,5-6,12-13,23H,4,7-10H2,(H,17,19)(H,21,22)/t12-,13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against recombinant human Leukotriene A4 hydrolase |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50285635

((S)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(O)=O Show InChI InChI=1S/C16H20N2O5/c19-14(17-23)10-12(9-11-5-2-1-3-6-11)15(20)18-8-4-7-13(18)16(21)22/h1-3,5-6,12-13,23H,4,7-10H2,(H,17,19)(H,21,22)/t12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit kidney neutral endopeptidase (NEP) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285636

((S)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCCC[C@H]1C(O)=O Show InChI InChI=1S/C17H22N2O5/c20-15(18-24)11-13(10-12-6-2-1-3-7-12)16(21)19-9-5-4-8-14(19)17(22)23/h1-3,6-7,13-14,24H,4-5,8-11H2,(H,18,20)(H,22,23)/t13-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme(ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50285637

((R)-2-((R)-2-Benzyl-3-hydroxycarbamoyl-propionylam...)Show SMILES CSCC[C@@H](NC(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C16H22N2O5S/c1-24-8-7-13(16(21)22)17-15(20)12(10-14(19)18-23)9-11-5-3-2-4-6-11/h2-6,12-13,23H,7-10H2,1H3,(H,17,20)(H,18,19)(H,21,22)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit kidney neutral endopeptidase (NEP) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285632

((S)-2-[((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)...)Show SMILES C[C@H](N(C)C(=O)[C@@H](CC(=O)NO)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C15H20N2O5/c1-10(15(20)21)17(2)14(19)12(9-13(18)16-22)8-11-6-4-3-5-7-11/h3-7,10,12,22H,8-9H2,1-2H3,(H,16,18)(H,20,21)/t10-,12+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme(ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285633

((R)-3-Benzyl-N-((S)-1-carboxy-ethyl)-succinamic ac...)Show SMILES C[C@H](NC(=O)[C@@H](CC(O)=O)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C14H17NO5/c1-9(14(19)20)15-13(18)11(8-12(16)17)7-10-5-3-2-4-6-10/h2-6,9,11H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/t9-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme(ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285638

((R)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCC[C@@H]1C(O)=O Show InChI InChI=1S/C16H20N2O5/c19-14(17-23)10-12(9-11-5-2-1-3-6-11)15(20)18-8-4-7-13(18)16(21)22/h1-3,5-6,12-13,23H,4,7-10H2,(H,17,19)(H,21,22)/t12-,13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme (ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50285641

((S)-2-((R)-2-Cyclohexylmethyl-4-mercapto-butyrylam...)Show InChI InChI=1S/C14H25NO3S/c1-10(14(17)18)15-13(16)12(7-8-19)9-11-5-3-2-4-6-11/h10-12,19H,2-9H2,1H3,(H,15,16)(H,17,18)/t10-,12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit kidney neutral endopeptidase (NEP) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285639

((S)-2-((R)-2-Cyclohexylmethyl-3-hydroxycarbamoyl-p...)Show SMILES C[C@H](NC(=O)[C@H](CC1CCCCC1)CC(=O)NO)C(O)=O Show InChI InChI=1S/C14H24N2O5/c1-9(14(19)20)15-13(18)11(8-12(17)16-21)7-10-5-3-2-4-6-10/h9-11,21H,2-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/t9-,11+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme(ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285630

((S)-2-((R)-2-Benzyl-3-hydroxycarbamoyl-propionylam...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(O)=O Show InChI InChI=1S/C20H28N2O5/c23-18(22-27)13-16(11-14-7-3-1-4-8-14)19(24)21-17(20(25)26)12-15-9-5-2-6-10-15/h1,3-4,7-8,15-17,27H,2,5-6,9-13H2,(H,21,24)(H,22,23)(H,25,26)/t16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme(ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Angiotensin-converting enzyme
(Oryctolagus cuniculus) | BDBM50285634

((R)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCCC[C@@H]1C(O)=O Show InChI InChI=1S/C17H22N2O5/c20-15(18-24)11-13(10-12-6-2-1-3-7-12)16(21)19-9-5-4-8-14(19)17(22)23/h1-3,6-7,13-14,24H,4-5,8-11H2,(H,18,20)(H,22,23)/t13-,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit lung angiotensin-converting enzyme(ACE) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |
Neprilysin
(Oryctolagus cuniculus (rabbit)) | BDBM50285630

((S)-2-((R)-2-Benzyl-3-hydroxycarbamoyl-propionylam...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(O)=O Show InChI InChI=1S/C20H28N2O5/c23-18(22-27)13-16(11-14-7-3-1-4-8-14)19(24)21-17(20(25)26)12-15-9-5-2-6-10-15/h1,3-4,7-8,15-17,27H,2,5-6,9-13H2,(H,21,24)(H,22,23)(H,25,26)/t16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against rabbit kidney neutral endopeptidase (NEP) |
Bioorg Med Chem Lett 5: 2517-2522 (1995)
Article DOI: 10.1016/0960-894X(95)00441-U
BindingDB Entry DOI: 10.7270/Q2959J1M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data